Apricot Bee Pollen Alleviates Deoxynivalenol-Induced Cellular Toxicity in Bovine Granulosa Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Reagents
2.3. Measurement of Total Phenolics
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Cell Cycle Assay
2.7. Cell Apoptosis Analysis
2.8. Quantitative Real-Time PCR (qRT-PCR) Assay
2.9. Western Blotting Assay
2.10. Steroid Hormone Detection
2.11. Reactive Oxygen Species (ROS) Assay
2.12. Malondialdehyde (MDA) Assay
2.13. Total Antioxidant Capacity (T-AOC) Assay
2.14. RNA-Seq Analysis
2.15. Statistical Analysis
3. Results
3.1. DON Suppresses Cell Proliferation and Induces Apoptosis of bGCs
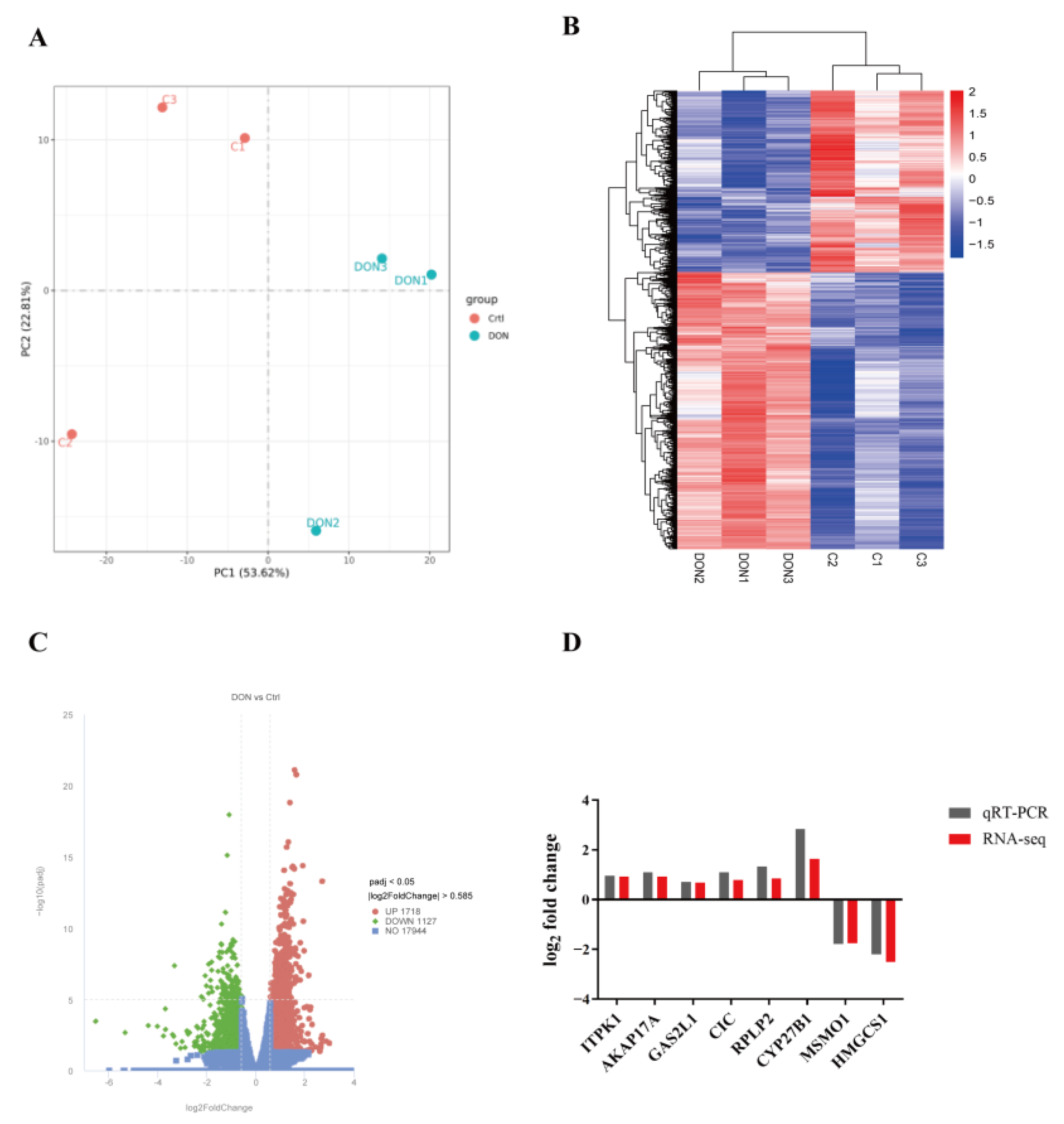
3.2. DON Alters Gene Expression Patterns of bGCs
3.3. DON Inhibits Steroid Hormone Synthesis and Induces Oxidative Stress in bGCs
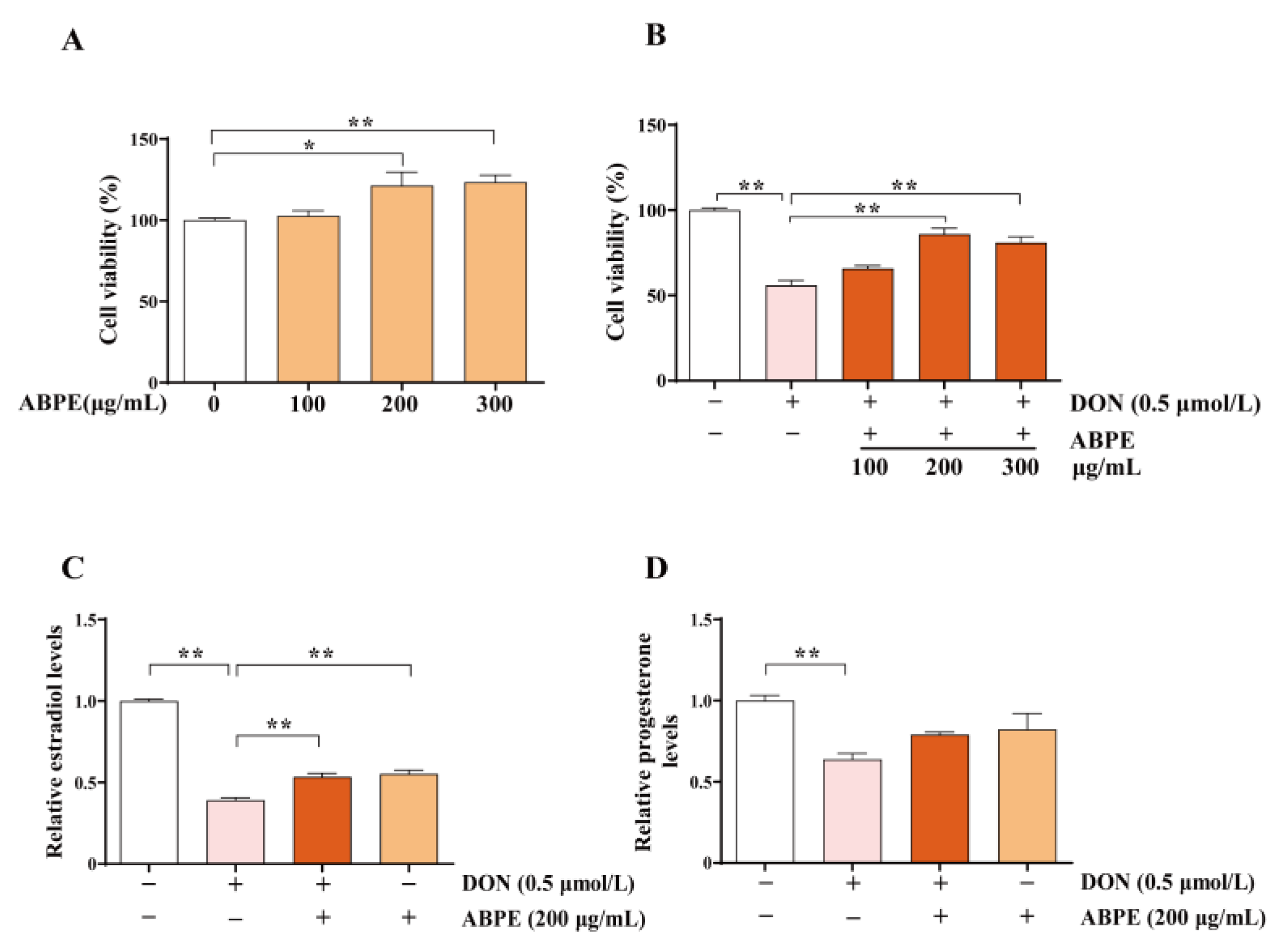
3.4. ABPE Alleviates the Decrease of Cell Viability and Steroid Hormones Caused by DON in bGCs
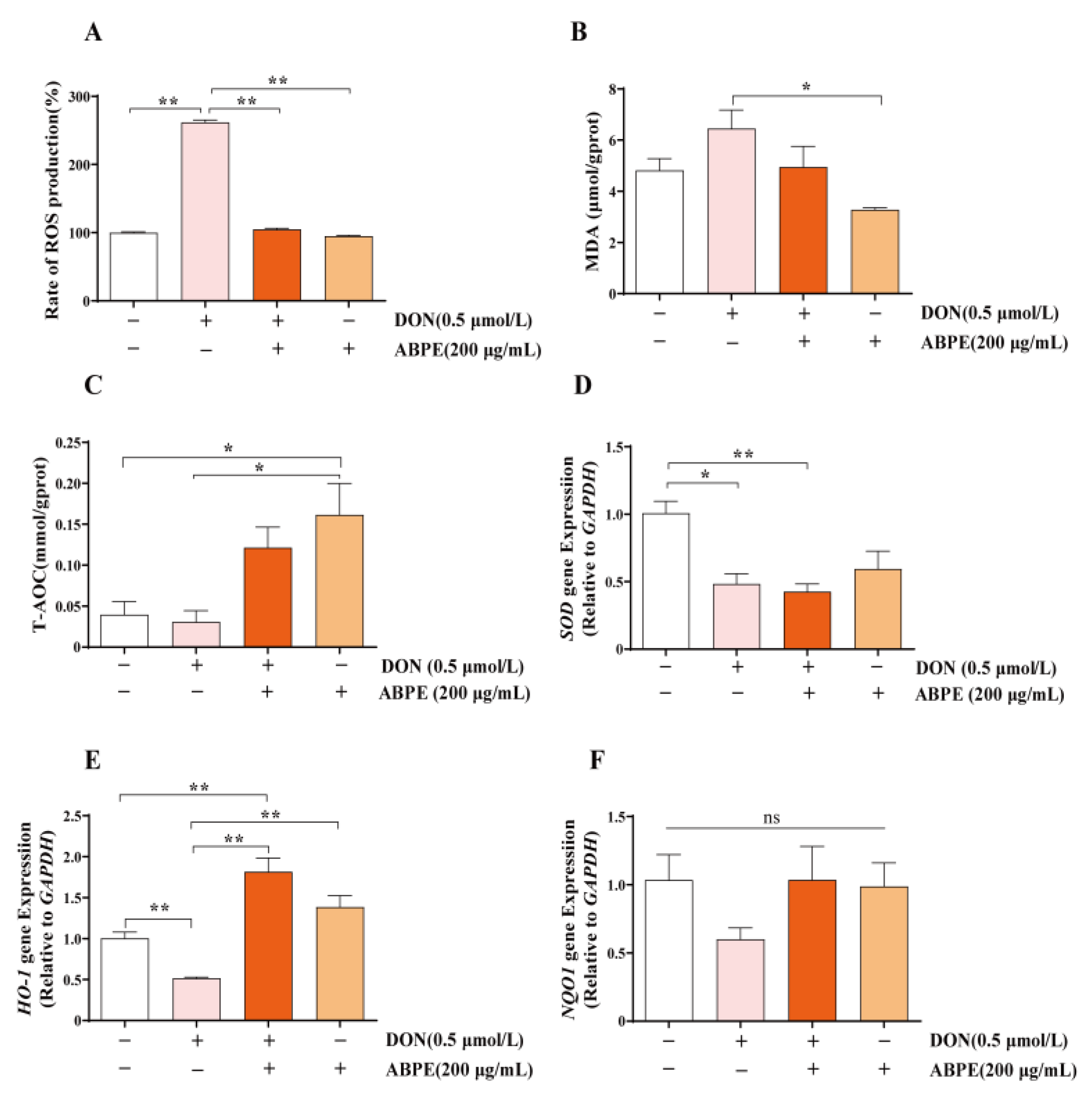
3.5. ABPE Alleviates Oxidative Stress Induced by DON in bGCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Genes | Sequence (5′-3′) | Length (bp) | Accession No. |
|---|---|---|---|
| GAPDH | F: GGTCACCAGGGCTGCTTTTA | 222 | NM_001034034.2 |
| R: CCAGCATCACCCCACTTGAT | |||
| SOD | F: GCTGGAAGCCATCAAACG | 107 | NM_201527.2 |
| R: TGAAGCCGAGCCAACCC | |||
| NQO1 | F: TTCAATCCCGTCATCTCC | 215 | NM_001034535.1 |
| R: TCAAACCAGCCTTTCAGG | |||
| HO-1 | F: CTGGTGATGGCGTCTTTGT | 215 | NM_001014912.1 |
| R: TCCTGGAGTCGCTGAACAT | |||
| MSMO1 | F: TTTGCCTGACAATCCT | 140 | NM_001098863.1 |
| R: ATCCAGGTAAACAGAACA | |||
| CYP27B1 | F: TCGGCTCGGTGTTCGTG | 171 | NM_001192284.1 |
| R: CAGACTGGTTCCTCATGGCTAC | |||
| ITPK1 | F: CTGCTCGCCACCCTTCA | 114 | NM_001192489.3 |
| R: CCATGAGCCACTCTGGTTTT | |||
| RPLP2 | F: GGCTCAACAAGGTCATCAGTG | 158 | NM_174788.4 |
| R: TGCGGCTGGAGCAGAAC | |||
| AKAP17A | F: GGAGAACAAGAGCCTCGTCAAGT | 147 | XM_024990316.2 |
| R: CGGAAGAAGGAGTCCCAGTCG | |||
| GAS2L1 | F: CCCAGTCCAGAGTTGAGCACA | 250 | NM_001083698.1 |
| R: GGACGCTGAGGGACGAAGA | |||
| CIC | F: TTTTGAGTTTGACGAGTGCGAAGC | 151 | XM_015458193.3 |
| R: AGGGCGGAAGCCGAAGAGTG | |||
| HMGCS1 | F: GAGGAGTCTGGGAATACG | 129 | NM_001206578.1 |
| R: TACCAGGGCATACCGT |
| Antibodies | Dilution Ratio | Supplier | RRID | Cat. No | MW (KD) |
|---|---|---|---|---|---|
| BAX | 1:4000 | ABmart, China | AB_2910262 | T40051 | 21 |
| PCNA | 1:1000 | Proteintech, USA | AB_2160330 | 10205-2-AP | 36–38 |
| StAR | 1:1000 | ABclonal, China | AB_2772418 | A16432 | 28 |
| CYP19A1 | 1:500 | Cell Signaling, USA | AB_2630344 | 14528s | 50 |
| CYP11A1 | 1:1000 | ABclonal, China | AB_2763764 | A1713 | 50 |
| GAPDH | 1:5000 | ABclonal, China | AB_2736879 | AC002 | 36 |
| HRP Goat Anti-Mouse IgG (H + L) | 1:8000 | ABclonal, China | AB_2769851 | AS003 | |
| HRP Goat Anti-Rabbit IgG (H + L) | 1:8000 | ABclonal, China | AB_2769854 | AS014 |
References
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Schatzmayr, G.; Streit, E. Global Occurrence of Mycotoxins in the Food and Feed Chain: Facts and Figures. World Mycotoxin J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- Sumarah, M.W. The Deoxynivalenol Challenge. J. Agric. Food Chem. 2022, 70, 9619–9624. [Google Scholar] [CrossRef]
- Xu, A.; Yu, S.; Li, Y.; Liu, H.; Yan, Z.; Wu, A.; Peng, S.; Liu, N. Total Deoxynivalenol Contamination of Wheat Products and Coarse Grains in Shanghai, China: Occurrence and Health Risk Assessment. Foods 2024, 13, 3373. [Google Scholar] [CrossRef]
- Marczuk, J.; Obremski, K.; Lutnicki, K.; Gajęcka, M.; Gajęcki, M. Zearalenone and Deoxynivalenol Mycotoxicosis in Dairy Cattle Herds. Pol. J. Vet. Sci. 2012, 15, 365–372. [Google Scholar] [CrossRef]
- Côté, L.-M.; Dahlem, A.M.; Yoshizawa, T.; Swanson, S.P.; Buck, W.B. Excretion of Deoxynivalenol and Its Metabolite in Milk, Urine, and Feces of Lactating Dairy Cows. J. Dairy Sci. 1986, 69, 2416–2423. [Google Scholar] [CrossRef]
- Seeling, K.; Dänicke, S.; Valenta, H.; Van Egmond, H.P.; Schothorst, R.C.; Jekel, A.A.; Lebzien, P.; Schollenberger, M.; Razzazi-Fazeli, E.; Flachowsky, G. Effects of Fusarium Toxin-Contaminated Wheat and Feed Intake Level on the Biotransformation and Carry-over of Deoxynivalenol in Dairy Cows. Food Addit. Contam. 2006, 23, 1008–1020. [Google Scholar] [CrossRef]
- Cortinovis, C.; Caloni, F.; Schreiber, N.B.; Spicer, L.J. Effects of Fumonisin B1 Alone and Combined with Deoxynivalenol or Zearalenone on Porcine Granulosa Cell Proliferation and Steroid Production. Theriogenology 2014, 81, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Li, S.; Zou, H.; Ji, D.; Lv, M.; Zhou, P.; Wei, Z.; Zhang, Z.; Cao, Y. Melatonin Alleviates Deoxynivalenol-Induced Apoptosis of Human Granulosa Cells by Reducing Mutually Accentuated FOXO1 and ER Stress. Biol. Reprod. 2021, 105, 554–566. [Google Scholar] [CrossRef]
- Fan, H.; Ren, Z.; Xu, C.; Wang, H.; Wu, Z.; Rehman, Z.U.; Wu, S.; Sun, M.A.; Bao, W. Chromatin Accessibility and Transcriptomic Alterations in Murine Ovarian Granulosa Cells upon Deoxynivalenol Exposure. Cells 2021, 10, 2818. [Google Scholar] [CrossRef]
- Guerrero-Netro, H.M.; Chorfi, Y.; Price, C.A. Effects of the Mycotoxin Deoxynivalenol on Steroidogenesis and Apoptosis in Granulosa Cells. Reproduction 2015, 149, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-H.; Jarvis, B.B.; Chung, Y.-J.; Pestka, J.J. Apoptosis Induction by the Satratoxins and Other Trichothecene Mycotoxins: Relationship to ERK, P38 MAPK, and SAPK/JNK Activation. Toxicol. Appl. Pharmacol. 2000, 164, 149–160. [Google Scholar] [CrossRef]
- Fan, H.; Wang, S.; Wang, H.; Sun, M.; Wu, S.; Bao, W. Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects. Antioxidants 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Kolesarova, A.; Capcarova, M.; Maruniakova, N.; Lukac, N.; Ciereszko, R.E.; Sirotkin, A.V. Resveratrol Inhibits Reproductive Toxicity Induced by Deoxynivalenol. J. Environ. Sci. Health Part A 2012, 47, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Halenar, M.; Medvedova, M.; Maruniakova, N.; Kolesarova, A. Assessment of a Potential Preventive Ability of Amygdalin in Mycotoxin-Induced Ovarian Toxicity. J. Environ. Sci. Health Part B 2015, 50, 411–416. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Alagawany, M.; Farag, M.R.; Elnesr, S.S. Beneficial Impacts of Bee Pollen in Animal Production, Reproduction and Health. J. Anim. Physiol. Anim. Nutr. 2019, 103, 477–484. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-Collected Pollen from Five Portuguese Natural Parks: Palynological Origin, Phenolic Content, Antioxidant Properties and Antimicrobial Activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Lv, J.-M.; Wang, R.; He, H.; Wu, M.; Zhang, R.; He, J. Preparation Technologies, Structural Characteristics and Biological Activities of Polysaccharides from Bee Pollen: A Review. Int. J. Biol. Macromol. 2025, 306, 141545. [Google Scholar] [CrossRef]
- El-Bialy, B.E.; Abdeen, E.E.; El-Borai, N.B.; El-Diasty, E.M. Experimental Studies on Some Immunotoxicological Aspects of Aflatoxins Containing Diet and Protective Effect of Bee Pollen Dietary Supplement. Pak. J. Biol. Sci. 2016, 19, 26–35. [Google Scholar] [CrossRef]
- Maruyama, H.; Sakamoto, T.; Araki, Y.; Hara, H. Anti-Inflammatory Effect of Bee Pollen Ethanol Extract from Cistus Sp. of Spanish on Carrageenan-Induced Rat Hind Paw Edema. BMC Complement. Altern. Med. 2010, 10, 30. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Xiao, G.; Liu, G.; Dong, H.; Liu, R.; Lu, Q. Phenolamide Extract of Apricot Bee Pollen Alleviates Glucolipid Metabolic Disorders and Modulates the Gut Microbiota and Metabolites in High-Fat Diet-Induced Obese Mice. Food Funct. 2023, 14, 4662–4680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, P.; Liu, M.; Liu, S.; Huo, L.; Ding, Z.; Liu, M.; Wang, S.; Lv, C.; Wu, H.; et al. Deoxynivalenol Exposure Inhibits Biosynthesis of Milk Fat and Protein by Impairing Tight Junction in Bovine Mammary Epithelial Cells. Ecotoxicol. Env. Environ. Saf. 2022, 237, 113504. [Google Scholar] [CrossRef] [PubMed]
- Liberato, M.d.C.T.C.; de Morais, S.M.; Siqueira, S.M.C.; de Menezes, J.E.S.A.; Ramos, D.N.; Machado, L.K.A.; Magalhães, I.L. Phenolic Content and Antioxidant and Antiacetylcholinesterase Properties of Honeys from Different Floral Origins. J. Med. Food 2011, 14, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Novak, B.; Vatzia, E.; Springler, A.; Pierron, A.; Gerner, W.; Reisinger, N.; Hessenberger, S.; Schatzmayr, G.; Mayer, E. Bovine Peripheral Blood Mononuclear Cells Are More Sensitive to Deoxynivalenol Than Those Derived from Poultry and Swine. Toxins 2018, 10, 152. [Google Scholar] [CrossRef]
- Wang, J.; Jin, Y.; Wu, S.; Yu, H.; Zhao, Y.; Fang, H.; Shen, J.; Zhou, C.; Fu, Y.; Li, R.; et al. Deoxynivalenol Induces Oxidative Stress, Inflammatory Response and Apoptosis in Bovine Mammary Epithelial Cells. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1663–1674. [Google Scholar] [CrossRef]
- Bailey, J.R.; Breton, J.; Panic, G.; Cogan, T.A.; Bailey, M.; Swann, J.R.; Lee, M.R.F. The Mycotoxin Deoxynivalenol Significantly Alters the Function and Metabolism of Bovine Kidney Epithelial Cells In Vitro. Toxins 2019, 11, 554. [Google Scholar] [CrossRef]
- Yang, M.; Wu, X.; Zhang, W.; Ye, P.; Wang, Y.; Zhu, W.; Tao, Q.; Xu, Y.; Shang, J.; Zhao, D.; et al. Transcriptional Analysis of Deoxynivalenol-induced Apoptosis of Sow Ovarian Granulosa Cell. Reprod. Domest. Anim. 2020, 55, 217–228. [Google Scholar] [CrossRef]
- Medvedova, M.; Kolesarova, A.; Capcarova, M.; Labuda, R.; Sirotkin, A.; Kovacik, J.; Bulla, J. The Effect of Deoxynivalenol on the Secretion Activity, Proliferation and Apoptosis of Porcine Ovarian Granulosa Cells in Vitro. J. Environ. Sci. Health Part B 2011, 46, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ndossi, D.G.; Frizzell, C.; Tremoen, N.H.; Fæste, C.K.; Verhaegen, S.; Dahl, E.; Eriksen, G.S.; Sørlie, M.; Connolly, L.; Ropstad, E. An in Vitro Investigation of Endocrine Disrupting Effects of Trichothecenes Deoxynivalenol (DON), T-2 and HT-2 Toxins. Toxicol. Lett. 2012, 214, 268–278. [Google Scholar] [CrossRef]
- Urbanek, K.A.; Habrowska-Górczyńska, D.E.; Kowalska, K.; Stańczyk, A.; Domińska, K.; Piastowska-Ciesielska, A.W. Deoxynivalenol as Potential Modulator of Human Steroidogenesis. J. Appl. Toxicol. 2018, 38, 1450–1459. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative Stress-Induced Apoptosis in Granulosa Cells Involves JNK, P53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef] [PubMed]
- Kolesarova, A.; Capcarova, M.; BakovA, Z.; Galik, B.; Juracek, M.; Simko, M.; Sirotkin, A. The Effect of Bee Pollen on Secretion Activity, Markers of Proliferation and Apoptosis of Porcine Ovarian Granulosa Cells in Vitro. J. Environ. Sci. Health Part B 2011, 46, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, A.A.; Abdella, E.M.; Ahmed, R.R.; Ahmed, Y.K. Assessment of Anti-Mutagenic, Anti-Histopathologic and Antioxidant Capacities of Egyptian Bee Pollen and Propolis Extracts. Cytotechnology 2014, 66, 283–297. [Google Scholar] [CrossRef]
- Roberts, R.A.; Laskin, D.L.; Smith, C.V.; Robertson, F.M.; Allen, E.M.G.; Doorn, J.A.; Slikker, W. Nitrative and Oxidative Stress in Toxicology and Disease. Toxicol. Sci. 2009, 112, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lv, Y.; He, C.; Lin, X.; Li, C.; Xu, W.; Duan, N.; Wu, S.; Wang, Z. Application of PEG-CdSe@ZnS Quantum Dots for ROS Imaging and Evaluation of Deoxynivalenol-Mediated Oxidative Stress in Living Cells. Food Chem. Toxicol. 2020, 146, 111834. [Google Scholar] [CrossRef]
- Yeligar, S.M.; Machida, K.; Kalra, V.K. Ethanol-Induced HO-1 and NQO1 Are Differentially Regulated by HIF-1α and Nrf2 to Attenuate Inflammatory Cytokine Expression. J. Biol. Chem. 2010, 285, 35359–35373. [Google Scholar] [CrossRef]
- Naseri, L.; Khazaei, M.R.; Khazaei, M. Potential Therapeutic Effect of Bee Pollen and Metformin Combination on Testosterone and Estradiol Levels, Apoptotic Markers and Total Antioxidant Capacity in A Rat Model of Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2021, 15, 101–107. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Zheng, X.; Wu, H.; Sun, P.; Lu, Q.; Fang, F.; Liu, M.; Zhou, S.; Liu, R.; Li, X.; et al. Apricot Bee Pollen Alleviates Deoxynivalenol-Induced Cellular Toxicity in Bovine Granulosa Cells. Animals 2025, 15, 1580. https://doi.org/10.3390/ani15111580
Lv C, Zheng X, Wu H, Sun P, Lu Q, Fang F, Liu M, Zhou S, Liu R, Li X, et al. Apricot Bee Pollen Alleviates Deoxynivalenol-Induced Cellular Toxicity in Bovine Granulosa Cells. Animals. 2025; 15(11):1580. https://doi.org/10.3390/ani15111580
Chicago/Turabian StyleLv, Ce, Xiaoxue Zheng, Hanxiao Wu, Peihao Sun, Qun Lu, Fang Fang, Mingxiao Liu, Shuo Zhou, Rui Liu, Xiang Li, and et al. 2025. "Apricot Bee Pollen Alleviates Deoxynivalenol-Induced Cellular Toxicity in Bovine Granulosa Cells" Animals 15, no. 11: 1580. https://doi.org/10.3390/ani15111580
APA StyleLv, C., Zheng, X., Wu, H., Sun, P., Lu, Q., Fang, F., Liu, M., Zhou, S., Liu, R., Li, X., Yang, L., & Liang, A. (2025). Apricot Bee Pollen Alleviates Deoxynivalenol-Induced Cellular Toxicity in Bovine Granulosa Cells. Animals, 15(11), 1580. https://doi.org/10.3390/ani15111580








