Dietary Supplementation with Algae Powders and Carotenoids Enhances Growth Performance and Tissue-Specific Carotenoid Accumulation in Penaeus Vannamei
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Feeds
2.2. Shrimp Rearing and Sampling
2.3. Growth Performance and Colorimetric Analysis
2.4. Free Carotenoids Analysis in Algae Powders and Feeds
2.5. Carotenoids Analysis in Shrimps
2.6. Correlation and Statistical Analysis
3. Results
3.1. Growth Performance and Colorimetric Analysis
3.2. Carotenoids Profile in Algae and Feeds
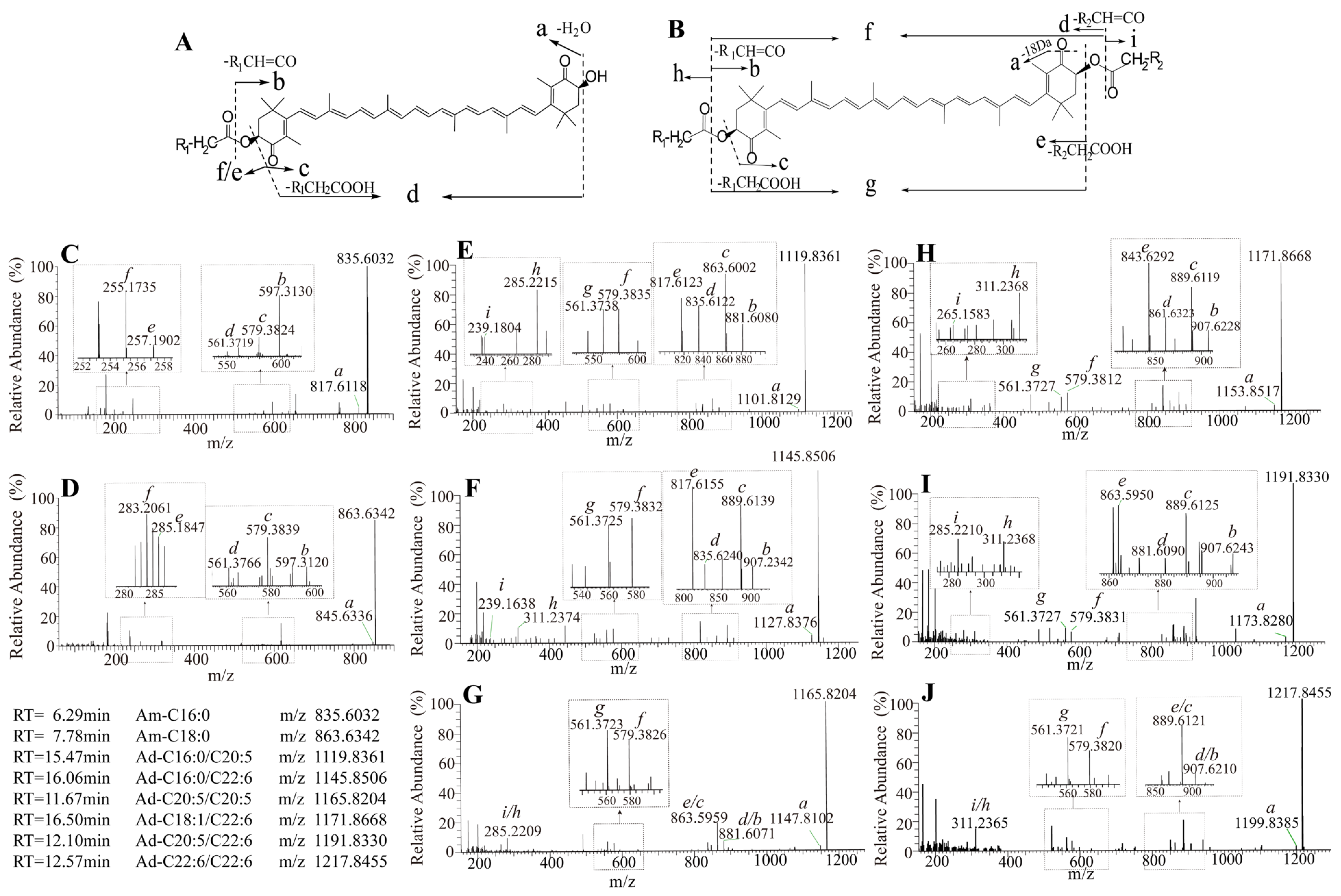
3.3. Carotenoids Identification in Shrimp
3.4. Carotenoid Distribution Across the Shrimp Tissues Influenced by Algal-Supplemented Feed
3.5. Carotenoid Distribution Across the Shrimp Tissues Influenced by Carotenoid-Supplemented Feed
3.6. Correlation Analysis Between the Carotenoid Composition of Feeds and Shrimp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Supamattaya, K.; Kiriratnikom, S.; Boonyaratpalin, M.; Borowitzka, L. Effect of a Dunaliella extract on growth performance, health condition, immune response and disease resistance in black tiger shrimp (Penaeus monodon). Aquaculture 2005, 248, 207–216. [Google Scholar] [CrossRef]
- Vernon-Carter, E.; Ponce-Palafox, J.; Pedroza-Islas, R. Pigmentation of pacific white shrimp (Penaeus vannamei) using aztec marigold (Tagetes erecta) extracts as the carotenoid source. Arch. Latinoam. Nutr. 1996, 46, 243–246. [Google Scholar] [CrossRef]
- Matsuno, T. Aquatic animal carotenoids. Fish. Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Fawzy, S.; Wang, W.; Zhou, Y.; Xue, Y.; Yi, G.; Wu, M.; Huang, X. Can dietary β-carotene supplementation provide an alternative to astaxanthin on the performance of growth, pigmentation, biochemical, and immuno-physiological parameters of Litopenaeus vannamei? Aquacult. Res. 2022, 23, 101054. [Google Scholar] [CrossRef]
- Ponce-Palafox, J.T.; Arredondo-Figueroa, J.L.; Vernon-Carter, E.J. Carotenoids from plants used in diets for the culture of the pacific white shrimp (Litopenaeus vannamei). Rev. Mex. Ing. Quim. 2006, 5, 157–165. [Google Scholar]
- Lin, Y.; Chang, J.; Huang, H.; Lee, C.; Hu, Y.; Wu, M.; Huang, C.; Nan, F. Improving red-color performance, immune response and resistance to Vibrio parahaemolyticus on white shrimp Penaeus vannamei by an engineered astaxanthin yeast. Sci. Rep. 2023, 13, 2248. [Google Scholar] [CrossRef]
- Alcaíno, J.; Baeza, M.; Cifuentes, V. Carotenoid Distribution in Nature. In Carotenoids in Nature: Biosynthesis, Regulation and Function; Stange, C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–33. [Google Scholar] [CrossRef]
- Niu, J.; Xie, S.; Fang, H.; Xie, J.; Guo, T.; Zhang, Y.; Liu, Z.; Liao, S.; He, J.; Tian, L. Dietary values of macroalgae Porphyra haitanensis in Litopenaeus vannamei under normal rearing and WSSV challenge conditions: Effect on growth, immune response and intestinal microbiota. Fish. Shellfish. Immunol. 2018, 81, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Varjani, S.; Lee, D.; Chang, J. Sustainable aquaculture and animal feed from microalgae–nutritive value and techno-functional components. Renew. Sustain. Energy Rev. 2021, 150, 111549. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Hu, Q.; Sommerfeld, M.; Li, Y.; Han, D. A new paradigm for producing astaxanthin from the unicellular green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2016, 113, 2088–2099. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, M.; Wang, B.; Xu, R.; Pang, T.; Liu, J. Dietary Haematococcus pluvialis powder supplementation affect carotenoid content, astaxanthin isomer, antioxidant capacity and immune-related gene expression in pacific white shrimp, Litopenaeus vannamei. Aquacult. Res. 2021, 52, 2403–2414. [Google Scholar] [CrossRef]
- Nègre, D.; Aite, M.; Belcour, A.; Frioux, C.; Brillet-Guéguen, L.; Liu, X.; Bordron, P.; Godfroy, O.; Lipinska, A.P.; Leblanc, C. Genome–scale metabolic networks shed light on the carotenoid biosynthesis pathway in the brown algae Saccharina japonica and Cladosiphon okamuranus. Antioxidants 2019, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, X.; Hang, Y.; Deng, Y.; Lu, Q.; Lu, S. The p450-type carotene hydroxylase puchy1 from Porphyra suggests the evolution of carotenoid metabolism in red algae. J. Integr. Plant Biol. 2014, 56, 902–915. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Wade, N.M.; Gabaudan, J.; Glencross, B.D. A review of carotenoid utilisation and function in crustacean aquaculture. Rev. Aquac. 2017, 9, 141–156. [Google Scholar] [CrossRef]
- Yamada, S.; Tanaka, Y.; Sameshima, M.; Ito, Y. Pigmentation of prawn (Penaeus japonicus) with carotenoids: I. effect of dietary astaxanthin, β-carotene and canthaxanthin on pigmentation. Aquaculture 1990, 87, 323–330. [Google Scholar] [CrossRef]
- Liao, I.C.; Chien, Y.H. The pacific white shrimp, Litopenaeus vannamei, in Asia: The world’s most widely cultured alien crustacean. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 489–519. [Google Scholar] [CrossRef]
- CFS. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2023. [Google Scholar]
- Sookying, D.; Davis, D.; Soller Dias da Silva, F. A review of the development and application of soybean-based diets for pacific white shrimp Litopenaeus vannamei. Aquacult. Nutr. 2013, 19, 441–448. [Google Scholar] [CrossRef]
- Fang, H.; He, X.; Zeng, H.; Liu, Y.; Tian, L.; Niu, J. Replacement of astaxanthin with lutein in diets of juvenile Litopenaeus vannamei: Effects on growth performance, antioxidant capacity, and immune response. Front. Mar. Sci. 2021, 8, 803748. [Google Scholar] [CrossRef]
- Shen, Q.; Li, S.; Zhang, S.; Xu, J.; Chen, H.; Luo, Q.; Yang, R.; Chen, J. The impact of Neoporphyra haitanensis dietary supplement on astaxanthin esters and fatty acids accumulation associated with immune promotion in pacific white shrimp (Litopenaeus vannamei). Aquaculture 2024, 593, 741347. [Google Scholar] [CrossRef]
- Okpala, C.O.R. The physicochemical changes of farm-raised pacific white shrimp (Litopenaeus vannamei) as influenced by iced storage. Food Nutr. Sci. 2015, 6, 906. [Google Scholar] [CrossRef][Green Version]
- Niu, J.; Li, C.; Liu, Y.; Tian, L.; Chen, X.; Huang, Z.; Lin, H. Dietary values of astaxanthin and canthaxanthin in Penaeus monodon in the presence and absence of cholesterol supplementation: Effect on growth, nutrient digestibility and tissue carotenoid composition. Br. J. Nutr. 2012, 108, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wen, H.; Li, C.; Supamattaya, K.; Kiriratnikom, S.; Boonyaratpalin, M.; Borowitzka, L. Comparison effect of dietary astaxanthin and β-carotene in the presence and absence of cholesterol supplementation on growth performance, antioxidant capacity and gene expression of Penaeus monodon under normoxia and hypoxia condition. Aquaculture 2014, 422, 8–17. [Google Scholar] [CrossRef]
- Wang, H.; Dai, A.; Liu, F.; Guan, Y. Effects of dietary astaxanthin on the immune response, resistance to white spot syndrome virus and transcription of antioxidant enzyme genes in pacific white shrimp Litopenaeus vannamei. Iran. J. Fish. Sci. 2015, 14, 699–718. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Typ, B.; Ym, C. Oxidation and thermal degradation of carotenoids. J. Oil Palm Res. 1999, 11, 62–78. [Google Scholar]
- Niu, J.; Tian, L.; Liu, Y.; Yang, H.; Ye, C.; Gao, W.; Mai, K. Effect of dietary astaxanthin on growth, survival, and stress tolerance of postlarval shrimp, Litopenaeus vannamei. J. World Aquacult. Soc. 2009, 40, 795–802. [Google Scholar] [CrossRef]
- Ettefaghdoost, M.; Haghighi, H. Impact of different dietary lutein levels on growth performance, biochemical and immuno-physiological parameters of oriental river prawn (Macrobrachium nipponense). Fish Shellfish Immunol. 2021, 115, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Mimoun-Benarroch, M.; Hugot, C.; Rhazi, L.; Niamba, C.; Depeint, F. The bioavailability of astaxanthin is dependent on both the source and the isomeric variants of the molecule. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73, 61–69. [Google Scholar] [CrossRef]
- Goodwin, T. The carotenoids of the berries of Lonicera japonica. Biochem. J. 1952, 51, 458. [Google Scholar] [CrossRef]
- Schiedt, K.; Bischof, S.; Glinz, E. Metabolism of Carotenoids and In Vivo Racemization of (3S, 3′S)-astaxanthin in the Crustacean Penaeus. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1993; Volume 214, pp. 148–168. [Google Scholar] [CrossRef]
- Castillo, R.; Nègre-Sadargues, G.; Lenel, R. General survey of the carotenoids in Crustacea. In Carotenoid Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 1982; pp. 211–224. [Google Scholar] [CrossRef]
- Wade, N.M.; Cheers, S.; Bourne, N.; Irvin, S.; Blyth, D.; Glencross, B.D. Dietary astaxanthin levels affect colour, growth, carotenoid digestibility and the accumulation of specific carotenoid esters in the giant tiger shrimp, Penaeus monodon. Aquacult. Res. 2017, 48, 395–406. [Google Scholar] [CrossRef]
- Petit, H.; Nègre-Sadargues, G.; Castillo, R.; Trilles, J.-P. The Effects of Dietary Astaxanthin on Growth and Moulting Cycle of Postlarval Stages of the Prawn, Penaeus japonicus (Crustacea, Decapoda). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1997, 117, 539–544. [Google Scholar] [CrossRef]
- Latscha, T. The role of astaxanthin in shrimp pigmentation. In Proceedings of the Advances in Tropical Aquaculture, Workshop, Tahiti, French Polynesia, 20 February–4 March 1989. [Google Scholar]
- Liao, W.; Nur-E-Borhan, S.A.; Okada, S.; Matsui, T.; Yamaguchi, K. Pigmentation of cultured black tiger prawn by feeding with a Spirulina-supplemented diet. Nippon. Suisan Gakkaishi 1993, 59, 165–169. [Google Scholar] [CrossRef]
- Parisenti, J.; Beirão, L.H.; Tramonte, V.L.; Ourique, F.; da Silveira Brito, C.C.; Moreira, C.C. Preference ranking of colour in raw and cooked shrimps. Int. J. Food Sci. Technol. 2011, 46, 2558–2561. [Google Scholar] [CrossRef]
- Cianci, M.; Rizkallah, P.J.; Olczak, A.; Raftery, J.; Chayen, N.E.; Zagalsky, P.F.; Helliwell, J.R. The molecular basis of the coloration mechanism in lobster shell: β-crustacyanin at 3.2-Å resolution. Proc. Natl. Acad. Sci. USA 2002, 99, 9795–9800. [Google Scholar] [CrossRef]
- Babin, A.; Moreau, J.; Moret, Y. Storage of carotenoids in crustaceans as an adaptation to modulate immunopathology and optimize immunological and life-history strategies. BioEssays 2019, 41, 1800254. [Google Scholar] [CrossRef]
- Nickell, D.; Bromage, N. The effect of timing and duration of feeding astaxanthin on the development and variation of fillet colour and efficiency of pigmentation in rainbow trout (Oncorhynchus mykiss). Aquaculture 1998, 169, 233–246. [Google Scholar] [CrossRef]
- Wade, N.M.; Anderson, M.; Sellars, M.J.; Tume, R.K.; Preston, N.P.; Glencross, B.D. Mechanisms of colour adaptation in the prawn Penaeus monodon. J. Exp. Biol. 2012, 215, 343–350. [Google Scholar] [CrossRef]
- Wade, N.; Goulter, K.C.; Wilson, K.J.; Hall, M.R.; Degnan, B.M. Esterified astaxanthin levels in lobster epithelia correlate with shell colour intensity: Potential role in crustacean shell colour formation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 141, 307–313. [Google Scholar] [CrossRef]
- Wade, N.M.; Budd, A.; Irvin, S.; Glencross, B.D. The combined effects of diet, environment and genetics on pigmentation in the giant tiger prawn, Penaeus monodon. Aquaculture 2015, 449, 78–86. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, Q.; Yang, L.; Xue, Y.; Xu, J.; Xue, C. Effect of thermal processing on astaxanthin and astaxanthin esters in pacific white shrimp Litopenaeus vannamei. J. Oleo Sci. 2015, 64, 243–253. [Google Scholar] [CrossRef]
- Han, C.; Xiao, Y.; Guo, X.; Zhang, H.; Ren, J.; Yang, J. Authentication of pacific white shrimp (Litopenaeus vannamei) reared in freshwater and seawater areas using fatty acid profiles combined with chemometrics. Food Control 2025, 168, 110897. [Google Scholar] [CrossRef]
- Miao, F.; Geng, Y.; Lu, D.; Zuo, J.; Li, Y. Stability and changes in astaxanthin ester composition from Haematococcus pluvialis during storage. Chin. J. Oceanol. Limnol. 2013, 31, 1181–1189. [Google Scholar] [CrossRef]


| Ingredients (%) | Control | SJ | SF | NH | ZX | FX |
|---|---|---|---|---|---|---|
| Peruvian fish meal | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 |
| Domestic fish meal | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Shrimp meal | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Yeast powder | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Soybean meal | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| Peanut meal | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Concentrated protein a | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Flour | 19.97 | 19.97 | 19.97 | 19.97 | 19.97 | 19.97 |
| Cholesterol | 0.5. | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Fish oil | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Soybean oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Phospholipids | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Dicalcium phosphate | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Antioxidants | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Vitamin premix b | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral mixture c | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Cellulose | 5.00 | 2.00 | 2.00 | 2.00 | 4.90 | 4.90 |
| Algae product meal | 0.00 | 3.00 | 3.00 | 3.00 | 0.10 | 0.10 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Index | Control | SJ | SF | NH | ZT | FX |
|---|---|---|---|---|---|---|
| Initial weight (g) | 2.70 ± 0.09 a | 2.51 ± 0.09 a | 2.49 ± 0.13 a | 2.71 ± 0.37 a | 2.45 ± 0.06 a | 2.48 ± 0.10 a |
| Final weight (g) | 11.22 ± 1.08 b | 13.08 ± 1.10 ab | 13.03 ± 1.59 ab | 14.22 ± 2.24 a | 15.41 ± 1.04 a | 15.11 ± 0.58 a |
| WG (g) | 8.52 ± 1.04 c | 10.57 ± 1.01 b | 10.54 ± 1.47 b | 11.51 ± 1.88 ab | 12.96 ± 0.98 a | 12.63 ± 0.48 ab |
| WGR (%) | 315.62 ± 28.86 c | 421.12 ± 8.19 b | 423.29 ± 14.13 b | 424.72 ± 12.93 b | 528.98 ± 19.17 a | 509.27 ± 10.34 a |
| SGR (%) | 2.37 ± 0.15 c | 2.75 ± 0.02 b | 2.75 ± 0.04 b | 2.76 ± 0.05 b | 3.06 ± 0.05 a | 3.01 ± 0.03 a |
| Survival rate (%) | 64.29 ± 4.29 a | 56.67 ± 4.71 ab | 68.33 ± 15.46 a | 46.67 ± 5.87 b | 61.67 ± 2.36 ab | 53.33 ± 12.47 ab |
| L* | 37.63 ± 0.46 a | 35.82 ± 0.66 b | 35.68 ± 0.24 b | 36.01 ± 0.53 b | 35.55 ± 0.44 b | 36.04 ± 0.41 b |
| a* | −0.42 ± 0.09 b | −1.14 ± 0.13 e | −1.13 ± 0.05 e | −0.53 ± 0.01 c | −0.29 ± 0.06 a | −0.69 ± 0.02 d |
| b* | 5.21 ± 0.26 a | 3.71 ± 0.36 c | 3.87 ± 0.03 c | 4.50 ± 0.12 b | 4.37 ± 0.26 b | 4.01 ± 0.15 c |
| W | 62.59 ± 0.44 b | 64.29 ± 0.67 a | 64.23 ± 0.55 a | 64.15 ± 0.52 a | 64.60 ± 0.43 a | 64.09 ± 0.41 a |
| Carotenoids | Algae Powder (mg/kg) | Feeds (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S. japonica | S. fusiforme | N. haitanensis | Control | SJ | SF | NH | ZX | FX | |
| α-carotene | ND | ND | 17.29 ± 0.62 a | ND | 0.04 ± 0.002 c | 0.13 ± 0.02 c | 0.75 ± 0.08 b | 0.12 ± 0.01 c | 0.13 ± 0.02 c |
| β-carotene | 3.70 ± 0.15 b | 5.23 ± 0.25 b | 72.62 ± 3.80 a | 0.31 ± 0.09 c | 0.42 ± 0.04 c | 0.34 ± 0.1 c | 3.75 ± 0.45 b | 0.32 ± 0.04 c | 0.40 ± 0.1 c |
| α-cryptoxanthin | ND | ND | 2.20 ± 0.32 a | ND | ND | ND | 0.76 ± 0.08 b | 0.22 ± 0.02 c | 0.24 ± 0.04 c |
| β-cryptoxanthin | ND | ND | 31.11 ± 1.23 a | ND | ND | ND | 1.02 ± 0.17 b | 0.26 ± 0.06 c | 0.23 ± 0.04 c |
| leutin | ND | ND | 82.16 ± 4.12 a | 8.62 ± 0.30 c | 7.29 ± 0.03 c | 8.76 ± 0.94 c | 12.02 ± 0.03 b | 6.64 ± 0.81 c | 3.40 ± 0.69 d |
| zeaxanthin | 11.27 ± 0.44 b | 5.62 ± 0.14 d | 76.28 ± 0.80 a | 0.88 ± 0.08 g | 0.53 ± 0.04 h | 1.99 ± 0.07 f | 2.82 ± 0.02 e | 9.41 ± 0.80 c | 1.28 ± 0.15 g |
| antheraxanthin | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| fucoxanthin | 3.58 ± 0.09 a | 2.40 ± 0.36 bc | ND | ND | 0.70 ± 0.08 d | 2.21 ± 0.47 c | ND | ND | 2.85 ± 0.18 b |
| Total | 18.55 ± 0.40 b | 13.20 ± 0.41 c | 281.66 ± 7.53 a | 9.81 ± 0.32 cd | 8.98 ± 0.03 d | 13.43 ± 0.93 c | 21.12 ± 0.45 b | 16.97 ± 0.14 b | 8.53 ± 0.75 d |
| Tissue | Carotenoids | Control | SJ | SF | NH | ZT | FX |
|---|---|---|---|---|---|---|---|
| (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | ||
| Exoskeleton | α-carotene | 0.81 ± 0.02 c | 1.48 ± 0.12 b | 1.74 ± 0.23 b | 7.74 ± 1.32 a | 0.86 ± 0.06 c | 0.81 ± 0.06 c |
| β-carotene | 0.92 ± 0.07 d | 1.68 ± 0.05 c | 2.74 ± 0.12 b | 11.35 ± 0.35 a | 0.94 ± 0.04 d | 0.81 ± 0.03 d | |
| Free astaxanthin | 3.58 ± 0.15 d | 4.16 ± 0.04 c | 4.94 ± 0.07 b | 8.12 ± 0.41 a | 4.91 ± 0.3 b | 3.30 ± 0.34 d | |
| Zeaxanthin | ND | ND | ND | ND | 2.76 ± 0.12 a | ND | |
| Fucoxanthin | ND | ND | ND | ND | ND | 1.35 ± 0.14 a | |
| Ad-C20:5/16:0 | 3.05 ± 0.05 b | 3.59 ± 0.12 b | 5.12 ± 0.22 a | 5.37 ± 0.54 a | 5.39 ± 0.53 a | 3.15 ± 0.05 b | |
| Ad-C22:6/16:0 | 6.85 ± 0.35 d | 6.67 ± 0.23 d | 12.35 ± 1.06 c | 24.35 ± 0.86 a | 16.19 ± 1.43 b | 6.85 ± 0.35 d | |
| Ad-C20:5/20:5 | 2.62 ± 0.59 d | 2.85 ± 0.09 d | 2.21 ± 0.13 d | 10.95 ± 0.25 b | 12.35 ± 0.5 a | 3.95 ± 0.99 c | |
| Ad-C22:6/18:1 | 3.72 ± 0.15 c | 2.95 ± 0.18 c | 5.42 ± 0.43 b | 13.85 ± 1.16 a | 13.72 ± 0.15 a | 3.05 ± 0.59 c | |
| Ad-C22:6/20:5 | 6.82 ± 0.07 de | 7.43 ± 0.31 d | 9.37 ± 0.19 c | 23.88 ± 0.59 a | 16.82 ± 0.07 b | 6.16 ± 1.46 e | |
| Ad-C22:6/22:6 | 4.72 ± 1.00 e | 4.25 ± 0.16 e | 9.85 ± 0.17 c | 18.46 ± 0.55 a | 14.72 ± 0.23 b | 5.72 ± 0.23 d | |
| Total content | 33.09 ± 1.00 d | 35.06 ± 0.51 d | 53.74 ± 0.67 c | 124.07 ± 3.94 a | 88.66 ± 1.96 b | 35.15 ± 1.67 d | |
| Hepatopancreas | Free astaxanthin | 0.28 ± 0.03 d | 0.42 ± 0.02 c | 0.25 ± 0.04 d | 0.98 ± 0.02 b | 1.08 ± 0.13 a | 0.32 ± 0.04 c |
| Zeaxanthin | ND | ND | ND | ND | 1.19 ± 0.21 a | ND | |
| Fucoxanthin | ND | ND | ND | ND | ND | 1.20 ± 0.10 a | |
| Ad-C22:6/16:0 | 0.14 ± 0.01 d | 1.18 ± 0.18 c | 1.69 ± 0.18 b | 3.55 ± 0.31 a | 3.46 ± 0.41 a | 0.44 ± 0.11 d | |
| Ad-C22:6/18:1 | 2.15 ± 0.38 c | 0.51 ± 0.07 d | 1.04 ± 0.13 d | 8.72 ± 0.83 a | 4.46 ± 0.74 b | 2.15 ± 0.11c | |
| Ad-C22:6/20:5 | 2.18 ± 0.12 c | 2.14 ± 0.15 c | 3.18 ± 0.21 b | 4.68 ± 0.73 a | 4.4 ± 0.62 a | 2.27 ± 0.55 c | |
| Ad-C22:6/22:6 | 1.14 ± 0.08 c | 1.93 ± 0.25 c | 4.32 ± 0.25 b | 10.28 ± 1.05 a | 3.23 ± 1.44 b | 1.10 ± 0.08 c | |
| Total content | 5.89 ± 0.41 e | 6.18 ± 0.41 d | 10.48 ± 1.08 c | 28.21 ± 1.95 a | 17.82 ± 0.07 b | 7.48 ± 0.6 d | |
| Muscle | Free astaxanthin | 0.08 ± 0.01 d | 0.21 ± 0.04 d | 0.42 ± 0.08 c | 0.58 ± 0.01 b | 0.78 ± 0.21 a | 0.11 ± 0.01 d |
| Am-C16:0 | 14.79 ± 0.29 bc | 17.22 ± 2.41 b | 19.25 ± 3.02 b | 14.79 ± 0.29 bc | 24.79 ± 0.29 a | 13.79 ± 1.87 c | |
| Am-C18:0 | 18.22 ± 2.19 b | 20.11 ± 2.15 b | 22.25 ± 3.33 ab | 18.22 ± 2.19 b | 26.89 ± 1.73 a | 18.56 ± 2.57 b | |
| Total content | 33.09 ± 2.07 c | 37.55 ± 3.34 bc | 41.92 ± 4.82 b | 33.59 ± 2.07 c | 52.46 ± 1.66 a | 32.46 ± 2.41 c | |
| Total content in three tissues | 72.07 ± 2.07 d | 78.79 ± 4.26 d | 106.14 ± 6.57 c | 185.87 ± 1.96 a | 158.94 ± 3.14 b | 73.09 ± 3.38 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Huang, C.; Shen, Q.; Luo, Q.; Yang, R.; Chen, H.; Wu, W.; Chen, J. Dietary Supplementation with Algae Powders and Carotenoids Enhances Growth Performance and Tissue-Specific Carotenoid Accumulation in Penaeus Vannamei. Animals 2025, 15, 1550. https://doi.org/10.3390/ani15111550
Liu P, Huang C, Shen Q, Luo Q, Yang R, Chen H, Wu W, Chen J. Dietary Supplementation with Algae Powders and Carotenoids Enhances Growth Performance and Tissue-Specific Carotenoid Accumulation in Penaeus Vannamei. Animals. 2025; 15(11):1550. https://doi.org/10.3390/ani15111550
Chicago/Turabian StyleLiu, Pujiang, Chengwei Huang, Qian Shen, Qijun Luo, Rui Yang, Haimin Chen, Wei Wu, and Juanjuan Chen. 2025. "Dietary Supplementation with Algae Powders and Carotenoids Enhances Growth Performance and Tissue-Specific Carotenoid Accumulation in Penaeus Vannamei" Animals 15, no. 11: 1550. https://doi.org/10.3390/ani15111550
APA StyleLiu, P., Huang, C., Shen, Q., Luo, Q., Yang, R., Chen, H., Wu, W., & Chen, J. (2025). Dietary Supplementation with Algae Powders and Carotenoids Enhances Growth Performance and Tissue-Specific Carotenoid Accumulation in Penaeus Vannamei. Animals, 15(11), 1550. https://doi.org/10.3390/ani15111550





