Effects of Rumen-Protected 5-Hydroxytryptophan and Melatonin Supplementation on Antioxidant Capacity, Meat Quality, and Shelf Life of Hu Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Materials
2.3. Diets, Experimental Design, and Animal Management
2.4. Growth Performance
2.5. Slaughter Performance and Muscle Sampling
2.6. Sample Preparation and Determination
2.6.1. Determination of MT Content
2.6.2. Determination of Antioxidant Activity
2.6.3. Determination of Meat Quality
2.6.4. Determination of Amino Acids and Fatty Acids
2.6.5. Determination of Shelf Life
2.7. Statistical Analysis
3. Results
3.1. Effects of RPT 5-HTP and MT Supplementation on Growth Performance of Hu Sheep
3.2. Effects of RPT 5-HTP and MT Supplementation on Slaughter Performance of Hu Sheep
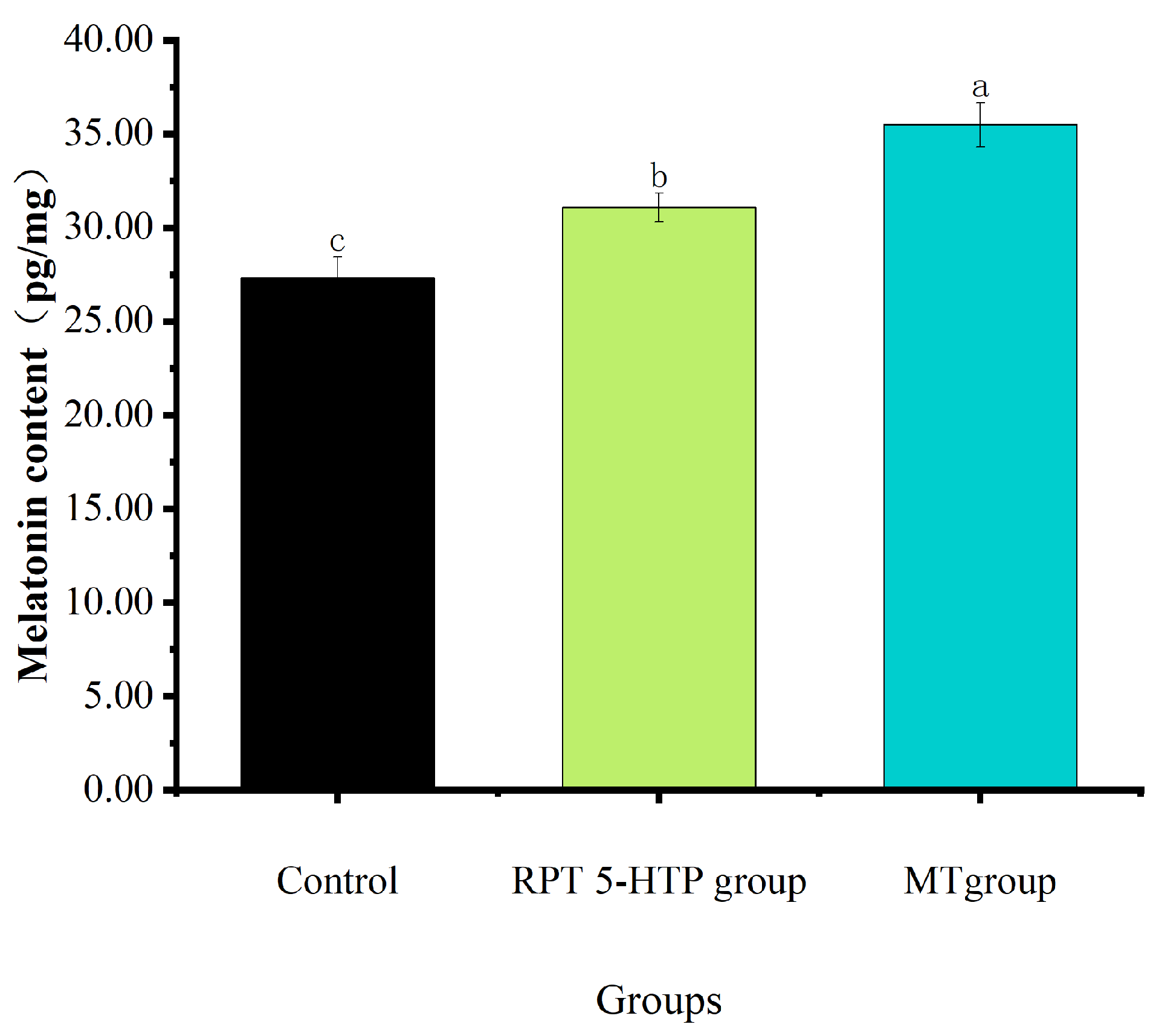
3.3. MT Content
3.4. Antioxidation
3.5. Meat Quality
3.6. Amino Acid
3.7. Fatty Acid
3.8. Shelf Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murariu, O.C.; Murariu, F.; Frunză, G.; Ciobanu, M.M.; Boișteanu, P.C. Fatty acid indices and the nutritional properties of karakul sheep meat. Nutrients 2023, 15, 1061. [Google Scholar] [CrossRef]
- Ripoll, G.; Joy, M.; Panea, B. Consumer perception of the quality of lamb and lamb confit. Foods 2018, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.J.; Dou, M.L.; Wang, X.Y.; Li, Z.; Shi, X.E.; Li, X. Relationship between antioxidant capacity of postmortem muscle and meat quality. Chin. J. Anim. Nutr. 2018, 30, 1676–1680. [Google Scholar]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Bubenik, G.A. Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Neurosignals 2001, 10, 350–366. [Google Scholar] [CrossRef]
- Vasey, C.; McBride, J.; Penta, K. Circadian rhythm dysregulation and restoration: The role of melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Yin, Y. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Cui, Y.F.; Qiu, X.J. Effects and Mechanism of Melatonin on Body Antioxidant Capacity and Lipid Metabolism. Chin. J. Anim. Sci. 2023, 59, 1–9. [Google Scholar] [CrossRef]
- Feng, L.; Jiang, X.; Kitazawa, H.; Wang, X.; Guo, Y.; Li, L.; Wang, J. Characterization of bioactive films loaded with melatonin and regulation of postharvest ROS scavenging and ascorbate-glutathione cycle in Agaricus bisporus. Postharvest Biol. Technol. 2022, 194, 112107. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Z.; Wang, J.; Fu, Y.; Zhang, Z.; Khan, M.R.; Cong, X. Effect of exogenous melatonin on postharvest storage quality of passion fruit through antioxidant metabolism. LWT 2024, 194, 115835. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Z.; Ding, X.; Jiang, X.; Tan, L.; Lin, C.; Li, M. RP58 knockdown contributes to hypoxia-ischemia-induced pineal dysfunction and circadian rhythm disruption in neonatal rats. J. Pineal Res. 2023, 75, e12885. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.A.; Forcada, F.; Vázquez, M.I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Pérez-Pe, R.; Casao, A. Role of melatonin on embryo viability in sheep. Reprod. Fertil. Dev. 2019, 31, 82–92. [Google Scholar] [CrossRef]
- Yang, C.H.; Wu, Z.Y.; Li, Y.; Zhang, W. Effect of melatonin administration to lactating cashmere goats on milk production of dams and on hair follicle development in their offspring. Animal 2020, 14, 1241–1248. [Google Scholar] [CrossRef]
- Viola, I.; Canto, F.; Abecia, J.A. Effects of melatonin implants on locomotor activity, body temperature, and growth of lambs fed a concentrate-based diet. J. Vet. Behav. 2023, 68, 24–31. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Gonzalez-Candia, A.; Veliz, M.; Carrasco-Pozo, C.; Castillo, R.L.; Cárdenas, J.C.; Ebensperger, G.; Herrera, E.A. Antenatal melatonin modulates an enhanced antioxidant/pro-oxidant ratio in pulmonary hypertensive newborn sheep. Redox Biol. 2019, 22, 101128. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.K.; Ishwar, A.K.; Kumar, R.; Niyogi, D.; Kumar, M. Effect of exogenous melatonin and different photoperiods on oxidative status and antioxidant enzyme activity in Chhotanagpuri ewe. Vet. World 2018, 11, 130–134. [Google Scholar] [CrossRef]
- Pezeshk, S.; Rezaei, M.; Hosseini, H. Effects of turmeric, shallot extracts, and their combination on quality characteristics of vacuum-packaged rainbow trout stored at 4 ± 1 C. J. Food Sci. 2011, 76, 387–391. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, G.; Li, X.; Zhao, G.; Chen, H.; Ma, C.; Yang, K. Effect of diet supplemented with rumen-protected 5-hydroxytryptophan on the concentration of 5-hydroxytryptophan and melatonin in the plasma of sheep. Pak. J. Zool 2022, 54, 777–783. [Google Scholar] [CrossRef]
- Hou, C.X.; Sun, S.; Mei, Y.Y.; Ma, X.C.; Zhang, W.J.; Deng, H.F.; Zhao, G.D.; Yang, K.L. Effects of supplementation with different levels of melatonin on plasma melatonin content, urine 6-hydroxymelatonin content and antioxidant capacity of sheep. China Herbiv. Sci. 2024, 44, 37–41. [Google Scholar] [CrossRef]
- GB 5009.228-2016; National Food Safety Standard Determination of Volatile Basic Nitrogen in Foods. National Health and Family Planning Commission: Beijing, China, 2016.
- GB 2707-2016; National Food Safety Standard for Fresh (Frozen) Livestock and Poultry Products. National Health and Family Planning Commission: Beijing, China, 2016.
- Rodríguez, A.B.; Bodas, R.; Landa, R.; López-Campos, Ó.; Mantecón, A.R.; Giráldez, F.J. Animal performance, carcass traits and meat characteristics of Assaf and Merino× Assaf growing lambs. Livest. Sci. 2011, 138, 13–19. [Google Scholar] [CrossRef]
- Sahin, N.; Onderci, M.; Sahin, K.; Gursu, M.F.; Smith, M.O. Ascorbic acid and melatonin reduce heat-induced performance inhibition and oxidative stress in Japanese quails. Br. Poult. Sci. 2004, 45, 116–122. [Google Scholar] [CrossRef]
- Duan, T.; Wu, Z.; Zhang, H.; Liu, Y.; Li, Y.; Zhang, W. Effects of melatonin implantation on carcass characteristics, meat quality and tissue levels of melatonin and prolactin in Inner Mongolian cashmere goats. J. Anim. Sci. Biotechnol. 2019, 10, 70. [Google Scholar] [CrossRef]
- Li, S.; Cao, J.; Wang, Z.; Dong, Y.; Wang, W.; Chen, Y. Melatonin Mediates Monochromatic Light-induced Insulin-like Growth Factor 1 Secretion of Chick Liver: Involvement of Membrane Receptors. Photochem. Photobiol. 2016, 92, 595–603. [Google Scholar] [CrossRef]
- Yue, L.; Qin, X.; Liu, X.; Wang, Z.; Dong, Y.; Chen, Y.; Cao, J. Melatonin receptor Mel1b-and Mel1c-mediated green light induced the secretion of growth hormone in anterior pituitary of chicks. Photochem. Photobiol. 2019, 95, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Aschalew, N.D.; Cheng, L.; Xia, Y.; Zhang, L.; Yin, G.; Zhen, Y. Dietary 5-hydroxytryptophan improves sheep growth performance by enhancing ruminal functions, antioxidant capacity, and tryptophan metabolism: In vitro and in vivo studies. Front. Immunol. 2024, 15, 1398310. [Google Scholar] [CrossRef]
- Sharma, M.; Palacios-Bois, J.; Schwartz, G.; Iskandar, H.; Thakur, M.; Quirion, R.; Nair, N.P.V. Circadian rhythms of melatonin and cortisol in aging. Biol. Psychiatry 1989, 25, 305–319. [Google Scholar] [CrossRef]
- Zemdegs, I.Z.; McMillen, I.C.; Walker, W.; Thorburn, G.D.; Nowak, R. Diurnal rhythms in plasma melatonin concentrations in the fetal sheep and pregnant ewe during late gestation. Endocrinology 1988, 123, 284–289. [Google Scholar] [CrossRef]
- Huether, G.; Messner, M.; Rodenbeck, A.; Hardeland, R. Effect of continuous melatonin infusions on steady-state plasma melatonin levels in rats under near physiological conditions. J. Pineal Res. 1998, 24, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Shindo, H.; Komai, T.; Kawai, K. Mechanism of intestinal absorption and brain uptake of L-5-hydroxytryptophan in rats, as compared to those of L-3, 4-dihydroxyphenylalanine. Chem. Pharm. Bull. 1977, 25, 1417–1425. [Google Scholar] [CrossRef]
- Mukherjee, S.; Moniruzzaman, M.; Maitra, S.K. Daily and seasonal profiles of gut melatonin and their temporal relationship with pineal and serum melatonin in carp Catla catla under natural photo-thermal conditions. Biol. Rhythm Res. 2014, 45, 301–315. [Google Scholar] [CrossRef]
- Namboodiri, M.A.A.; Sugden, D.; Klein, D.C.; Mefford, I.N. 5-Hydroxytryptophan elevates serum melatonin. Science 1983, 221, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Sugden, D.; Namboodiri, M.A.A.; Klein, D.C.; Grady Jr, R.K.; Mefford, I.N. Ovine pineal indoles: Effects of l-tryptophan or L-5-hydroxytryptophan administration. J. Neurochem. 1985, 44, 769–772. [Google Scholar] [CrossRef]
- Rawding, R.S.; Hutchison, V.H. Effect of chlorpromazine, 5-hydroxytryptophan, and benserazide on plasma melatonin in the mudpuppy, Nectvrus maculosus. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1993, 104, 159–164. [Google Scholar] [CrossRef]
- Torres, F.; González-Candia, A.; Montt, C.; Ebensperger, G.; Chubretovic, M.; Serón-Ferré, M.; Herrera, E.A. Melatonin reduces oxidative stress and improves vascular function in pulmonary hypertensive newborn sheep. J. Pineal Res. 2015, 58, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, F.; Wang, C.; Li, X.; Zhang, S.; Zhang, W.; Yang, K. Effects of duodenal 5-hydroxytryptophan perfusion on melatonin synthesis in GI tract of sheep. Molecules 2021, 26, 5275. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Tan, D.X.; Zanghi, B.M.; Manchester, L.C.; Reiter, R.J. Melatonin identified in meats and other food stuffs: Potentially nutritional impact. J. Pineal Res. 2014, 57, 213–218. [Google Scholar] [CrossRef]
- Appiah, M.O.; He, B.; Lu, W.; Wang, J. Antioxidative effect of melatonin on cryopreserved chicken semen. Cryobiology 2019, 89, 90–95. [Google Scholar] [CrossRef] [PubMed]
- She, Q.; Han, Z.; Liang, S.; Xu, W.; Li, X.; Zhao, Y.; Li, Y. Impacts of circadian rhythm and melatonin on the specific activities of immune and antioxidant enzymes of the Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2019, 89, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghi, V.; Mehri, M.; Mehri, M. Response surface model to illustrate the benefits of tryptophan, melatonin, and N, N-dimethylglycine in quail chicks exposed to aflatoxin B1. Poult. Sci. 2023, 102, 102803. [Google Scholar] [CrossRef]
- Ashok Kumar, A.K.; Mehrotra, S.; Singh, G.; Narayanan, K.; Das, G.K.; Soni, Y.K.; Verma, M.R. Sustained delivery of exogenous melatonin influences biomarkers of oxidative stress and total antioxidant capacity in summer-stressed anestrous water buffalo (Bubalus bubalis). Theriogenology 2015, 83, 1402–1407. [Google Scholar] [CrossRef]
- Kanyar, I.M.; Karadaş, F. Effect of melatonin and vitamin E as antioxidants on body weight, carcass traits of Awassi lambs fed a high-energy and normal diet. Iraqi J. Agric. Sci. 2023, 54, 1339–1350. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, L.; Huang, Y.; Wang, Y.; Song, W.; Zhen, L. Effect of dietary supplementation with melatonin on meat quality, fatty acid profiles and metabolome differences in broiler breast. J. Food Compos. Anal. 2023, 121, 105410. [Google Scholar] [CrossRef]
- Song, P.; Huo, G.; Feng, J.; Zhang, W.; Li, X.; Zhao, J. Intramuscular vitamin A injection in newborn lambs enhances antioxidant capacity and improves meat quality. Front. Vet. Sci. 2023, 10, 1272874. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Cooper, B.; Sobreira, T.; Kim, Y.H.B. Utilizing pork exudate metabolomics to reveal the impact of aging on meat quality. Foods 2021, 10, 668. [Google Scholar] [CrossRef]
- Tian, X.; Li, D.; Zhao, X.; Xiao, Z.; Sun, J.; Yuan, T.; Yu, T. Dietary grape pomace extract supplementation improved meat quality, antioxidant capacity, and immune performance in finishing pigs. Front. Microbiol. 2023, 14, 1116022. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Falvo, M.J. Protein–which is best? J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar]
- Ge, F.; Li, J.; Gao, H.; Wang, X.; Zhang, X.; Gao, H.; Chen, Y. Comparative analysis of carcass traits and meat quality in indigenous Chinese cattle breeds. J. Food Compos. Anal. 2023, 124, 105645. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; World Health Organization; United Nations University. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. In Proceedings of the Joint FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements, Rome, Italy, 5–17 October 1981; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef]
- Ruiz-Núñez, B.; Kuipers, R.S.; Luxwolda, M.F.; De Graaf, D.J.; Breeuwsma, B.B.; Dijck-Brouwer, D.J.; Muskiet, F.A. Saturated fatty acid (SFA) status and SFA intake exhibit different relations with serum total cholesterol and lipoprotein cholesterol: A mechanistic explanation centered around lifestyle-induced low-grade inflammation. J. Nutr. Biochem. 2014, 25, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; He, J.; Yu, B. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. 2022, 367, 130781. [Google Scholar] [CrossRef] [PubMed]
- Howes, N.L.; Bekhit, A.E.D.A.; Burritt, D.J.; Campbell, A.W. Opportunities and implications of pasture-based lamb fattening to enhance the long-chain fatty acid composition in meat. Compr. Rev. Food Sci. Food Saf. 2015, 14, 22–36. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Holman, B.W.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Chikwanha, O.C.; Moelich, E.; Gouws, P.; Muchenje, V.; Nolte, J.V.E.; Dugan, M.E.; Mapiye, C. Effects of feeding increasing levels of grape (Vitis vinifera cv. Pinotage) pomace on lamb shelf-life and eating quality. Meat Sci. 2019, 157, 107887. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | Content (%) | Nutrient Levels | Content (%) |
|---|---|---|---|
| Corn silage | 29.31 | Neutral detergent fiber | 42.38 |
| Corn | 26.79 | Acid detergent fiber | 25.76 |
| Tofu pulp | 15.00 | Crude protein | 12.99 |
| Cotton hull | 10.00 | Ash | 4.35 |
| Cottonseed meal | 7.60 | Ether extract | 3.29 |
| Soybean meal | 2.74 | Calcium | 0.68 |
| Rapeseed meal | 2.35 | Phosphorus | 0.33 |
| Linseed meal | 1.72 | ||
| Wheat bran | 1.72 | ||
| Lysine | 1.58 | ||
| Premix 1 | 0.84 | ||
| NaCl | 0.35 | ||
| Total | 100.00 |
| Items | Control Group | RPT 5-HTP Group | MT Group | SEM | p-Value |
|---|---|---|---|---|---|
| Dry matter intake (kg/d) | 1.15 | 1.16 | 1.16 | 0.023 | 0.982 |
| Initial body weight (kg) | 30.91 | 30.83 | 30.79 | 0.212 | 0.974 |
| Final body weight (kg) | 35.85 | 35.93 | 36.01 | 0.306 | 0.978 |
| Average daily gain (g/d) | 164.50 | 170.00 | 174.00 | 5.128 | 0.770 |
| Ratio of feed intake to gain | 7.10 | 6.88 | 6.72 | 0.102 | 0.329 |
| Items | Control Group | RPT 5-HTP Group | MT Group | SEM | p-Value |
|---|---|---|---|---|---|
| Greyhound rib (mm) | 12.15 | 12.42 | 12.80 | 0.248 | 0.589 |
| Eye muscle area (cm2) | 18.14 | 18.14 | 19.76 | 0.345 | 0.079 |
| Live weight (kg) | 36.12 | 36.18 | 36.12 | 0.404 | 0.997 |
| Carcass weight (kg) | 18.45 | 18.67 | 18.63 | 0.146 | 0.913 |
| Slaughter rate (%) | 51.12 | 51.61 | 51.57 | 0.146 | 0.277 |
| Items | Control Group | RPT 5-HTP Group | MT Group | SEM | p-Value |
|---|---|---|---|---|---|
| Superoxide dismutase U/mg | 224.9 | 221.78 | 225.06 | 4.146 | 0.952 |
| Malondialdehyde nmol/mg | 1.44 a | 1.12 b | 1.12 b | 0.058 | 0.020 |
| Glutathione peroxidase U/mg | 130.14 | 123.72 | 129.60 | 3.712 | 0.805 |
| Catalase U/mg | 0.39 b | 0.45 ab | 0.50 a | 0.012 | 0.009 |
| Total antioxidant capacity mmol/g | 0.08 | 0.08 | 0.07 | 0.002 | 0.404 |
| Items | Control Group | RPT 5-HTP Group | MT Group | SEM | p-Value |
|---|---|---|---|---|---|
| pH45min | 6.31 | 6.19 | 6.22 | 0.059 | 0.700 |
| Brightness L* | 34.16 | 34.22 | 33.44 | 0.411 | 0.717 |
| Infrared a* | 14.99 | 15.27 | 13.93 | 0.354 | 0.278 |
| Yellowness b* | 5.97 | 5.95 | 5.73 | 0.110 | 0.642 |
| pH24h | 5.84 | 5.81 | 5.71 | 0.030 | 0.177 |
| Shear force (N) | 45.85 | 45.76 | 45.98 | 0.266 | 0.950 |
| Drip loss (%) | 11.84 a | 9.64 b | 7.83 c | 0.441 | <0.01 |
| Cooking loss (%) | 39.38 | 37.25 | 38.37 | 0.522 | 0.264 |
| Items | Amino Acid | Control Group | RPT 5-HTP Group | MT Group | SEM | p-Value |
|---|---|---|---|---|---|---|
| Essential amino acids | Trp | 0.11 | 0.18 | 0.19 | 0.020 | 0.278 |
| Lysine | 13.88 | 13.15 | 14.32 | 0.344 | 0.399 | |
| Leucine | 18.76 c | 22.13 b | 24.43 a | 0.690 | <0.01 | |
| Histidine | 3.58 b | 3.71 ab | 4.22 a | 0.116 | 0.046 | |
| Methionine | 5.47 c | 6.72 b | 7.62 a | 0.268 | <0.01 | |
| Valine | 12.50 c | 14.75 b | 16.25 a | 0.442 | <0.01 | |
| Threonine | 7.05 c | 9.21 b | 11.22 a | 0.453 | <0.01 | |
| Isoleucine | 11.46 c | 13.73 b | 15.10 a | 0.429 | <0.01 | |
| Phenylalanine | 8.75 b | 10.38 a | 11.27 a | 0.312 | <0.01 | |
| Non-essential amino acids | Glycine | 3.03 | 3.02 | 2.53 | 0.126 | 0.192 |
| Alanine | 14.18 c | 18.27 b | 21.07 a | 0.782 | <0.01 | |
| Tyrosine | 6.74 b | 8.24 a | 8.96 a | 0.291 | <0.01 | |
| Proline | 11.15 c | 13.63 b | 15.10 a | 0.463 | <0.01 | |
| Aspartic acid | 6.4 | 5.08 | 5.80 | 0.279 | 0.142 | |
| Glutamic acid | 22.22 c | 27.82 b | 33.68 a | 1.242 | <0.01 | |
| Serine | 3.16 c | 6.91 b | 9.30 a | 0.739 | <0.01 | |
| Arginine | 8.55 b | 9.73 b | 11.75 a | 0.393 | <0.01 | |
| Essential amino acids | 81.55 c | 93.96 b | 104.62 a | 2.722 | <0.01 | |
| Non-essential amino acids | 75.46 c | 92.70 b | 108.20 a | 3.579 | <0.01 | |
| Umami amino acids | 65.20 c | 83.38 b | 96.79 a | 3.507 | <0.01 | |
| Sweet amino acids | 61.35 c | 72.81 b | 83.32 a | 2.403 | <0.01 | |
| Items | Control Group | RPT 5-HTP Group | MT Group | SEM | p-Value |
|---|---|---|---|---|---|
| C4:0 | 0.037 | 0.042 | 0.045 | 0.004 | 0.816 |
| C6:0 | 0.026 | 0.021 | 0.021 | 0.003 | 0.697 |
| C8:0 | 0.180 | 0.126 | 0.135 | 0.021 | 0.569 |
| C10:0 | 1.567 | 1.152 | 1.040 | 0.244 | 0.667 |
| C11:0 | 0.055 | 0.036 | 0.051 | 0.005 | 0.217 |
| C12:0 | 0.848 | 0.498 | 0.465 | 0.130 | 0.436 |
| C13:0 | 0.071 | 0.037 | 0.045 | 0.009 | 0.326 |
| C14:0 | 24.371 | 15.189 | 15.280 | 3.778 | 0.551 |
| C15:0 | 2.628 | 1.412 | 1.844 | 0.336 | 0.346 |
| C16:0 | 329.631 | 220.571 | 229.061 | 42.503 | 0.536 |
| C17:0 | 12.026 | 6.755 | 7.645 | 1.544 | 0.349 |
| C18:0 | 204.886 a | 97.632 b | 110.154 ab | 18.350 | 0.022 |
| C20:0 | 0.602 | 0.393 | 0.285 | 0.103 | 0.469 |
| C21:0 | 0.100 | 0.105 | 0.119 | 0.004 | 0.129 |
| Saturated Fatty Acids | 577.03 | 343.97 | 366.19 | 63.886 | 0.273 |
| C14:1 | 0.765 | 0.536 | 0.649 | 0.095 | 0.647 |
| C16:1 | 17.706 | 12.052 | 14.900 | 2.435 | 0.665 |
| C18:1n9c | 439.291 a | 265.987 ab | 228.701 b | 34.518 | 0.018 |
| C20:1 | 0.886 | 0.689 | 0.647 | 0.083 | 0.485 |
| Monounsaturated Fatty Acids | 458.65 a | 279.26 b | 244.90 b | 36.328 | 0.024 |
| C18:2n6c | 89.019 | 66.251 | 59.555 | 7.121 | 0.217 |
| C18:3n6 | 0.529 | 0.408 | 0.269 | 0.066 | 0.294 |
| C18:3n3 | 2.736 | 1.884 | 1.521 | 0.299 | 0.247 |
| C20:2 | 2.114 Aa | 1.840 Aab | 1.326 Ab | 0.076 | 0.076 |
| C20:3n6 | 1.391 | 1.277 | 1.153 | 0.068 | 0.377 |
| C20:4n6 | 16.685 | 17.688 | 14.728 | 0.847 | 0.371 |
| C20:5n3 | 0.482 | 0.535 | 0.429 | 0.023 | 0.161 |
| C22:6 | 0.489 | 0.555 | 0.497 | 0.018 | 0.268 |
| Polyunsaturated Fatty Acids | 204.89 | 200.36 | 102.55 | 35.324 | 0.216 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Yang, H.; Ainiwaer, Z.; Fan, W.; Xu, T.; Yang, K.; Wang, C. Effects of Rumen-Protected 5-Hydroxytryptophan and Melatonin Supplementation on Antioxidant Capacity, Meat Quality, and Shelf Life of Hu Sheep. Animals 2025, 15, 1582. https://doi.org/10.3390/ani15111582
Xu S, Yang H, Ainiwaer Z, Fan W, Xu T, Yang K, Wang C. Effects of Rumen-Protected 5-Hydroxytryptophan and Melatonin Supplementation on Antioxidant Capacity, Meat Quality, and Shelf Life of Hu Sheep. Animals. 2025; 15(11):1582. https://doi.org/10.3390/ani15111582
Chicago/Turabian StyleXu, Shiheng, Honghai Yang, Zulibina Ainiwaer, Wenpeng Fan, Tongxiang Xu, Kailun Yang, and Caidie Wang. 2025. "Effects of Rumen-Protected 5-Hydroxytryptophan and Melatonin Supplementation on Antioxidant Capacity, Meat Quality, and Shelf Life of Hu Sheep" Animals 15, no. 11: 1582. https://doi.org/10.3390/ani15111582
APA StyleXu, S., Yang, H., Ainiwaer, Z., Fan, W., Xu, T., Yang, K., & Wang, C. (2025). Effects of Rumen-Protected 5-Hydroxytryptophan and Melatonin Supplementation on Antioxidant Capacity, Meat Quality, and Shelf Life of Hu Sheep. Animals, 15(11), 1582. https://doi.org/10.3390/ani15111582






