Assessing the Genetic and Environmental Factors on Egg Amino Acid Traits in Chickens: A Review
Simple Summary
Abstract
1. Introduction
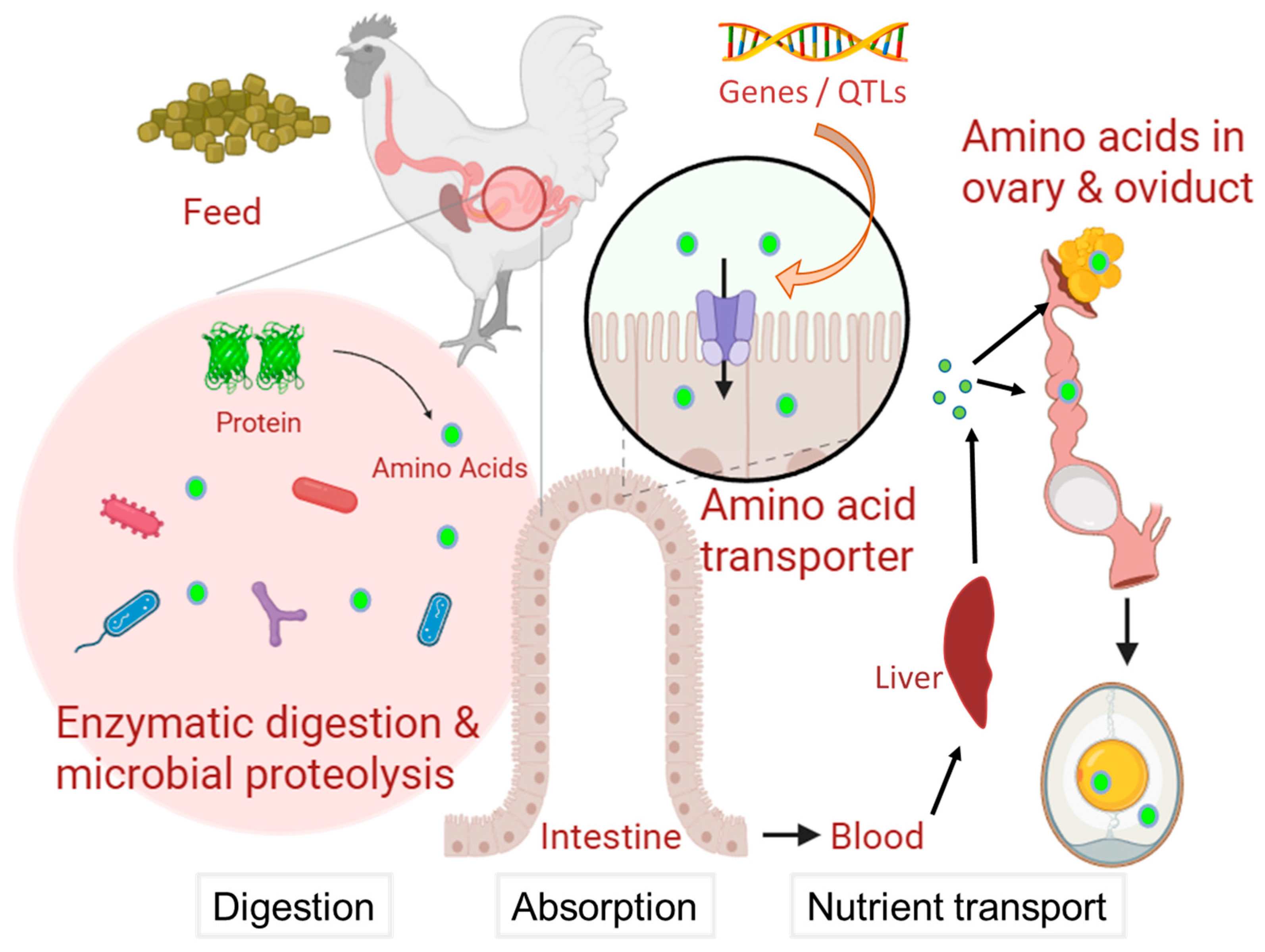
2. Amino Acid Metabolism in the Eggs
3. Analytic Methods
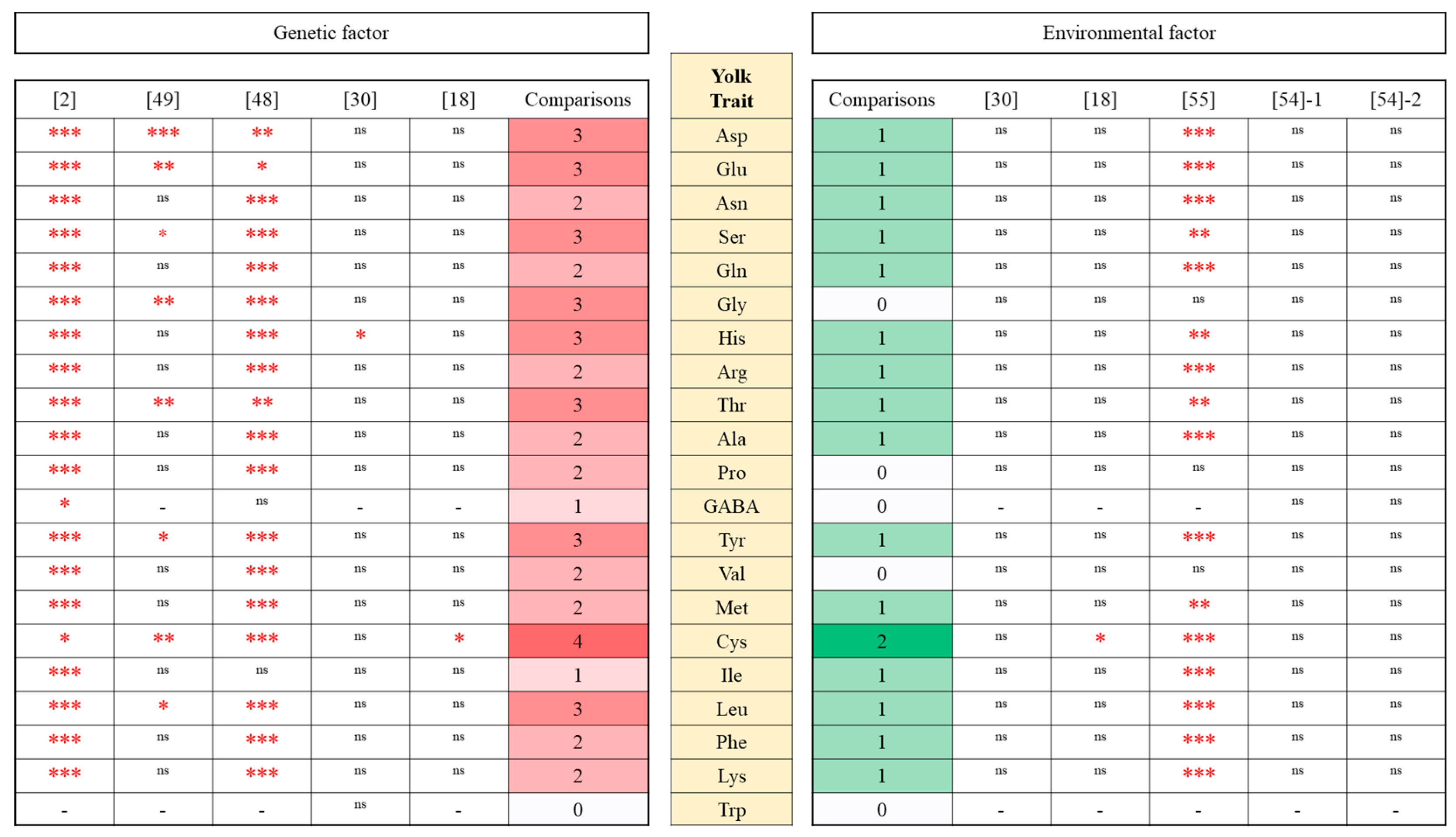
4. Genetic Factors
4.1. Breed/Strain Comparisons
4.2. Yolk Amino Acids
4.3. Albumen Amino Acids
4.4. Nutrient Transport
5. Environmental Factors
5.1. Yolk and Albumen Amino Acids
5.2. Microbiota Influence in Amino Acid Metabolism
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The golden egg: Nutritional value, bioactivities, and emerging benefits for human health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Shimamoto, S.; Takaya, M.; Sato, S.; Takahashi, K.; Nishimura, K.; Ijiri, D. Impact on genetic differences among various chicken breeds on free amino acid contents of egg yolk and albumen. Sci. Rep. 2021, 11, 2270. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Rodriguez, N.R. Egg protein as a source of power, strength, and energy. Nutr. Today 2009, 44, 43–48. [Google Scholar] [CrossRef]
- Andersen, C.J. Bioactive egg components and inflammation. Nutrients 2015, 7, 7889–7913. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Kalaivani, M. Designer foods and their benefits: A review. J. Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Kralik, G.; Kralik, Z. Poultry products enriched with nutricines have beneficial effects on human health. Med. Glas 2017, 14, 1. [Google Scholar] [CrossRef]
- Surai, P.F.; Sparks, N.H.C. Designer eggs: From improvement of egg composition to functional food. Trends Food Sci. Technol. 2001, 12, 7–16. [Google Scholar] [CrossRef]
- Roberts, J.R. Factors affecting egg internal quality and egg shell quality in laying hens. J. Poult. Sci. 2004, 41, 161–177. [Google Scholar] [CrossRef]
- Goto, T.; Tsudzuki, M. Genetic mapping of quantitative trait loci for egg production and egg quality traits in chickens: A review. J. Poult. Sci. 2017, 54, 1–12. [Google Scholar] [CrossRef]
- Goto, T.; Ishikawa, A.; Yoshida, M.; Goto, N.; Umino, T.; Nishibori, M.; Tsudzuki, M. Quantitative trait loci mapping for external egg traits in F2 chickens. J. Poult. Sci. 2014, 51, 118–129. [Google Scholar] [CrossRef]
- Goto, T.; Ishikawa, A.; Goto, N.; Nishibori, M.; Umino, T.; Tsudzuki, M. Mapping of main-effect and epistatic quantitative trait loci for internal egg traits in an F2 resource population of chickens. J. Poult. Sci. 2014, 51, 375–386. [Google Scholar] [CrossRef]
- Goto, T.; Shiraishi, J.I.; Bungo, T.; Tsudzuki, M. Characteristics of egg-related traits in the Onagadori (Japanese Extremely Long Tail) breed of chickens. J. Poult. Sci. 2015, 52, 81–87. [Google Scholar] [CrossRef]
- Lay, D.C., Jr.; Fulton, R.M.; Hester, P.Y.; Karcher, D.M.; Kjaer, J.B.; Mench, J.A.; Mullens, B.A.; Newberry, R.C.; Nicol, C.J.; O’Sullivan, N.P.; et al. Hen welfare in different housing systems. Poult. Sci. 2011, 90, 278–294. [Google Scholar] [CrossRef]
- Arulnathan, V.; Turner, I.; Bamber, N.; Ferdous, J.; Grassauer, F.; Doyon, M.; Pelletier, N. A systematic review of potential productivity, egg quality, and animal welfare implications of extended lay cycles in commercial laying hens in Canada. Poult. Sci. 2024, 103, 103475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Ning, Z.H.; Xu, G.Y.; Hou, Z.C.; Yang, A.N. Heritabilities and genetic and phenotypic correlations of egg quality traits in brown-egg dwarf layers. Poult. Sci. 2005, 84, 1209–1213. [Google Scholar] [CrossRef]
- Wolc, A.; White, I.M.S.; Hill, W.G.; Olori, V.E. Inheritance of hatchability in broiler chickens and its relationship to egg quality traits. Poult. Sci. 2010, 89, 2334–2340. [Google Scholar] [CrossRef]
- Wolc, A.; Arango, J.; Settar, P.; O’sullivan, N.P.; Olori, V.E.; White, I.M.S.; Hill, W.G.; Dekkers, J.C.M. Genetic parameters of egg defects and egg quality in layer chickens. Poult. Sci. 2012, 91, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Takaya, M.; Nishimura, K.; Goto, T. Breed and feed affect amino acid contents of egg yolk and eggshell color in chickens. Poult. Sci. 2020, 99, 172–178. [Google Scholar] [CrossRef]
- Goto, T.; Mori, H.; Shiota, S.; Tomonaga, S. Metabolomics approach reveals the effects of breed and feed on the composition of chicken eggs. Metabolites 2019, 9, 224. [Google Scholar] [CrossRef]
- Dermane, A.; Eloh, K.; Palanga, K.K.; Adjito, D.T.; N’nanle, O.; Karou, D.S.; Kpanzou, T.A.; Caboni, P. Comparative metabolomic profiling of eggs from 3 diverse chicken breeds using GC-MS analysis. Poult. Sci. 2024, 103, 103616. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Liu, F.; Li, R.; Azzam, M.M.; Dong, X. Characterization and evaluation of Taihe black-boned silky fowl eggs based on physical properties, nutritive values, and flavor profiles. Foods 2024, 13, 3308. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Wakayama, M.; Ashino, Y.; Kadowaki, R.; Sato, M.; Soga, T.; Tomita, M. Effects of feed crops and boiling on chicken egg yolk and white determined by a metabolome analysis. Food Chem. 2020, 327, 127077. [Google Scholar] [CrossRef]
- Giannenas, I.; Grigoriadou, K.; Sidiropoulou, E.; Bonos, E.; Cheilari, A.; Vontzalidou, A.; Karaiskou, C.; Aligiannis, N.; Florou-Paneri, P.; Christaki, E. Untargeted UHPLC-MS metabolic profiling as a valuable tool for the evaluation of eggs quality parameters after dietary supplementation with oregano, thyme, sideritis tea and chamomile on brown laying hens. Metabolomics 2021, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Z.; Shi, P.P.; Tan, G.; Zeng, J.; Huang, P. Metabolome analysis of egg yolk and white following dietary supplementation with Ampelopsis grossedentata extract. Poult. Sci. 2024, 103, 104110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; You, M.; Ma, N.; Lv, J. Advance in the application of metabolomics technology in poultry. Front. Vet. Sci. 2024, 11, 1501630. [Google Scholar] [CrossRef]
- Narukawa, M. Physiological responses to taste signals of functional food components. Biosci. Biotechnol. Biochem. 2018, 82, 200–206. [Google Scholar] [CrossRef]
- Kirimura, J.; Shimizu, A.; Kimizuka, A.; Ninomiya, T.; Katsuya, N. Contribution of peptides and amino acids to the taste of foods. J. Agric. Food Chem. 1969, 17, 689–695. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations–A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Free aromatic amino acids in egg yolk show antioxidant properties. Food Chem. 2011, 129, 155–161. [Google Scholar] [CrossRef]
- Goto, T.; Shimamoto, S.; Ohtsuka, A.; Ijiri, D. Analyses of free amino acid and taste sensor traits in egg albumen and yolk revealed potential of value-added eggs in chickens. Anim. Sci. J. 2021, 92, e13510. [Google Scholar] [CrossRef]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- Apajalahti, J.; Vienola, K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed. Sci. Technol. 2016, 221, 323–330. [Google Scholar] [CrossRef]
- Bröer, S. Intestinal amino acid transport and metabolic health. Annu. Rev. Nutr. 2023, 43, 73–99. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, P.; Wu, G. Amino acid nutrition and metabolism in chickens. In Amino Acids in Nutrition and Health. Advances in Experimental Medicine and Biology: Volume 1285; Wu, G., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 109–131. [Google Scholar] [CrossRef]
- Chandel, N.S. Amino acid metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040584. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Hakeem, W.G.A.; Selvaraj, R.K.; Shanmugasundaram, R. Beyond protein synthesis: The emerging role of arginine in poultry nutrition and host-microbe interactions. Front. Physiol. 2024, 14, 1326809. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, D.; Zhou, Y.; Sun, Y.; Ao, X.; Hao, R.; Gao, M.; Xu, Y.; Li, P.; Jia, C.; et al. Yolk precursor synthesis and deposition in hierarchical follicles and effect on egg production performance of hens. Poult. Sci. 2023, 102, 102756. [Google Scholar] [CrossRef] [PubMed]
- Bain, M.M.; Nys, Y.; Dunn, I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016, 57, 330–338. [Google Scholar] [CrossRef]
- Yin, Z.T.; Lian, L.; Zhu, F.; Zhang, Z.H.; Hincke, M.; Yang, N.; Hou, Z.D. The transcriptome landscapes of ovary and three oviduct segments during chicken (Gallus gallus) egg formation. Genomics 2020, 112, 243–251. [Google Scholar] [CrossRef]
- Torres, N.; Tobón-Cornejo, S.; Velazquez-Villegas, L.A.; Noriega, L.G.; Alemán-Escondrillas, G.; Tovar, A.R. Amino acid catabolism: An overlooked area of metabolism. Nutrients 2023, 15, 3378. [Google Scholar] [CrossRef]
- Sabry, M.I.E.; Yalcin, S. Factors influencing the development of gastrointestinal tract and nutrient transporters’ function during the embryonic life of chickens-A review. J. Anim. Physiol. Anim. Nutr. 2023, 107, 1419–1428. [Google Scholar] [CrossRef]
- Shibata, M.; Takahashi, T.; Endo, K.; Kozakai, T.; Azuma, Y.; Kurose, Y. Age-related regulation of active amino acid transport in the ileum of broiler chickens. J. Poult. Sci. 2020, 57, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 2018, 43, 752–789. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yin, Y.; Tu, Q.; Yang, H. Glucose and amino acid in enterocyte: Absorption, metabolism and maturation. Front. Biosci. 2018, 23, 1721–1739. [Google Scholar] [CrossRef]
- Wong, E.A.; Uni, Z. Centennial review: The chicken yolk sac is a multifunctional organ. Poult. Sci. 2021, 100, 100821. [Google Scholar] [CrossRef]
- Sah, N.; Mishra, B. Regulation of egg formation in the oviduct of laying hen. Worlds. Poult. Sci. J. 2018, 74, 1–13. [Google Scholar] [CrossRef]
- Xu, W.; Zhong, C.; Zou, C.; Wang, B.; Zhang, N. Analytical methods for amino acid determination in organisms. Amino Acids 2020, 52, 1071–1088. [Google Scholar] [CrossRef]
- Goto, T.; Ohya, K.; Takaya, M. Genotype affects free amino acids of egg yolk and albumen in Japanese indigenous breeds and commercial Brown layer chickens. Poult. Sci. 2022, 101, 101582. [Google Scholar] [CrossRef]
- Nishimura, K.; Ijiri, D.; Shimamoto, S.; Takaya, M.; Ohtsuka, A.; Goto, T. Genetic effect on free amino acid contents of egg yolk and albumen using five different chicken genotypes under floor rearing system. PLoS ONE 2021, 16, e0258506. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.H. Protein and amino acid content in four brands of commercial table eggs in retail markets in relation to human requirements. Animals 2020, 10, 406. [Google Scholar] [CrossRef]
- Gao, J.; Xu, W.; Zeng, T.; Tian, Y.; Wu, C.; Liu, S.; Zhao, Y.; Zhou, S.; Lin, X.; Cao, H.; et al. Genome-wide association study of egg-laying traits and egg quality in LingKun chickens. Front. Vet. Sci. 2022, 9, 877739. [Google Scholar] [CrossRef]
- Shiraishi, J.I.; Ijiri, D.; Katafuchi, A.; Tomonaga, S.; Shimamoto, S.; Do, H.; Ishihara, S.; Ohtsuka, A. Quantification of Nτ-methylhistidine and Nπ-methylhistidine in chicken plasma by liquid chromatography–tandem mass spectrometry. J. Poult. Sci. 2023, 60, 2023017. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, Y.; Qi, J.; Xi, Y.; Shen, Z.; Twumasi, G.; Bai, L.; Hu, J.; Wang, J.; Li, L.; et al. Genome-wide association analysis explores the genetic loci of amino acid content in duck’s breast muscle. BMC Genom. 2024, 25, 486. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, N.; Takaya, M.; Hayashi, H.; Goto, T. Housing systems affect eggshell lightness and free amino acid contents of egg albumen in Tosa-jidori chickens: A preliminary research. Animals 2023, 13, 1837. [Google Scholar] [CrossRef]
- Kawamura, N.; Yokoyama, R.; Takaya, M.; Ono, R.; Goto, T. Combined effect of feed and housing system affects free amino acid content of egg yolk and albumen in brown layer chickens. J. Poult. Sci. 2023, 60, 2023007. [Google Scholar] [CrossRef]
- Ferréa, S.; González-Ruiza, V. Guillarmea, D.; Rudaza, S. Analytical strategies for the determination of amino acids: Past, present and future trends. J. Chromatogr. B 2019, 1132, 121819. [Google Scholar] [CrossRef]
- Song, Y.; Xu, C.; Kuroki, H.; Liao, Y.; Tsunoda, M. Recent trends in analytical methods for the determination of amino acids in biological samples. J. Pharm. Biomed. Anal. 2018, 147, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, H.; Dettmer, K.; Gronwald, W.; Oefner, P.J. Advances in amino acid analysis. Anal. Bioanal. Chem. 2009, 393, 445–452. [Google Scholar] [CrossRef]
- Gałęzowska, G.; Ratajczyk, J.; Wolska, L. Determination of amino acids in human biological fluids by high-performance liquid chromatography: Critical review. Amino Acids 2021, 53, 993–1009. [Google Scholar] [CrossRef]
- Yin, X.; Adams, E.; Schepdael, A.V. Overview of chromatographic and electrophoretic methods for the determination of branched-chain amino acids. J. Sep. Sci. 2023, 46, e2300213. [Google Scholar] [CrossRef]
- Yogeswari, M.S.; Selamat, J.; Jambari, N.N.; Khatib, A.; Amin, M.H.M.; Murugesu, S. Metabolomics for quality assessment of poultry meat and eggs. Food Qual. Saf. 2024, 8, fyae004. [Google Scholar] [CrossRef]
- Blanco, A.E.; Icken, W.; Ould-Ali, D.; Cavero, D.; Schmutz, M. Genetic parameters of egg quality traits on different pedigree layers with special focus on dynamic stiffness. Poult. Sci. 2014, 93, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, Y.; Yang, X.; Shan, M.; Gao, X.; Zhang, Y.; Hu, J.; Shan, A. Dietary intake of broiler breeder hens during the laying period affects amino acid and fatty acid profiles in eggs. R. Bras. Zootec. 2019, 48, e20180292. [Google Scholar] [CrossRef]
- Zeng, P.L.; Li, X.G.; Wang, X.Q.; Zhang, D.X.; Shu, G.; Luo, Q.B. The relationship between gene expression of cationic and neutral amino acid transporters in the small intestine of chick embryos and chick breed, development, sex, and egg amino acid concentration. Poult. Sci. 2011, 90, 2548–2556. [Google Scholar] [CrossRef]
- Li, X.G.; Chen, X.L.; Wang, X.Q. Changes in relative organ weights and intestinal transporter gene expression in embryos from White Plymouth Rock and WENS yellow feather chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 368–375. [Google Scholar] [CrossRef]
- Su, S.; Miska, K.B.; Fetterer, R.H.; Jenkins, M.C.; Wong, E.A. Expression of digestive enzymes and nutrient transporters in Eimeria acervulina-challenged layers and broilers. Poult. Sci. 2014, 93, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Miska, K.B.; Fetterer, R.H. Expression of amino acid and sugar transporters, aminopeptidase, and the di- and tri-peptide transporter PepT1; differences between modern fast growing broilers and broilers not selected for rapid growth. Poult. Sci. 2019, 98, 2272–2280. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Hul, M.V.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Jang, L.G.; Choi, G.; Kim, S.W.; Kim, B.Y.; Lee, S.; Park, H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef]
- Kohl, K.D. Diversity and function of the avian gut microbiota. J. Comp. Physiol. B 2012, 182, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Yan, S.; Li, P.; Li, G.; Gao, M.; Yan, L.; Lv, Z.; Guo, Y. Comparison and correlation analysis of immune function and gut microbiota of broiler chickens raised in double-layer cages and litter floor pens. Microbiol. Spectr. 2022, 10, e00045-22. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, S.; Li, X.; Yang, X.; Long, F.; Yang, X. Effects of encapsulated essential oils and organic acids on laying performance, egg quality, intestinal morphology, barrier function, and microflora count of hens during the early laying period. Poult. Sci. 2019, 98, 6751–6760. [Google Scholar] [CrossRef]
- Li, W.; Yang, M.; Luo, Y.; Liu, W.; Wang, Z.; Ning, Z. Effects of dietary rosemary ultrafine powder supplementation on aged hen health and productivity: A randomized controlled trial. Poult. Sci. 2024, 103, 104133. [Google Scholar] [CrossRef]
- Liu, S.; Hu, J.; Li, L.; Xing, S.; Yang, Y.; Liao, X. Sodium butyrate reduces ammonia production in the cecum of laying hens by regulating ammonia-producing bacteria. Poult. Sci. 2023, 102, 102241. [Google Scholar] [CrossRef]
- Wan, Y.; Ma, R.; Zhang, H.; Li, L.; Chai, L.; Qi, R.; Liu, W.; Li, J.; Li, Y.; Zhan, K. Different non-cage housing systems alter duodenal and cecal microbiota composition in Shendan chickens. Front. Vet. Sci. 2021, 8, 728538. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; Vuyst, L.D.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Kumar, D.; Lal, M.K.; Dutt, S.; Raigond, P.; Changan, S.S.; Tiwari, R.K.; Chourasia, K.N.; Mangal, V.; Singh, B. Functional fermented probiotics, prebiotics, and synbiotics from non-dairy products: A perspective from nutraceutical. Mol. Nutr. Food Res. 2022, 66, e2101059. [Google Scholar] [CrossRef]
- Biesalski, H.K. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016, 1372, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Luasiri, P.; Li, J.; Laosam, P.; Sangsawad, P. Research advancements on the diversity and host interaction of gut microbiota in chickens. Front. Vet. Sci. 2024, 11, 1492545. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, H.; Xie, J.; Zeng, T.; Hao, L.; Xu, W.; Lu, L. The effects of fermented feed on the growth performance, antioxidant activity, immune function, intestinal digestive enzyme activity, morphology, and microflora of yellow-feather chickens. Animals 2023, 13, 3545. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Li, S.; Zhang, H.; Liu, Z. Effects of tea residues-fermented feed on production performance, egg quality, antioxidant capacity, caecal microbiota, and ammonia emissions of laying hens. Front. Vet. Sci. 2023, 10, 1195074. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Luise, D.; Floc’h, N.L.; Tesseraud, S.; Lambert, W.; Bosi, P.; Trevisi, P.; Beaumont, M.; Corrent, E. Functional amino acids in pigs and chickens: Implication for gut health. Front. Vet. Sci. 2021, 8, 663727. [Google Scholar] [CrossRef]
- Long, A.D.; Macdonald, S.J.; King, E.G. Dissecting complex traits using the Drosophila synthetic population resource. Trends Genet. 2014, 30, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.F.; Ladejobi, O.; Amer, S.; Bentley, A.R.; Biernaskie, J.; Boden, S.A.; Clark, M.; Dell’Acqua, M.; Dixon, L.E.; Filippi, C.V.; et al. Multi-parent populations in crops: A toolbox integrating genomics and genetic mapping with breeding. Heredity 2020, 125, 396–416. [Google Scholar] [CrossRef]
- Song, B.; Li, P.; Xu, H.; Wang, Z.; Yuan, J.; Zhang, B.; Lv, Z.; Song, Z.; Guo, Y. Effects of rearing system and antibiotic treatment on immune function, gut microbiota and metabolites of broiler chickens. J. Anim. Sci. Biotechnol. 2022, 13, 144. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Zhang, J.; Wang, X.; Wang, R.; Bao, J.; Zhang, R. Effects of dustbathing environment on gut microbiota and expression of intestinal barrier and immune-related genes of adult laying hens housed individually in modified traditional cage. Poult. Sci. 2023, 102, 103097. [Google Scholar] [CrossRef]
- Yan, L.; Lv, Z.Z.; An, S.; Xing, K.; Wang, Z.G.; Lv, M.B.; Choct, M.; Guo, Y.M.; Zhou, G.L. Effects of rearing system and narasin on growth performance, gastrointestinal development, and gut microbiota of broilers. Poult. Sci. 2021, 100, 100840. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyawali, D.; Goto, T. Assessing the Genetic and Environmental Factors on Egg Amino Acid Traits in Chickens: A Review. Animals 2025, 15, 1554. https://doi.org/10.3390/ani15111554
Gyawali D, Goto T. Assessing the Genetic and Environmental Factors on Egg Amino Acid Traits in Chickens: A Review. Animals. 2025; 15(11):1554. https://doi.org/10.3390/ani15111554
Chicago/Turabian StyleGyawali, Dipson, and Tatsuhiko Goto. 2025. "Assessing the Genetic and Environmental Factors on Egg Amino Acid Traits in Chickens: A Review" Animals 15, no. 11: 1554. https://doi.org/10.3390/ani15111554
APA StyleGyawali, D., & Goto, T. (2025). Assessing the Genetic and Environmental Factors on Egg Amino Acid Traits in Chickens: A Review. Animals, 15(11), 1554. https://doi.org/10.3390/ani15111554





