The Relationship Between Protein Fraction Contents and Immune Cells in Milk
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Prediction of Milk Protein Fraction Contents
2.3. Transformation of Cell Score
2.4. Statistical Analysis
3. Results
3.1. Physiological Secretion Characteristics of Milk Protein Fraction Content
3.1.1. Secretion Profile of Milk Protein Fraction Content with Lactation
3.1.2. Secretion Profile of Milk Protein Fraction Content with Parity
3.2. Association of Milk Protein Fraction Content with Mastitis and SCS
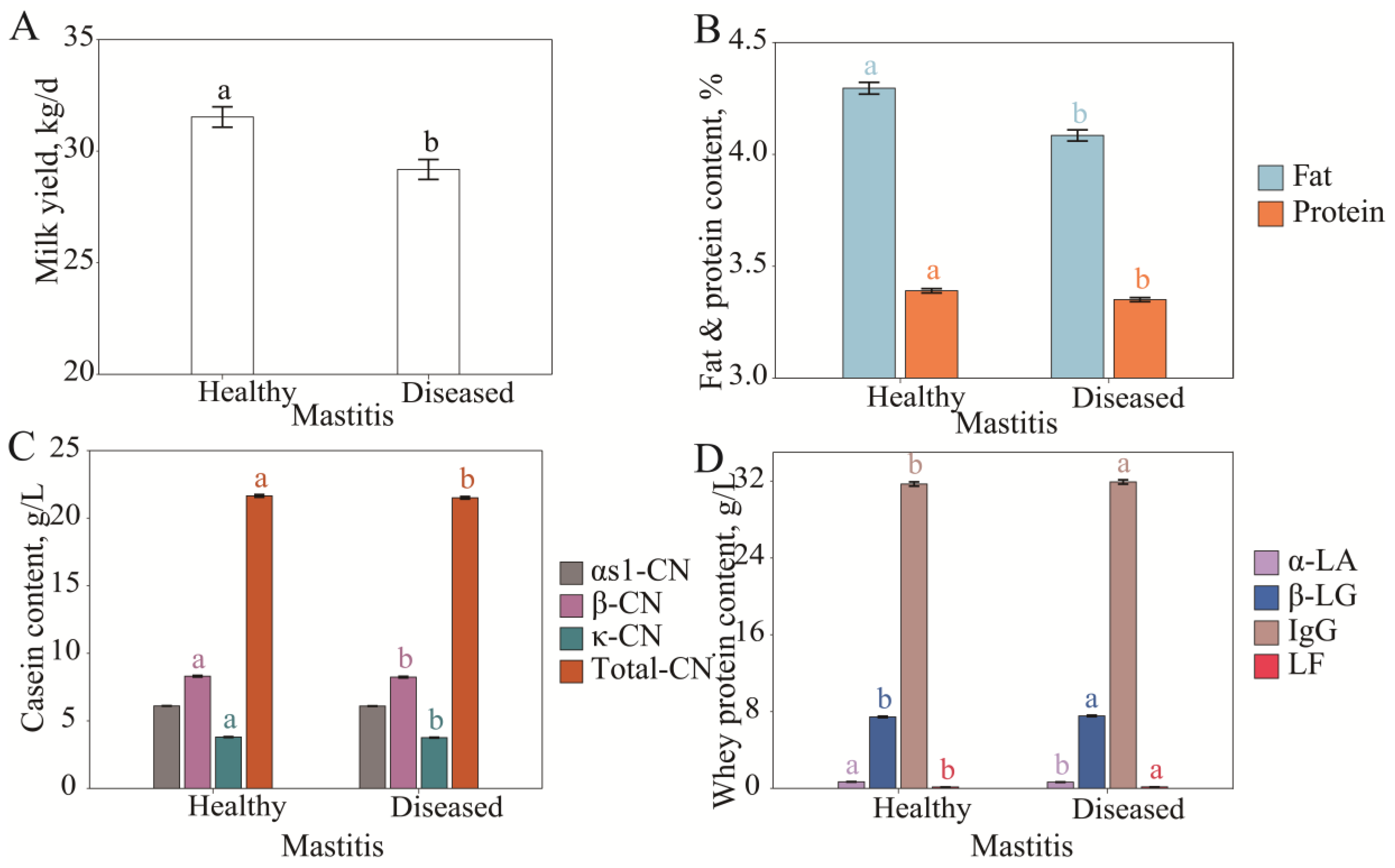
3.2.1. Differences in Milk Protein Fraction Content Under Different Mammary Gland Health Conditions
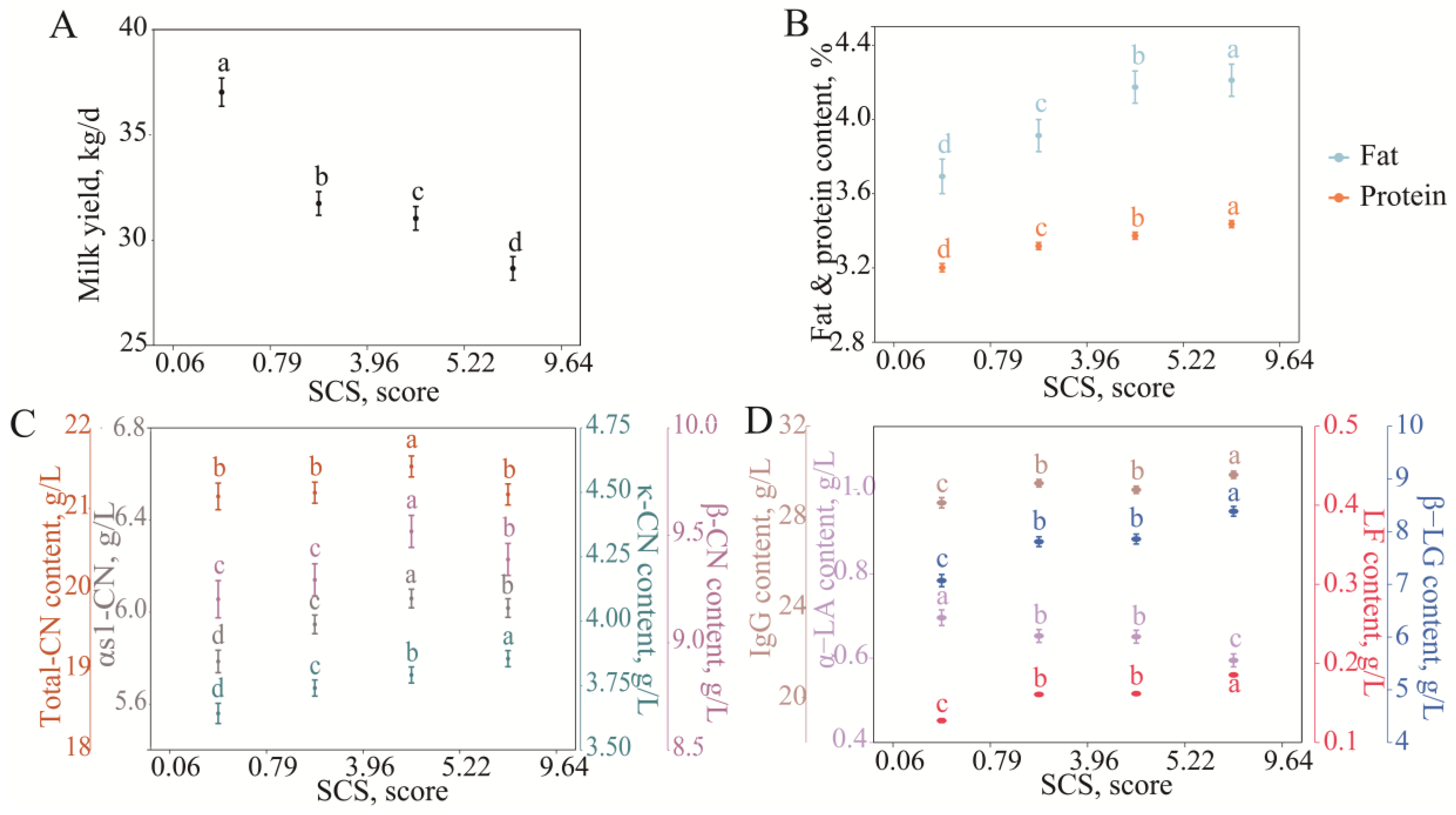
3.2.2. Variation in Milk Protein Fraction Content with SCS
3.3. Characteristics of Changes in Milk Protein Fraction Content with DSCCs
3.4. Characteristics of Changes in Milk Protein Fraction Content with Immune Cell Score
3.4.1. Variation in Milk Protein Fraction Content with PMN + LYM Score
3.4.2. Variation in Milk Protein Fraction Content with MAC Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMNs | Polymorphonuclear leukocytes |
| MACs | Macrophages |
| PMN + LYMS | PMN + LYM score |
| MACS | MAC score |
References
- Visioli, F.; Strata, A. Milk, Dairy Products, and Their Functional Effects in Humans: A Narrative Review of Recent Evidence. Adv. Nutr. 2014, 5, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, A.; Guo, A.C.; Vazquez-Fresno, R.; Lipfert, M.; Zhang, L.; Zheng, J.; Badran, H.; Budinski, Z.; Mandal, R.; Ametaj, B.N.; et al. Chemical Composition of Commercial Cow’s Milk. J. Agric. Food Chem. 2019, 67, 4897–4914. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, Z.; Erdem, Y.K. Detection of the milk proteins by RP-HPLC. J. Food 2015, 35, 5–11. [Google Scholar]
- Lemal, P.; Grelet, C.; Dehareng, F.; Soyeurt, H.; Schroyen, M.; Gengler, N. Residual analysis for the identification of potential MIR-derived biomarkers of heat stress in dairy cattle. J. Dairy Sci. 2024, 108, 1714–1729. [Google Scholar] [CrossRef]
- Ledesma-Martinez, E.; Aguiniga-Sanchez, I.; Weiss-Steider, B.; Rivera-Martinez, A.R.; Santiago-Osorio, E. Casein and Peptides Derived from Casein as Antileukaemic Agents. J. Oncol. 2019, 2019, 8150967. [Google Scholar] [CrossRef]
- Tenenbaum, M.; Deracinois, B.; Dugardin, C.; Auger, J.; Baniel, A.; Boulier, A.; Flahaut, C.; Ravallec, R.; Cudennec, B. Digested casein phosphopeptides impact intestinal calcium transport in vitro. Food Funct. 2024, 15, 8104–8115. [Google Scholar] [CrossRef]
- Rehman, S.U.; Feng, T.; Wu, S.; Luo, X.; Lei, A.; Luobu, B.; Hassan, F.U.; Liu, Q. Comparative Genomics, Evolutionary and Gene Regulatory Regions Analysis of Casein Gene Family in Bubalus bubalis. Front. Genet. 2021, 12, 662609. [Google Scholar] [CrossRef]
- Auestad, N.; Layman, D.K. Dairy bioactive proteins and peptides: A narrative review. Nutr. Rev. 2021, 79 (Suppl. S2), 36–47. [Google Scholar] [CrossRef]
- Creamer, L.K.; Plowman, J.E.; Liddell, M.J.; Smith, M.H.; Hill, J.P. Micelle Stability: κ-Casein Structure and Function. J. Dairy Sci. 1998, 81, 3004–3012. [Google Scholar] [CrossRef]
- Runthala, A.; Mbye, M.; Ayyash, M.; Xu, Y.; Kamal-Eldin, A. Caseins: Versatility of Their Micellar Organization in Relation to the Functional and Nutritional Properties of Milk. Molecules 2023, 28, 2023. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Subirade, M. β-Lactoglobulin/Folic Acid Complexes: Formation, Characterization, and Biological Implication. J. Phys. Chem. B 2010, 114, 6707–6712. [Google Scholar] [CrossRef] [PubMed]
- Chaneton, L.; Pérez Sáez, J.M.; Bussmann, L.E. Antimicrobial activity of bovine β-lactoglobulin against mastitis-causing bacteria. J. Dairy Sci. 2011, 94, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Gapper, L.W.; Copestake, D.E.; Otter, D.E.; Indyk, H.E. Analysis of bovine immunoglobulin G in milk, colostrum and dietary supplements: A review. Anal. Bioanal. Chem. 2007, 389, 93–109. [Google Scholar] [CrossRef]
- Ulfman, L.H.; Leusen, J.H.W.; Savelkoul, H.F.J.; Warner, J.O.; van Neerven, R.J.J. Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef]
- Gruden, S.; Poklar Ulrih, N. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef]
- Azooz, M.F.; El-Wakeel, S.A.; Yousef, H.M. Financial and economic analyses of the impact of cattle mastitis on the profitability of Egyptian dairy farms. Vet. World 2020, 13, 1750–1759. [Google Scholar] [CrossRef]
- Bors, A.; Bors, S.I.; Floristean, V.C. Mastitis impact on high-yielding dairy farm’s reproduction and net present value. Front. Vet. Sci. 2023, 10, 1345782. [Google Scholar] [CrossRef]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef]
- Schwarz, D.; Diesterbeck, U.S.; Konig, S.; Brugemann, K.; Schlez, K.; Zschock, M.; Wolter, W.; Czerny, C.P. Microscopic differential cell counts in milk for the evaluation of inflammatory reactions in clinically healthy and subclinically infected bovine mammary glands. J. Dairy Res. 2011, 78, 448–455. [Google Scholar] [CrossRef]
- Bobbo, T.; Penasa, M.; Cassandro, M. Short communication: Genetic aspects of milk differential somatic cell count in Holstein cows: A preliminary analysis. J. Dairy Sci. 2019, 102, 4275–4279. [Google Scholar] [CrossRef]
- Lipkens, Z.; Piepers, S.; Verbeke, J.; De Vliegher, S. Infection dynamics across the dry period using Dairy Herd Improvement somatic cell count data and its effect on cow performance in the subsequent lactation. J. Dairy Sci. 2019, 102, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Wiggans, G.R.; Shook, G.E. A Lactation Measure of Somatic Cell Count. J. Dairy Sci. 1987, 70, 2666–2672. [Google Scholar] [CrossRef]
- Fonseca, M.; Kurban, D.; Roy, J.P.; Santschi, D.E.; Molgat, E.; Dufour, S. Usefulness of differential somatic cell count for udder health monitoring: Effect of intramammary infections, days in milk, quarter location, and parity on quarter-level differential somatic cell count and somatic cell score in apparently healthy dairy cows. J. Dairy Sci. 2024, 108, 3878–3899. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; Kurban, D.; Roy, J.P.; Santschi, D.E.; Molgat, E.; Yang, D.A.; Dufour, S. Usefulness of differential somatic cell count for udder health monitoring: Identifying referential values for differential somatic cell count in healthy quarters and quarters with subclinical mastitis. J. Dairy Sci. 2024, 108, 3917–3928. [Google Scholar] [CrossRef]
- Fonseca, M.; Kurban, D.; Roy, J.P.; Santschi, D.E.; Molgat, E.; Dufour, S. Usefulness of differential somatic cell count for udder health monitoring: Diagnostic performance of somatic cell count, and differential somatic cell count for diagnosing of intramammary infections in dairy herds with automated milking systems. J. Dairy Sci. 2024, 108, 3929–3941. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Kleinhans, S.; Reimann, G.; Stuckler, P.; Reith, F.; Ilves, K.; Pedastsaar, K.; Yan, L.; Zhang, Z.; Valdivieso, M.; et al. Investigation of dairy cow performance in different udder health groups defined based on a combination of somatic cell count and differential somatic cell count. Prev. Vet. Med. 2020, 183, 105123. [Google Scholar] [CrossRef]
- Bisutti, V.; Vanzin, A.; Toscano, A.; Pegolo, S.; Giannuzzi, D.; Tagliapietra, F.; Schiavon, S.; Gallo, L.; Trevisi, E.; Negrini, R.; et al. Impact of somatic cell count combined with differential somatic cell count on milk protein fractions in Holstein cattle. J. Dairy Sci. 2022, 105, 6447–6459. [Google Scholar] [CrossRef]
- Pegolo, S.; Mota, L.F.M.; Bisutti, V.; Martinez-Castillero, M.; Giannuzzi, D.; Gallo, L.; Schiavon, S.; Tagliapietra, F.; Revello Chion, A.; Trevisi, E.; et al. Genetic parameters of differential somatic cell count, milk composition, and cheese-making traits measured and predicted using spectral data in Holstein cows. J. Dairy Sci. 2021, 104, 10934–10949. [Google Scholar] [CrossRef]
- Giannuzzi, D.; Vanzin, A.; Pegolo, S.; Toscano, A.; Bisutti, V.; Gallo, L.; Schiavon, S.; Cecchinato, A. Novel insights into the associations between immune cell population distribution in mammary glands and milk minerals in Holstein cows. J. Dairy Sci. 2024, 107, 593–606. [Google Scholar] [CrossRef]
- Haxhiaj, K.; Wishart, D.S.; Ametaj, B.N. Mastitis: What It Is, Current Diagnostics, and the Potential of Metabolomics to Identify New Predictive Biomarkers. Dairy 2022, 3, 722–746. [Google Scholar] [CrossRef]
- Mondini, S.; Gislon, G.; Zucali, M.; Sandrucci, A.; Tamburini, A.; Bava, L. Factors influencing somatic cell count and leukocyte composition in cow milk: A field study. J. Dairy Sci. 2024, 108, 2721–2733. [Google Scholar] [CrossRef]
- Davis, S.R. Triennial Lactation Symposium/BOLFA: Mammary growth during pregnancy and lactation and its relationship with milk yield. J. Anim. Sci. 2017, 95, 5675–5688. [Google Scholar] [CrossRef] [PubMed]
- Lean, I.J.; Golder, H.M.; LeBlanc, S.J.; Duffield, T.; Santos, J.E.P. Increased parity is negatively associated with survival and reproduction in different production systems. J. Dairy Sci. 2023, 106, 476–499. [Google Scholar] [CrossRef] [PubMed]
- Bochniarz, M.; Błaszczyk, P.; Szczubiał, M.; Vasiu, I.; Adaszek, Ł.; Michalak, K.; Pietras-Ożga, D.; Wochnik, M.; Dąbrowski, R. Comparative analysis of total protein, casein, lactose, and fat content in milk of cows suffering from subclinical and clinical mastitis caused by Streptococcus spp. J. Vet. Res. 2023, 67, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hayes, K.D.; Mauer, L.J. Fluorescent Labeling Study of Plasminogen Concentration and Location in Simulated Bovine Milk Systems. J. Dairy Sci. 2006, 89, 58–70. [Google Scholar] [CrossRef]
- Rauh, V.M.; Sundgren, A.; Bakman, M.; Ipsen, R.; Paulsson, M.; Larsen, L.B.; Hammershøj, M. Plasmin activity as a possible cause for age gelation in UHT milk produced by direct steam infusion. Int. Dairy J. 2014, 38, 199–207. [Google Scholar] [CrossRef]
- Katsafadou, A.I.; Vasileiou, N.G.C.; Fthenakis, G.C. Use of Proteomics in the Study of Mastitis in Ewes. Pathogens 2019, 8, 134. [Google Scholar] [CrossRef]
- Wellnitz, O.; Bruckmaier, R.M. Invited review: The role of the blood-milk barrier and its manipulation for the efficacy of the mammary immune response and milk production. J. Dairy Sci. 2021, 104, 6376–6388. [Google Scholar] [CrossRef]
- Fox, P.F.; Brodkorb, A. The casein micelle: Historical aspects, current concepts and significance. Int. Dairy J. 2008, 18, 677–684. [Google Scholar] [CrossRef]
- Costa, A.; Egger-Danner, C.; Mészáros, G.; Fuerst, C.; Penasa, M.; Sölkner, J.; Fuerst-Waltl, B. Genetic associations of lactose and its ratios to other milk solids with health traits in Austrian Fleckvieh cows. J. Dairy Sci. 2019, 102, 4238–4248. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Bovenhuis, H.; Egger-Danner, C.; Fuerst-Waltl, B.; Boutinaud, M.; Guinard-Flament, J.; Obritzhauser, W.; Visentin, G.; Penasa, M. Mastitis has a cumulative and lasting effect on milk yield and lactose content in dairy cows. J. Dairy Sci. 2025, 108, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Wellnitz, O.; Arnold, E.T.; Lehmann, M.; Bruckmaier, R.M. Short communication: Differential immunoglobulin transfer during mastitis challenge by pathogen-specific components. J. Dairy Sci. 2013, 96, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.K.; Wellnitz, O.; Hernández-Castellano, L.E.; Ahmadpour, A.; Bruckmaier, R.M. Supraphysiological oxytocin increases the transfer of immunoglobulins and other blood components to milk during lipopolysaccharide- and lipoteichoic acid–induced mastitis in dairy cows. J. Dairy Sci. 2016, 99, 9165–9173. [Google Scholar] [CrossRef]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef]
- He, W.; Ma, S.; Lei, L.; He, J.; Li, X.; Tao, J.; Wang, X.; Song, S.; Wang, Y.; Wang, Y.; et al. Prevalence, etiology, and economic impact of clinical mastitis on large dairy farms in China. Vet. Microbiol. 2020, 242, 108570. [Google Scholar] [CrossRef]
- Petzer, I.M.; Etter, E.M.C.; Donkin, E.F.; Webb, E.C.; Karzis, J. Epidemiological and partial budget analysis for treatment of subclinical Staphylococcus aureus intramammary infections considering microbiological and cytological scenarios. Prev. Vet. Med. 2017, 148, 66–77. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ren, X.; Liu, L.; Yang, Z.; Li, C.; Bao, X.; Amantuer, A.; Wen, P.; Wang, D.; Zhang, S. The Relationship Between Protein Fraction Contents and Immune Cells in Milk. Animals 2025, 15, 1578. https://doi.org/10.3390/ani15111578
Wang H, Ren X, Liu L, Yang Z, Li C, Bao X, Amantuer A, Wen P, Wang D, Zhang S. The Relationship Between Protein Fraction Contents and Immune Cells in Milk. Animals. 2025; 15(11):1578. https://doi.org/10.3390/ani15111578
Chicago/Turabian StyleWang, Haitong, Xiaoli Ren, Li Liu, Zhuo Yang, Chunfang Li, Xiangnan Bao, Ayihumaer Amantuer, Peipei Wen, Dongwei Wang, and Shujun Zhang. 2025. "The Relationship Between Protein Fraction Contents and Immune Cells in Milk" Animals 15, no. 11: 1578. https://doi.org/10.3390/ani15111578
APA StyleWang, H., Ren, X., Liu, L., Yang, Z., Li, C., Bao, X., Amantuer, A., Wen, P., Wang, D., & Zhang, S. (2025). The Relationship Between Protein Fraction Contents and Immune Cells in Milk. Animals, 15(11), 1578. https://doi.org/10.3390/ani15111578





