Effects of Grape By-Products on Oxidative Stress and Inflammation in Farm Animals: An Overview of Studies Performed in Pigs, Chickens, and Cattle
Simple Summary
Abstract
1. Introduction
2. Theoretical Background: Oxidative Stress, Inflammation, and Their Interlinkage, and Potential Effects of Polyphenols
2.1. Oxidative Stress: Role of Oxidants and Antioxidants
2.2. Inflammation: Regulation and Consequences
2.3. Link Between Oxidative Stress, Inflammation, and Stress of the Endoplasmic Reticulum
2.4. Effects of Polyphenols on Oxidative Stress and Inflammation
3. Polyphenols in Grapes and Grape By-Products
4. The Effects of Grape By-Products on Oxidative Stress and Inflammation in Farm Animals
4.1. Pigs
4.1.1. Studies in Weaned Pigs
4.1.2. Studies in Pigs with Body Weight Gains Greater than 30 kg and Growing-Finishing Pigs
| Species | Grape By-Product | Dose and Treatment Duration | Main Effects | Reference |
|---|---|---|---|---|
| Weaned pigs | Grape pomace | 5% in diet for 36 days | Duodenum: ↑ SOD activity Colon: ↑ CAT and GPx activities ↑ Total antioxidant status, ↓ TBARS | [158] |
| Weaned pigs | Ensiled grape pomace | Unspecified dose for 15 or 30 days | d1-d15: ↑ ADG, FCR d1-d30: ↑ GSH, TBARS, and protein carbonyls in different tissues | [160] |
| Weaned pigs | Grape seed meal | 8% for 30 days | Colon and lymph nodes: ↑ CAT, SOD, GPx gene expression and/or activity ↓ DNA oxidative damage and protein carbonylation | [161] |
| Weaned pigs | Grape seed and marc meal extract | 1% in diet for 28 days | Intestinal mucosa: ↓ NF-κB and Nrf2 transactivation, ↓ NF-κB and Nrf2 target gene expression, ↑ FCR | [157] |
| Weaned pigs | Grape seed and marc meal extract | 1% in diet for 28 days | ↓ Pro-inflammatory gene expression (TNF, IL8, IL1B, ICAM1) in the intestinal mucosa | [154] |
| Weaned pigs | Grape seed and marc meal extract | 1% in diet for 28 days | Liver and plasma: No effect on TEAC, α-tocopherol, TBARS Liver: No effect on expression of genes involved in inflammation, cytoprotection, and ER stress, and NF-kB-p50 protein level | [162] |
| Weaned pigs | Grape extract | 150 mg/kg diet for 27/28 or 55/56 days | No effects on GPx and SOD activity and TBARS in liver, jejunum, and ileum No effects on tissue repair or immune response-related gene expression in liver (HSP70, HSP90AA1, CYP8B1, MMP13, TNFRSF14, CCL4) No effect on SOD and MDA level and acute-phase proteins in plasma | [159] |
| Weaned pigs | Grape seed procyanidins | 50, 100, or 150 mg/kg diet for 28 days | ↓ MDA; ↑ GPx; ↑ SOD ↑ Microbiota diversity ↓ Diarrhea incidence ↑ Serum total antioxidant capacity | [155,156] |
| Weaned pigs | Grape seed-derived procyanidins | 100, 200, or 400 mg/kg diet for 56 days | ↓ IL-1β, IL-6, TNF-α levels in PBMCs after LPS challenge | [165] |
| Weaned pigs | Proanthocyanidins | 250 mg/kg for 28 days | ↑ GSH, SOD, GPx, and ↓ MDA in intestinal mucosa and serum ↑ ADG, FCR | [157] |
| Growing pigs | Grape seed anthocyanidins | 15, 30, 60, 120 mg/kg for 33 days | ↑ Plasma SOD, GPx, ↓ MDA ↑ ADG ↑ FCR (30–120 mg/kg groups) | [168] |
| Finishing pigs | Grape pomace | 1, 5, 10, or 15 g/kg diet for 90 days | ↓ Intestinal NF-κB p65 and pro-inflammatory target gene expression ↑ Intestinal Nrf2 expression ↑ ADG, ADFI | [170] |
| Finishing pigs | Grape seed cake | 5% in diet for 24 days | Liver: ↓ Expression of cytokines (IL-1ß, IL-8, IL-6, TNF-α, IFN-γ) and NF-κB and target genes ↓ CAT expression and activity No effect on SOD and GPx activities and total antioxidant capacity | [169] |
| Finishing pigs | Grape pomace | 5% in diet for 24 days | Liver: ↓ Expression of cytokines and NF-κB target genes; (IL-8, IL-6, IFN-γ, eNOS, and COX2) ↓ Protein concentrations of IL-8, TNF-α, and interferon (IFN)-γ; No effect on SOD, CAT, GPx, and total antioxidant capacity; ↓ TBARS | [163] |
| Finishing pigs | Dried grape pomace powder | 6% in diet for 75 days | ↑ Total antioxidant capacity, SOD ↓ MDA and ROS in pork | [173] |
| Finishing pigs | Fermented grape pomace | 30 g/kg for 105 days | ↓ TBARS in pork ↑ ADG during grower phase | [171] |
| Sows | Grape seed polyphenols | 200 or 300 mg/kg for 56 days | ↑ SOD, GPx in plasma; ↑ IgG and IgM in colostrum; ↑ Farrowing and pre-weaning piglet survivability | [176] |
4.1.3. Studies in Sows
4.2. Chickens
4.2.1. Studies in Broilers
4.2.2. Studies in Laying Hens
4.3. Cattle
| Species | Grape By-Product | Dose and Treatment Duration | Main Effects | Reference |
|---|---|---|---|---|
| Broiler chickens | Grape pomace | 15, 30, or 60 g/kg for 21 days | ↑ Antioxidant capacity of ileal content No effect on ADG, ADFI, and FCR | [191] |
| Broiler chickens | Grape seed powder | 1, 2, or 3% in diet for 42 days | 2% and 3% in diet: ↑ Plasma GPx; ↓ Plasma MDA; ↑ ADG | [178] |
| Broiler chickens | Grape seed extract | 100 mg/kg for 42 days | Serum and liver: ↑ GPx and total antioxidant capacity, ↓ MDA; Liver: ↑ Nrf2 target genes ↑ ADG, ADFI, FCR | [177] |
| Broiler chickens | Grape seed extract after E. tenella challenge | 12 mg/kg for 21 days | Plasma: ↑ SOD activity, No effect on MDA conc. ↑ ADG | [185] |
| Broiler chickens | Grape seed proanthocyanidins | 200 or 400 mg/kg for 21 days | Serum: ↑ SOD and GPx, ↓ MDA Serum, Ileum und Jejunum mucosa: ↓ IL-1β ↑ ADG, ADFI, FCR | [180] |
| Broiler chickens | Grape seed proanthocyanidins after aflatoxin B1 challenge | 250 mg/kg for 28 days | Liver and serum: ↑ SOD, GPx, CAT, GR, GST, and GSH level ↓ MDA Spleen:↓ Inflammatory cytokines | [181] |
| Broiler chickens | Grape seed proanthocyanidins after aflatoxin B1 challenge | 250 mg/kg or 500 mg/kg for 28 days | Spleen: ↓ Cytokine expression (TNF-α, IFN-γ, IL-1β, IL-6) Liver: ↑ Expression of Nrf2 and some target genes (HO-1, GPx1, NQO1, GCLC); ↑ ADG, ADFI, FCR | [182] |
| Laying hens | Grape pomace | 3, 6, and 9% in diet for 8 weeks | ↑ Feed efficiency; ↑ Egg mass; ↑ Egg weight 6% and 9% in diet: ↑ Feed intake ↑ Egg production; ↑ GPx and ↓ MDA in serum and egg yolk | [202] |
| Laying hens | Grape pomace | 5% in diet for 4 weeks | No significant changes in egg tocopherol or polyphenol content | [204] |
| Laying hens | Grape pomace | 5% in diet for 12 weeks | ↓ Feed intake ↓ Egg mass No effect on serum GPx, SOD, MDA, and total antioxidant capacity | [201] |
| Laying hens | Grape pomace | 4% or 6% in diet for 12 weeks | ↓ MDA in plasma and yolk of eggs stored for 15 days | [208] |
| Laying hens | Grape pomace/Grape extract | 30 or 60 g/kg/0.5 or 1 g/kg for 4 weeks | 60 g/kg grape pomace: ↓ TBARS in eggs stored 4 months | [209] |
| Laying hens | Grape pomace flour under heat stress | 1%, 2%, or 3% for 35 days | ↓ TBARS in egg yolk (all inclusion level) and serum (2% and 3%); ↑ Total antioxidant capacity in egg yolk (all inclusion level) and serum (2% and 3%) ↑ Serum levels of GPx (all inclusion level) and SOD (2%) ↑ Egg-laying performance (1% inclusion level) ↑ Feed intake | [203] |
| Laying hens | Grape marc flour | 1% or 3% in diet for 34 days | ↓ MDA in eggs stored 30 days at room temperature No effect on MDA conc. in eggs stored 30 days under refrigeration | [210] |
| Laying hens | Grape seed extract | 250, 500, or 750 mg/kg for 5 weeks | ↓ MDA in plasma | [206] |
| Laying hens | Grape seed (GS) Grape seed extract (GSE) | 0.5%, 1%, or 1.5%/675, 1350, or 2025 mg/kg for 12 weeks | GS 1%, GSE 1350 mg and 2025 mg/kg: ↓ MDA in eggs stored 14 days; GS 1.5%, GSE 2025 mg/kg: ↑ MDA in eggs stored 7 days | [203] |
4.3.1. Studies in Dairy Cows
4.3.2. Studies in Calves and Beef Cattle
| Species | Grape By-Product | Dose and Treatment Duration | Main Effects | Reference |
|---|---|---|---|---|
| Dairy cows | Grape pomace | 15% for 12 weeks | ↑ Plasma polyphenol conc. No effect on milk polyphenol conc. | [214] |
| Dairy cows | Grape seed and marc meal extract | 1% in total mixed ration for 12 weeks | Plasma: No effects on concentrations of various antioxidants, TBARS, and total antioxidant capacity Liver: ↓ FGF21 expression | [229] |
| Dairy cows | Grape seed and marc meal extract | 1% in total mixed ration for 4 weeks | ↓ Plasma acute-phase proteins (SAA, HP) ↓ Expression of hepatic genes related to inflammation and ER stress | [231] |
| Dairy cows | Grape seed extract (dissolved in drinking water, 500 mL per os) | 20, 40, 60, or 80 mg/kg BW/day for 50 days | No effects on GPx, SOD, total antioxidant capacity in serum, and MDA in plasma; no effects on ADFI; ↑ Milk yield (20 mg/kg/BW/day group) | [234] |
| Calves | Grape seed extract | 25, 50, and 100 mg/kg BW/day for 60 days | ↓ Plasma MDA and TNF-α; ↑ Plasma SOD | [235] |
| Calves | Grape seed extract | 4 g/day for 60 days | ↑ Plasma SOD, CAT, total antioxidant capacity, IgG and IgA; ↓ Plasma MDA, TNF-α, IL-6; ↑ ADG | [236] |
| Beef cattle | Grape pomace flour | 10% in diet for 75 days | ↓ MDA in meat after 7 d storage; ↑ IL-1 and NF-κB signaling | [237] |
| Beef cattle | Grape pomace bran (GPB) or grape pomace silage (GPS) | 10% in diet for 21 days | GPB: ↓ TBARS in serum, TBARS in liver, GST and ROS in liver; ↓ ADG; ↑ FCR; GPS: ↓ GST in serum, intestine, liver | [238] |
| Beef cattle | Dried grape pomace | 100 or 200 g per kg TMR for 129 days | 200 g group: ↓ ADG; ↑ FCR | [239] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APP | Acute-phase protein |
| APR | Acute-phase reaction |
| CAT | Catalase |
| COX2 | Cyclooxygenase 2 |
| ER | Endoplasmic reticulum |
| ERAD | ER-associated degradation |
| FGF21 | Fibroblast growth factor 21 |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSPE | Grape seed proanthocyanidin extract |
| GST | Glutathione S-transferase |
| HO-1 | Heme oxygenase-1 |
| Ig | Immunoglobulin |
| IL | Interleukin |
| LPS | Lipopollysaccharide |
| MDA | Malondialdehyde |
| NF-kB | Nuclear factor kappa B |
| Nrf2 | Nuclear factor-erythroid 2-related factor-2 |
| PBMC | Peripheral blood mononuclear cells |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid reactive substances |
| TGF | Transforming growth factor |
| TNF-α | Tumor necrosis factor α |
| UPR | Unfolded protein response |
| UPS | Ubiquitin–proteasome system |
References
- Martinez-Miro, S.; Tecles, F.; Ramon, M.; Escribano, D.; Hernandez, F.; Madrid, J.; Orengo, J.; Martinez-Subiela, S.; Manteca, X.; Ceron, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Kikusato, M.; Toyomizu, M. Mechanisms underlying the effects of heat stress on intestinal integrity, inflammation, and microbiota in chickens. J. Poult. Sci. 2023, 60, 2023021. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Colonna, M.A.; Losacco, C.; Puvaca, N. Biological health markers associated with oxidative stress in dairy cows during lactation period. Metabolites 2023, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Lavecchia, T.; Rea, G.; Antonacci, A.; Giardi, M.T. Healthy and adverse effects of plant-derived functional metabolites: The need of revealing their content and bioactivity in a complex food matrix. Crit. Rev. Food Sci. Nutr. 2013, 53, 198–213. [Google Scholar] [CrossRef]
- Mei, H.; Li, Y.; Wu, S.; He, J. Natural plant polyphenols contribute to the ecological and healthy swine production. J. Anim. Sci. Biotechnol. 2024, 15, 146. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Cao, X.; Zou, T.; You, J.; Guan, W. Plant-derived polyphenols in sow nutrition: An update. Anim. Nutr. 2022, 12, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Al Rharad, A.; El Aayadi, S.; Avril, C.; Souradjou, A.; Sow, F.; Camara, Y.; Hornick, J.L.; Boukrouh, S. Meta-Analysis of Dietary Tannins in Small Ruminant Diets: Effects on Growth Performance, Serum Metabolites, Antioxidant Status, Ruminal Fermentation, Meat Quality, and Fatty Acid Profile. Animals 2025, 15, 596. [Google Scholar] [CrossRef]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Hornick, J.-L.; Chentouf, M.; Cabaraux, J.-F. Effects of Sulla flexuosa Hay as Alternative Feed Resource on Goat’s Milk Production and Quality. Animals 2023, 13, 709. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Saracila, M.; Panaite, T.D.; Papuc, C.P.; Criste, R.D. Heat stress in broiler chickens and the effect of dietary polyphenols, with special reference to willow (Salix spp.) bark supplements—A review. Antioxidants 2021, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary polyphenol supplementation in food producing animals: Effects on the quality of derived products. Animals 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Han, Y.; Tian, X.; Sajid, M.; Mehmood, S.; Wang, H.; Li, H. Phenolic composition of grape pomace and its metabolism. Crit. Rev. Food. Sci. Nutr. 2024, 64, 4865–4881. [Google Scholar] [CrossRef] [PubMed]
- Prata, C.; Zalambani, C.; Rossi, F.; Rossello, S.; Cerchiara, T.; Cappadone, C.; Malucelli, E. Nutrients and nutraceuticals from Vitis vinifera L. pomace: Biological activities, valorization, and potential application. Nutrients 2025, 17, 563. [Google Scholar] [CrossRef]
- Karastergiou, A.; Gancel, A.-L.; Jourdes, M.; Teissedre, P.-L. Valorization of grape pomace: A review of phenolic composition, bioactivity, and therapeutic potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef]
- Costa, M.M.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M.; Prates, J.A. Grape by-products as feedstuff for pig and poultry. Animals 2022, 12, 2239. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidant and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Gabai, G. Oxidant/antioxidant balance in the animal nutrition: The role of protein oxidation. Front. Vet. Sci. 2015, 2, 48. [Google Scholar] [CrossRef]
- Evans, P.; Halliwell, B. Micronutrients: Oxidant/antioxidant status. Br. J. Nutr. 2001, 85 (Suppl. 2), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kurokawa, H.; Matsui, H. Mitochondrial reactive oxygen species and heme, non-heme iron metabolism. Arch. Biochem. Biophys. 2021, 700, 108695. [Google Scholar] [CrossRef]
- Ayer, A.; Fazakerley, D.J.; James, D.E.; Stocker, R. The role of mitochondrial reactive oxygen species in insulin resistance. Free Radic. Biol. Med. 2022, 179, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12951. [Google Scholar] [CrossRef]
- Manoharan, R.R.; Prasad, A.; Pospisil, P.; Kzhyshkowska, J. ROS signalling in innate immunity via oxidative protein modifications. Front. Immunol. 2024, 15, 1359600. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Rad. Res. 1996, 25, 57–74. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Role of free radicals and catalytic metal ions in human disease. Methods Enzymol. 1990, 186, 1–85. [Google Scholar]
- Gutteridge, J.M.C. Biological origin of free radicals, and mechanisms of antioxidant protection. Chem.-Biol. Interact. 1994, 91, 133–140. [Google Scholar] [CrossRef]
- Raederstorff, D.; Wyss, A.; Calder, P.C.; Weber, P.; Eggersdorfer, M. Vitamin E function and requirements in relation to PUFA. Br. J. Nutr. 2015, 114, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Javouhey-Donzel, A.; Guenot, L.; Maupoil, V.; Rochette, L.; Rocquelin, G. Rat vitamin E status and heart lipid peroxidation: Effect of dietary alpha-linolenic acid and marine n-3 fatty acids. Lipids 1993, 28, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Grünthal, G.; Kluge, H.; Hirche, F.; Spilke, J.; Brandsch, C. Concentrations of cholesterol oxidation products in raw, heat-processed and frozen-stored meat of broiler chickens fed diets differing in the type of fat and vitamin E concentrations. Br. J. Nutr. 2005, 93, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Kirchgessner, M. The effect of dietary vitamin E supply and a moderately oxidized oil activities of hepatic lipogenic enzymes in rats. Lipids 1998, 33, 277–283. [Google Scholar] [CrossRef]
- Liu, J.F.; Huang, C.J. Dietary oxidised frying oil enhances tissue α-toccopherol depletion and radioisotope tracer excretion in vitamin E-deficient rats. J. Nutr. 1996, 126, 2227–2235. [Google Scholar] [CrossRef]
- Keller, U.; Brandsch, C.; Eder, K. Supplementation of vitamins C and E increases the vitamin E status but does not prevent the formation of oxysterols in the liver of guinea pigs fed an oxidised fat. Eur. J. Nutr. 2004, 43, 353–359. [Google Scholar] [CrossRef]
- Banerjee, B.D.; Seth, V.; Ahmed, R.S. Pesticide-induced oxidative stress: Perspectives and trends. Rev. Environ. Health 2001, 16, 1–40. [Google Scholar] [CrossRef]
- Alpsoy, L.; Yalvac, M.E. Key roles of vitamins A, C, and E in aflatoxin B1-induced oxidative stress. Vitam. Horm. 2011, 86, 287–305. [Google Scholar]
- Yanagi, T. Molecular mechanism of phase I and phase II drug-metabolizing enzymes: Implications for etoxification. Int. Rev. Cytol. 2007, 260, 35–112. [Google Scholar]
- Omura, T. Forty years of cytochrome P450. Biochem. Biophys. Res. Commun. 1999, 266, 690–698. [Google Scholar] [CrossRef]
- Goeptar, A.R.; Scheerens, H.; Vermeulen, N.P.E. Oxygen and xenobiotic reductase activities of cytochrome P450. Crit. Rev. Toxicol. 1995, 25, 25–65. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cocheme, H.M. Redox metabolism: ROS as specific molecular regulators of cell signalling and function. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35 Pt 5, 1147–1150. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cha, Y.-N.; Surh, Y.-J. A protective role of nuclear factor-erythroid-2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 2010, 690, 12–23. [Google Scholar] [CrossRef]
- Lu, M.C.; Ji, J.A.; Jiang, Z.Y.; You, Q.D. The Keap-1-Nrf2-ARE pathway as a potential preventive and therapeutic target: An update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef]
- Salman, S.; Paulet, V.; Hardonniere, K.; Kerdine-Römer, S. The role of NRF2 transcription factor in inflammatory skin diseases. BioFactors 2025, 51, e70013. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive ocygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Sidker, M.M.; Li, X.; Akumwami, S.; Labony, S.A. Reactive oxygene species: Role in pathophysiology, and mechanism of endogenous and dietary antioxidants during oxidative stress. Chonnam. Med. J. 2025, 61, 32–45. [Google Scholar]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Louvieaux, J.; Hornick, J.-L.; Chentouf, M.; Cabaraux, J.-F. Ecological, Morpho-Agronomical, and Nutritional Characteristics of Sulla flexuosa (L.) Medik. Ecotypes. Sci. Rep. 2023, 13, 13300. [Google Scholar] [CrossRef]
- Tapia, P.C. Subletal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “Mitohormesis” for health and vitality. Med. Hypotheses 2006, 66, 832–843. [Google Scholar] [PubMed]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Min, S.H.; Kang, G.M.; Park, J.W.; Kim, M.S. Beneficial effects of low-grade mitochondrial stress on metabolic diseases and aging. Yonsei Med. J. 2024, 65, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010, 45, 410–418. [Google Scholar] [CrossRef]
- Capozzi, A.; Saucier, C.; Bisbal, C.; Lambert, K. Grape polyphenols in the treatment of human skeletal muscle damage due to inflammation and oxidative stress during obesity and aging: Early outcomes and promises. Molecules 2022, 27, 6594. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Michalak, K.P.; Michalak, A.Z. Understanding chronic inflammation: Couplings between cytokines, ROS, NO, Cai2+, HIF-1α, Nrf2 and autophagy. Front. Immunol. 2025, 16, 1558263. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Schrödl, W.; Büchler, R.; Wendler, S.; Reinhold, P.; Muckova, P.; Reindl, J.; Rhode, H. Acute phase proteins as promising biomarkers: Perspectives and limitations for human and veterinary medicine. Proteom. Clin. Appl. 2016, 10, 1077–1092. [Google Scholar] [CrossRef]
- Venteclef, N.; Jakobsson, T.; Steffensen, K.R.; Treuter, E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends Endocrinol. Metabol. 2011, 22, 333–343. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef] [PubMed]
- Saco, Y.; Bassols, A. Acute phase proteins in cattle and swine: A review. Vet. Clin. Pathol. 2023, 52 (Suppl. 1), 50–63. [Google Scholar] [CrossRef]
- Jacobsen, N.; Weber, N.R.; Larsen, I.; Pedersen, K.S. Diagnostic utility of acute phase proteins and their ability to guide antibiotic usage in pigs, horses, and cattle: A mapping review. Acta Vet. Scand. 2024, 66, 45. [Google Scholar] [CrossRef]
- Petersen, H.H.; Nielsen, J.P.; Heegard, P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004, 35, 163–187. [Google Scholar] [CrossRef]
- Polepalle, T.; Moogala, S.; Boggarapu, S.; Pesala, D.S.; Palagi, F.B. Acute phase proteins and their role in periodontitis: A review. J. Clin. Diagn. Res. 2015, 9, ZE01–ZE05. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E. Use of liver activity index and other metabolic variables in the assessment of metabolic health in dairy herds. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 413–431. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin. Cell Dev. Biol. 2015, 54, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Dwarkasing, J.T.; Marks, D.L.; Witkamp, R.F.; van Norren, K. Hypothalamic inflammation and food intake regulation during chronic illness. Peptides 2016, 77, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Karrow, N.A. Activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine-immune axis during fetal and neonatal development: Lessons learned from the model inflammagen, lipopolysaccharide. Brain Behav. Immun. 2006, 20, 144–158. [Google Scholar]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcop. Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef]
- Klasing, K.C.; Johnstone, B.J. Monokines in growth and development. Poult. Sci. 1991, 70, 1781–1789. [Google Scholar] [CrossRef]
- Johnson, R.W. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 1997, 75, 1244–1255. [Google Scholar] [CrossRef]
- Andus, T.; Bauer, J.; Gerok, W. Effects of cytokines on the liver. Hepatology 1991, 13, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Choi, S.J.; Schwartz, M.W.; Wisse, B.E. Hypothalamic inflammation and energy homeostasis: Resolving the paradox. Front. Neuroendocrinol. 2010, 31, 79–84. [Google Scholar] [CrossRef]
- Langhans, W. Anorexia of infection: Current prospects. Nutrition 2000, 16, 996–1005. [Google Scholar] [CrossRef]
- Plata-Salaman, C.R. Anorexia during acute and chronic disease. Nutrition 1996, 12, 69–78. [Google Scholar] [CrossRef]
- Exton, M.S. Infection-induced anorexia: Active host defence strategy. Appetite 1997, 29, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Beisel, W.R. Metabolic effects of infection. Prog. Food Nutr. 1984, 8, 43–75. [Google Scholar]
- Ganz, T. Iron and infection. Int. J. Hematol. 2018, 107, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Parrow, N.L.; Fleming, R.E.; Minnick, M.F. Sequestration and scavenging of iron in infection. Infect. Immunol. 2013, 81, 3503–3514. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodriguez-Quinones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A.M. Hepcidin and the iron-infection axis. Science 2012, 338, 768–772. [Google Scholar] [CrossRef]
- Murray, M.J.; Murray, B.H. Anorexia of infection as a host defense. Am. J. Clin. Nutr. 1979, 32, 593–596. [Google Scholar] [CrossRef]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Ron, D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 2006, 86, 1133–1149. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Kaufman, R.J. A trip to the ER: Coping with stress. Trends Cell Biol. 2004, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, D.G.; Germain, M.; Mathai, J.P.; Nguyen, M.; Shore, G.C. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 2003, 22, 8608–8618. [Google Scholar] [CrossRef] [PubMed]
- Obaseki, I.; Ndolo, C.C.; Adedeji, A.A.; Popoola, H.O.; Kravats, A.N. The structural and functional dynamics of BiP and Grp94: Opportunities for therapeutic discovery. Trends Pharmacol. Sci. 2025, 46, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Integrated stress response stimulates FGF21 expression: Systemic enhancer of longevity. Cell. Signal. 2017, 40, 10–21. [Google Scholar] [CrossRef]
- Shimizu, M.; Sato, R. Endocrine fibroblast growth factors in relation to stress signalling. Cells 2022, 11, 505. [Google Scholar] [CrossRef]
- Carriquiry, M.; Weber, W.J.; Fahrenkrug, S.C.; Crooker, B.A. Hepatic gene expression in multiparous Holstein cows treated with bovine somatotropin and fed n-3 fatty acids in early lactation. J. Dairy Sci. 2009, 92, 4889–4900. [Google Scholar] [CrossRef]
- Schoenberg, K.M.; Giesy, S.L.; Harvatine, K.J.; Waldron, M.R.; Cheng, C.; Kharitonenkov, A.; Boisclair, Y.R. Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology 2011, 152, 4652–4661. [Google Scholar] [CrossRef]
- Schlegel, G.; Ringseis, R.; Keller, J.; Schwarz, F.J.; Windisch, W.; Eder, K. Expression of fibroblast growth factor 21 in the liver of dairy cows in the transition period and during lactation. J. Anim. Physiol. Anim. Nutr. 2013, 97, 820–829. [Google Scholar] [CrossRef]
- Drong, C.; Bühler, S.; Frahm, J.; Hüther, L.; Meyer, U.; von Soosten, D.; Gessner, D.K.; Eder, K.; Sauerwein, H.; Dänicke, S. Effects of body condition, monensin, and essential oils on ruminal lipopolysaccharide concentration, inflammatory markers, and endoplasmatic reticulum stress of transition dairy cows. J. Dairy Sci. 2017, 100, 2751–2764. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; She, G.; Kong, Y.; Guo, Y.; Wang, Z.; Liu, G.; Zhao, B. Serum hepatokines in dairy cows: Periparturient variation and changes in energy-related metabolic disorders. BMC Vet. Res. 2018, 14, 236. [Google Scholar] [CrossRef]
- Rosenbaum, S.; Ringseis, R.; Most, E.; Hillen, S.; Becker, S.; Erhardt, G.; Reiner, G.; Eder, K. Genes involved in carnitine synthesis and carnitine uptake are up-regulated in the liver of sows during lactation. Acta Vet. Scand. 2013, 55, 24. [Google Scholar] [CrossRef]
- Eder, K.; Gessner, D.K.; Ringseis, R. Fibroblast growth factor 21 in dairy cows: Current knowledge and potential relevance. J. Anim. Sci. Biotechnol. 2021, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.K.; Jun, W.; Lee, J. Mechanism of ER stress and inflammation for hepatic insulin resistance in obesity. Ann. Nutr. Metab. 2015, 67, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Lebeaupin, C.; Wu, N.N.; Kaufman, R.J.; Ren, J. ER stress and inflammation cross talk in obesity. J. Med. Res. Rev. 2023, 43, 5–30. [Google Scholar] [CrossRef]
- Drong, C.; Meyer, U.; von Soosten, D.; Frahm, J.; Rehage, J.; Breves, G.; Dänicke, S. Effect of monensin and essential oils on performance and energy metabolism of transition dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 537–551. [Google Scholar] [CrossRef]
- Schuh, K.; Sadri, H.; Häussler, S.; Webb, L.A.; Urh, C.; Wagner, M.; Koch, C.; Frahm, J.; Dänicke, S.; Dusel, G.; et al. Comparison of performance and metabolism from late pregnancy to early lactation in dairy cows with elevated v. normal body condition at dry-off. Animal 2019, 13, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Casaro, S.; Perez-Baez, J.; Bisinotto, R.S.; Chebel, R.C.; Prim, J.G.; Gonzalez, T.D.; Carvalho Gomes, G.; Tao, S.; Toledo, I.M.; do Amaral, B.C.; et al. Association between prepartum body condition score and prepartum and postpartum dry matter intake and energy balance in multiparous Holstein cows. J. Dairy Sci. 2024, 107, 4381–4393. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Alharti, A.; Zhou, Z.; Lopreiato, V.; Trevisi, E.; Loor, J.J. Body condition score prior to parturition is associated with plasma and adipose tissue biomarkers of lipid metabolism and inflammation in Holstein cows. J. Anim. Sci. Biotechnol. 2018, 9, 12. [Google Scholar] [CrossRef]
- Celik, C.; Lee, S.Y.T.; Yap, W.S.; Tibault, G. Endoplasmic reticulum stress and lipids in health and disease. Prog. Lipid Res. 2023, 89, 101198. [Google Scholar] [CrossRef]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Alotaibi, G.; Alkhammash, A. Pharmacological landscape of endoplasmc reticulum stress: Uncovering therapeutic avenues for metabolic diseases. Eur. J. Pharmacol. 2025, 998, 177509. [Google Scholar] [CrossRef]
- Ringseis, R.; Gessner, D.K.; Eder, K. Molecular insights into the mechanisms of liver-associated diseases in early-lactating dairy cows: Hypothetical role of endoplasmic reticulum stress. J. Anim. Physiol. Anim. Nutr. 2015, 99, 626–645. [Google Scholar] [CrossRef]
- Gessner, D.K.; Schlegel, G.; Ringseis, R.; Schwarz, F.J.; Eder, K. Up-regulation of endoplasmic reticulum stress induced genes of the unfolded protein response in the liver of periparturient dairy cows. BMC Vet. Res. 2014, 10, 46. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Garcia-Villanova, B.; Guerra-Hernandez, E.; Verardo, V. Grape seed proanthocyanidins: An overview of in vivo bioactivity in animal models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Pop, R.M.; Boarescu, P.M.; Bocsan, C.I.; Gherman, M.L.; Chedea, V.S.; Jianu, E.M.; Rosian, S.H.; Boarescu, I.; Ranga, F.; Tomoiaga, L.L.; et al. Anti-Inflammatory and Antioxidant Effects of White Grape Pomace Polyphenols on Isoproterenol-Induced Myocardial Infarction. Int. J. Mol. Sci. 2025, 26, 2035. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Magureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, S.O.; Patrasca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and anti-inflammatory actions of polyphenols from red and white grape pomace in ischemic heart disease. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Taladrid, D.; Rebollo-Hernanz, M.; Martin-Cabrejas, M.A.; Moreno-Arribas, M.V.; Bartolome, B. Grape pomace as a cardiometabolic health-promoting ingredient: Activity in the intestinal environment. Antioxidants 2023, 12, 979. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta 2012, 1822, 753–783. [Google Scholar] [CrossRef]
- Murakami, A. Dose-dependent functionality and toxicity of green tea polyphenols in exterimental rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and their role in oxidative stress, inflammation and human diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S.; Lin, Z.; Chen, X.; Jiao, Q.; Du, X.; Jiang, H. Targeting the Interplay between Autophagy and the Nrf2 Pathway in Parkinson’s Disease with Potential Therapeutic Implications. Biomolecules 2025, 19, 149. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- Kim, H.S.; Montana, V.; Jang, H.J.; Parpura, V.; Kim, J.A. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: A potential role for reducing lipid accumulation. J. Biol. Chem. 2013, 288, 22693–22705. [Google Scholar] [CrossRef]
- Pandey, P.; Lakhanpal, S.; Mahmood, D.; Kang, H.N.; Kim, B.; Kang, S.; Choi, J.; Choi, M.; Pandey, S.; Bhat, M.; et al. An updated review summarizing the anticancer potential of flavonoids via targeting NF-KB pathway. Front. Pharmacol. 2025, 15, 1513422. [Google Scholar] [CrossRef]
- Terra, X.; Pallares, V.; Ardevol, A.; Blade, C.; Fernandez-Larrea, J.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.; Liu, X.; Lan, Z. Grape seed proanthocyanidin extract supplementation affects exhaustive exercise-induced fatigue in mice. Food Nutr. Res. 2018, 62, 1421. [Google Scholar]
- Crescenzi, E.; Leonardi, A.; Pacifico, F. NF-KB in thyroid cancer: An update. Int. J. Mol. Sci. 2024, 25, 11464. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Shi, C.; Li, X.; Jiang, Y.; Mao, R. NF-KB: A mediator that promotes or inhibits angiogenesis in human diseases? Expert Rev. Mol. Med. 2023, 25, e25. [Google Scholar] [CrossRef]
- Keleku-Lukwete, N.; Suzuki, M.; Yamamoto, M. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Antioxid. Redox Signal. 2018, 29, 1746–1755. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overviewof Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ni, P.; Song, Y.; Gao, M.J.; Guo, X.Y.; Zhao, B.Q. Effective protective mechanisms of HO-1 in diabetic complications: A narrative review. Cell Death Discov. 2024, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; McIntosh, M.K. Potential mechanisms by which polyphenol-rich grapes prevent obesity mediated inflammation and metabolic diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). “FAOSTAT”. Available online: https://www.fao.org/faostat/en/#home (accessed on 25 April 2025).
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscl. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Zhou, F.; Deng, S.; Luo, Y.; Liu, Z.; Liu, C. Research progress on the protective effect of green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) on the liver. Nutrients 2025, 17, 1101. [Google Scholar] [CrossRef]
- Uifalean, A.; Schneider, S.; Ionescu, C.; Lalk, M.; Iuga, C.A. Soy isoflavones and breast cancer cell lines: Molecular mechanisms and future perspectives. Molecules 2015, 21, E13. [Google Scholar] [CrossRef]
- Chedea, V.S.; Macovei, S.O.; Bocsan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape Pomace Polyphenols as a Source of Compounds for Management of Oxidative Stress and Inflammation-A Possible Alternative for Non-Steroidal Anti-Inflammatory Drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef]
- Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape pomace valorization by extraction of phenolic polymeric pigments: A review. Processes 2022, 10, 469. [Google Scholar] [CrossRef]
- Winkler, A.; Weber, F.; Ringseis, R.; Eder, K.; Dusel, G. Determination of polyphenol and crude nutrient content and nutrient digestibility of dried and ensiled white and red grape pomace cultivars. Arch. Anim. Nutr. 2015, 69, 187–200. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour. Technol. 2007, 98, 2963–2970. [Google Scholar] [CrossRef]
- Ky, I.; Teissedre, P.L. Characterisation of Mediterranean grape pomace seed and skin extracts: Polyphenolic content and antioxidant activity. Molecules 2015, 20, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Centeno, M.R.; Jourdes, M.; Femenia, A.; Siml, S.; Rossello, C.; Teissedre, P.-L. Characterization of polyphenols and antioxidant properties of white grape pomace byproducts (Vitis vinifera L.). J. Agric. Food Chem. 2013, 61, 11579–11587. [Google Scholar] [CrossRef] [PubMed]
- De la Cerda-Carrasco, A.; Lopez-Solis, R.; Nunez-Kalasic, H.; Pena-Neira, A.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef]
- De Bellis, P.; Maggiolino, A.; Albana, C.; De Palo, P.; Blando, F. Ensiling grape pomace with and without addition of Lactiplantibacillus plantarum strain: Effect on polyphenols and microbiological characteristics, in vitro nutrient apparent digestibility, and gas emission. Front. Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varities (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2024, 52, 4360–4367. [Google Scholar] [CrossRef]
- Tapia-Quiros, P.; Montenegro-Landivar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of agri-food by products: The olive oil and winery industries cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Guaita, M.; Bosso, A. Polyphenolic characterization of grape skins and seeds of four Italian red cultivars at harvest and fermentative maceration. Foods 2019, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Zhao, C.N.; Liu, Q.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, H.B. Potential of grape wastes as a natural source of bioactive compounds. Molecules 2018, 23, 2598. [Google Scholar] [CrossRef]
- Pie, S.; Lalles, J.P.; Blazy, F.; Laffitte, J.; Seve, B.; Oswald, I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar]
- Kiernan, D.P.; O’Doherty, J.V.; Sweeney, T. The effect of prebiotic supplements on the gastrointestinal microbiota and associated health parameters in pigs. Animals 2023, 13, 3012. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18. [Google Scholar] [CrossRef]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, L.; Yan, H.; Li, Q.; Gao, J.; Hao, R. Grape seed procyanidins improve intestinal health by modulating gut microbiota and enhancing intestinal antioxidant capacity in weaned piglets. Livest. Sci. 2022, 264, 105066. [Google Scholar] [CrossRef]
- Hao, R.R.; Li, Q.H.; Zhao, J.Q.; Li, H.F.; Wang, W.W.; Gao, J.J. Effects of rape seed procyanidins on growth performance, immune function and antioxidative capacity in weaned piglets. Livest. Sci. 2015, 178, 237–242. [Google Scholar] [CrossRef]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.; Ma, X. Dietary grape seed procyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 2016, 7, 80313–80326. [Google Scholar] [CrossRef]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef]
- Rajkovic, E.; Schwarz, C.; Kapsamer, S.B.; Schedle, K.; Reisinger, N.; Emsenhuber, C.; Ocelova, V.; Roth, N.; Frieten, D.; Dusel, G.; et al. Evaluation of a dietary grape extract on oxidative status, intestinal morphology, plasma acute-phase proteins and inflammation parameters of weaning piglets at various points of time. Antioxidants 2022, 11, 1428. [Google Scholar] [CrossRef]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Bulgaru, V.C.; Anghel, A.C.; Saracila, M.; Vlassa, M.; Filip, M.; Taranu, I. Grape seed meal by-product is able to counteract oxidative stress induced by lipopolysaccharide and dextran sulphate in IPEC cells and piglets after weaning. PLoS ONE 2023, 18, e0283607. [Google Scholar] [CrossRef]
- Gessner, D.K.; Bonarius, M.; Most, E.; Fiesel, A.; Eder, K. Effects of polyphenol-rich plant products from grape or hop as feed supplements on the expression of inflammatory, antioxidative, cytoprotective and endoplasmic reticulum stress-related genes and the antioxidative status in the liver of piglets. J. Anim. Physiol. Anim. Nutr. 2017, 101, e185–e194. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Habeanu, M.; Gras, M.A.; Pistol, G.C.; Lefter, N.; Palade, M.; Ropota, M.; Chedea, V.S.; Marin, D.E. Assessment of the effect of grape seed cake inclusion in the diet of healthy fattening-finishing pigs. J. Physiol. Anim. Nutr. 2018, 102, e30–e42. [Google Scholar] [CrossRef]
- Pistol, G.C.; Bulgaro, V.C.; Grosu, I.A.; Ciupitu, A.-M.; Taranu, I. The effect of a diet containing grape seed meal on inflammatory and antioxidant markers in spleen of weaned piglets. Arch. Zootechn. 2024, 27, 75–89. [Google Scholar] [CrossRef]
- Park, J.C.; Lee, S.H.; Hong, J.K.; Cho, J.H.; Kim, I.H.; Park, S.K. Effect of dietary supplementation of procyanidin on growth performance and immune response in pigs. Asian Aust. J. Anim. Sci. 2014, 27, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, H.; Fang, H.; Jin, Y.; Zhao, Y.; Shen, J.; Zhou, C.; Li, R.; Wang, J.; Fu, Y.; et al. Effects of dietary grape pomace on the intestinal microbiota and growth performance of weaned piglets. Arch. Anim. Nutr. 2020, 74, 296–308. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, N.; Song, P.; He, T.; Levesque, C.; Bai, Y.; Zhang, A.; Ma, X. Grape seed proanthocyanidin affects lipid metabolism via changing gut microflora and enhancing propionate production in weaned pigs. J. Nutr. 2019, 149, 1523–1532. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Tan, H.; Luo, J.; et al. Grape seed proanthocyanidins improves growth performance, antioxidative capacity, and intestinal microbiota in growing pigs. Front. Microbiol. 2024, 15, 1501211. [Google Scholar] [CrossRef]
- Taranu, I.; Habeanu, M.; Gras, M.A.; Pistol, G.C.; Lefter, N.; Palade, M.; Ropota, M.; Chedea, V.S.; Marin, D.E. Effect of xenobiotic compounds from grape waste on liver function and oxidative status in pigs. Arch. Zootech. 2017, 20, 5–24. [Google Scholar]
- Horodincu, L.; Proca, A.C.; Slencu, B.G.; Trifan, A.; Pavel, G.; Solcan, G.; Solcan, C. Modulating effects of grape pomace on the intestinal antioxidative and inflammatory status in fattening pigs. Agriculture 2025, 15, 740. [Google Scholar] [CrossRef]
- Yan, L.; Kim, I.H. Effect of dietary grape pomace fermented by Saccharomyces boulardii on the growth performance, nutrient digestibility and meat quality in finishing pigs. Asian Austr. J. Anim. Sci. 2011, 24, 1763–1770. [Google Scholar] [CrossRef]
- Trombetta, F.; Fruet, A.P.B.; Stefanello, F.S.; Fonseca, P.A.F.; De Souza, A.N.M.; Tonetto, C.J.; Rosado Junior, A.G.; Nörnberg, J.L. Effects of the dietary inclusion of linseed oil and grape pomace on weight gain, carcass characteristics, and met quality of swine. Int. Food Res. J. 2019, 26, 1741–1749. [Google Scholar]
- Tian, X.; Li, D.; Zhao, X.; Xiao, Z.; Sun, J.; Yuan, T.; Wang, Y.; Zuo, X.; Yang, G.; Yu, T. Dietary grape pomace extract supplementation improved meat quality, antioxidant capacity, and immune performance in finishing pigs. Front. Microbiol. 2023, 14, 1116022. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Bak, K.H.; Goran, G.V.; Tatiyaborntham, N. Inhibitory mechanisms of polyphenols on heme protein-mediated lipid oxidation in muscle food: New insights and advances. Crit. Rev. Food Sci. Nutr. 2024, 64, 4921–4939. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Han, M.; Bu, Y.; Li, X.; Yi, S.; Xu, Y.; Li, J. Plant polyphenols regulating myoglobin oxidation and color stability in red meat and certain fish: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2276–2288. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, G.; Kebreab, E.; Yu, Q.; Li, J.; Zhang, X.; He, H.; Fang, R.; Dai, Q. Effect of dietary grapeseed polyphenols supplementation during late gestation and lactation on antioxidant status in serum and immunoglobulin content in colostrum of multiparous sows. J. Anim. Sci. 2019, 97, 2515–2523. [Google Scholar] [CrossRef]
- Wang, M.; He, Z.; Xiong, Z.; Liu, H.; Zhou, X.; He, J. Effects of dietary supplementation of grape seed extract in comparison with excessive level of vitamin E on growth performance and antioxidant function of broilers. Anim. Biotechnol. 2024, 35, 2331640. [Google Scholar] [CrossRef]
- Noor, S.; Al-Mashhdani, E.; Kadhim, H. Effects of grape seed powder on productive performance, lipid profile and total bacteria in duodenum and ceca of broiler chickens. Arch. Razi Inst. 2022, 77, 2159–2164. [Google Scholar]
- Gungor, E.; Altop, A.; Erener, G. Effect of raw and fermented grape seed on growth performance, antioxidant capacity, and cecal microflora in broiler chickens. Animal 2021, 15, 100194. [Google Scholar] [CrossRef]
- Cao, G.; Zeng, X.; Liu, J.; Xiang, Z.; Wang, Y.; Tao, F.; Yang, C. Change of serum metabolome and cecal microflora in broiler chickens supplemented with grape seed extract. Front. Immunol. 2020, 11, 610934. [Google Scholar] [CrossRef]
- Rajput, S.A.; Sun, L.; Zhang, N.Y.; Khalil, M.M.; Ling, Z.; Chong, L.; Wang, S.; Rajput, I.R.; Bloch, D.M.; Khan, F.A.; et al. Grape seed proanthocyanidin extract alleviates aflatoxin B1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins 2019, 11, 23. [Google Scholar] [CrossRef]
- Rajput, S.A.; Sun, L.; Zhang, N.; Khalil, M.M.; Gao, X.; Ling, Z.; Zhu, L.; Khan, F.A.; Zhang, J.; Qi, D. Ameliorative effects of grapeseed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histpathology and aflatoxin residues in broiler exposed to aflatoxin B1. Toxins 2017, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Abu Hafsa, S.H.; Ibrahim, S.A. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J. Anim. Physiol. Anim. Nutr. 2018, 102, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Farahat, M.H.; Abdallah, F.M.; Ali, H.A.; Hernandez-Santana, A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal 2017, 11, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Suo, X.; Gu, J.H.; Zhang, W.W.; Fang, Q.; Wang, X. Influence of grape seed proanthocyanidin extract in broiler chickens: Effect on chicken coccidiosis and antioxidant status. Poult. Sci. 2008, 87, 2273–2280. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhang, H.J.; Wang, J.; Wu, S.G.; Yue, H.Y.; Jiang, X.R.; Qi, G.H. Effects of dietary grape proanthocyanidins on the growth performance, jejunum morphology and plasma biochemical indices of broiler chicks. Animal 2017, 11, 762–770. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Giamouri, E.; Myrtsi, E.D.; Evergetis, E.; Filippi, K.; Papapostolou, H.; Koulocheri, S.D.; Zoidis, E.; Pappas, A.C.; Koutinas, A.; et al. Antioxidant status of broiler chickens fed diets supplemented with vinification by-products: A valorization approach. Antioxidants 2021, 10, 1250. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Simitzis, P.E.; Kyriakaki, P.; Giamouri, E.; Myrtsi, E.D.; Evergetis, E.; Filippi, K.; Papapostolou, H.; Koulocheri, S.D.; Pappas, A.C.; et al. Immune-related gene expression profiling of broiler chickens fed diets supplemented with vinification byproducts: A valorization approach II. Animals 2021, 11, 3038. [Google Scholar] [CrossRef]
- Duangnumsawang, Y.; Zentek, J.; Vahjen, W.; Tarradas, J.; Boroojeni, F.G. Alterations in bacterial metabolites, cytokines, and mucosal integrity in the caecum of broilers caused by feed additives and host-related factors. Front. Physiol. 2022, 13, 935870. [Google Scholar] [CrossRef]
- Duangnumsawang, Y.; Zentek, J.; Vahjen, W.; Tarradas, J.; Boroojeni, F.G. Impact of feed additives and host-related factors on bacterial metabolites, mucosal integrity and immune response in the ileum of broilers. Vet. Res. Commun. 2023, 47, 1861–1878. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goni, I.; Centeno, C.; Sayago-Ayerdy, S.G.; Arija, I.; Saura-Calixto, F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult. Sci. 2008, 87, 307–316. [Google Scholar] [CrossRef]
- Makri, S.; Kafantaris, I.; Stagos, D.; Chamokeridou, T.; Petrotos, K.; Gerasopoulos, K.; Mpesios, A.; Goutzourelas, N.; Kokkas, S.; Goulas, P.; et al. Novel feed including bioactive compounds from winery wastes improved broilers’ redox status in blood and tissues of vital organs. Food Chem. Toxicol. 2017, 102, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Gungor, E.; Altop, A.; Erener, G. Effect of raw and fermented grape pomace on the growth performance, antioxidant status, intestinal morphology, and selected bacterial species in broiler chicks. Animals 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- de-Cara, A.; Saldana, B.; Vázquez, P.; Rey, A.I. Dietary protected sodium butyrate and/or olive leaf and grape-based by-product supplementation modifies productive performance, antioxidant status and meat quality in broilers. Antioxidants 2023, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Nardoia, M.; Romero, C.; Brenes, A.; Arija, I.; Viveros, A.; Ruiz-Capillas, C.; Chamorro, S. Addition of fermented and unfermented grape skin in broilers’ diets: Effect on digestion, growth performance, intestinal microbiota and oxidative stability of meat. Animal 2020, 14, 1371–1381. [Google Scholar] [CrossRef]
- Romero, C.; Nardoia, M.; Arija, I.; Viveros, A.; Rey, A.I.; Prodanov, M.; Chamorro, S. Feeding broiler chickens with grape seed and skin meals to enhance α- and γ-tocopherol content and meat oxidative stability. Antioxidants 2021, 10, 699. [Google Scholar] [CrossRef]
- Romero, C.; Nardoia, M.; Brenes, A.; Arija, I.; Viveros, A.; Chamorro, S. Combining grape byproducts to maximise biological activity of polyphenols in chickens. Animals 2021, 11, 3111. [Google Scholar] [CrossRef]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Soica, C.; Iuga, M.; Mironeasa, S. Effects of supplementing grape pomace to broilers fed polyunsaturated fatty acids enriched diets on meat quality. Animals 2020, 10, 947. [Google Scholar] [CrossRef]
- Aditya, S.; Ohh, S.-J.; Ahammed, M.; Lohakare, J. Supplementation of grape pomace (Vitis vinifera) in broiler diets and its effect on growth performance, apparent total tract digestibility of nutrients, blood profile and meat quality. Anim. Nutr. 2018, 4, 210–214. [Google Scholar] [CrossRef]
- Bennato, F.; Di Luca, A.; Martino, C.; Ianni, A.; Marone, E.; Grotta, L.; Ramazzotti, S.; Cichelli, A.; Martino, G. Influence of grape pomace intake on nutritional value, lipid oxidation and volatile profile of poultry meat. Foods 2020, 9, 508. [Google Scholar] [CrossRef]
- Tufarelli, V.; Baghban-Kanani, P.; Azimi-Youvalari, S.; Hosseintabar-Ghasemabad, B.; Slozhenkina, M.; Gorlov, I.; Viktoronova, F.M.; Seidavi, A.; Laudadio, V. Effect of dietary flaxseed meal supplemented with dried tomato and grape pomace on performance traits and antioxidant status of laying hens. Anim. Biotechnol. 2022, 33, 1525–1532. [Google Scholar] [CrossRef]
- Selim, S.; Abdel-Megeid, N.S.; Alhotan, R.A.; Ebrahim, A.; Hussein, E. Grape pomace: Agrifood by-product with potential to enhance performance, yolk quality, antioxidant capacity, and eggshell ultrastructure in laying hens. Vet. Sci. 2023, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.H.; Gebert, R.R.; Barreta, M.; Boiago, M.M.; Souza, C.F.; Baldissera, M.D.; Santos, I.D.; Wagner, R.; Laporta, L.V.; Stefani, L.M.; et al. Addition of grape pomace flour in the diet on laying hens in heat stress: Impacts on health and performance as well as the fatty acid profile and total antioxidant capacity in the egg. Therm. Biol. 2019, 80, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Herranz, B.; Romero, C.; Sanchez-Roman, I.; Lopez-Torres, M.; Viveros, A.; Arija, I.; Alvarez, M.D.; de Pascual-Teresa, S.; Chamorro, S. Enriching eggs with bioactive compounds through the inclusion of grape pomace in laying hens diet: Effect on internal and external egg quality parameters. Foods 2024, 13, 1553. [Google Scholar] [CrossRef]
- Hafeez, A.; Hassni, S.F.; Naz, S.; Alonaizan, R.; Al-Akeel, R.K.; Sifa, D.; Shamsi, S.; Khan, R.U. Impact of grape (Vitis vinifera) seed extract on egg production traits, nutrient digestibility, lipid peroxidation and fertility of golden hens (Gallus gallus) during early stage production. Vet Q. 2023, 43, 1–7. [Google Scholar] [CrossRef]
- Hafeez, A.; Ullah, S.; Naz, S.; Alrefaei, A.F.; Khan, R.U.; Abdelrahman, S.H.; Losacco, C.; Selvaggi, M. Effect of dietary polyphenol rich grape (Vitis vinifera) seed extract supplementation on production performance, egg quality, plasma MDA, reproductive performance and faecal microbiota of golden laying hens. J. Appl. Anim. Res. 2024, 52, 2365748. [Google Scholar] [CrossRef]
- Kaya, A.; Yildirim, B.A.; Kaya, H.; Gül, M.; Celebi, S. The effects of diets supplemented with crushed and extracted grape seed on performance, egg quality parameters, yolk peroxidation and serum traits in laying hens. Eur. Poult. Sci. 2014, 78, 1–10. [Google Scholar] [CrossRef]
- Kara, K.; Güclü, B.K.; Baytok, E.; Sentürk, M. Effects of grape pomace supplementation to laying hen diet on performance, egg quality, egg lipid peroxidation and some biochemical parameters. J. Appl. Anim. Res. 2016, 44, 303–310. [Google Scholar] [CrossRef]
- Romero, C.; Arija, I.; Viveros, A.; Chamorro, S. Productive performance, egg quality and yolk lipid peroxidation in laying hens fed diets including grape pomace or grape extract. Animals 2022, 12, 1076. [Google Scholar] [CrossRef]
- Grigorova, S.; Gjorgovska, N.; Todorova, M.; Levkov, V. Use of grape marc flour supplementation in laying hens’ diet on laying productivity, egg quality and biochemical parameters. Vet. Zootechnika 2023, 81, 62–69. [Google Scholar]
- Akter, A.; Li, X.; Grey, E.; Wang, S.C.; Kebreab, E. Grape pomace supplementation reduced methane emissions and improvedmilk quality in lactating dairy cows. J. Dairy Sci. 2025, 108, 2468–2480. [Google Scholar] [CrossRef]
- Moate, P.J.; Jacobs, J.L.; Hixson, J.L.; Deighton, M.H.; Hannah, M.C.; Morris, G.L.; Ribaux, B.E.; Wales, W.J.; Williams, S.R.O. Effects of feeding either red or white grape marc on milk production and methane emissions from early-lactation dairy cows. Animals 2020, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Moate, P.J.; Williams, S.R.; Torok, V.A.; Hannah, M.C.; Ribaux, B.E.; Tavendale, M.H.; Eckard, R.J.; Jacobs, J.L.; Auldist, M.J.; Wales, W.J. Grape marc reduces methane emissions when fed to dairy cows. J. Dairy Sci. 2014, 97, 5073–5087. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Pelmus, R.S.; Lazar, C.; Pistol, G.C.; Calin, L.G.; Toma, S.M.; Dragomir, C.; Taranu, I. Effects of a diet containing dried grape pomace on blood metabolites and milk composition of dairy cows. J. Sci. Food Agric. 2017, 97, 2516–2523. [Google Scholar] [CrossRef]
- Ianni, A.; Di Maio, G.; Pittia, P.; Grotta, L.; Perpetuini, G.; Tofalo, R.; Cichelli, A.; Martino, G. Chemical–nutritional quality and oxidative stability of milk and dairy products obtained from Friesian cows fed with a dietary supplementation of dried grape pomace. J. Sci. Food Agric. 2019, 99, 3635–3643. [Google Scholar] [CrossRef]
- Ianni, A.; Innosa, D.; Martino, C.; Bennato, F.; Martino, G. Compositional characteristics and aromatic profile of caciotta cheese obtained from Friesian cows fed with a dietary supplementation of dried grape pomace. J. Dairy Sci. 2019, 102, 1025–1032. [Google Scholar] [CrossRef]
- Ianni, A.; Martino, G. Dietary grape pomace supplementation in dairy cows: Effect on nutritional quality of milk and its derived dairy products. Foods 2020, 9, 168. [Google Scholar] [CrossRef]
- Manso, T.; Gallardo, B.; Salvá, A.; Guerra-Rivas, C.; Mantecón, A.R.; Lavín, P.; De la Fuente, M.A. Influence of dietary grape pomace combined with linseed oil on fatty acid profile and milk composition. J. Dairy Sci. 2016, 99, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lungu, S.E.; Danso, F.; Dzou, C.F.; Chen, Y.; Zheng, X.; Nie, F.; Lin, H.; Chen, J.; Zhou, G. Animal health and nutrition: Metabolic disorders in cattle and improvement strategies. Front. Vet. Sci. 2025, 12, 1470391. [Google Scholar] [CrossRef]
- Tufarelli, V.; Puvaca, N.; Glamocic, D.; Pugliese, G.; Colonna, M.A. The most important metabolic diseases in dairy cattle during the transition period. Animals 2024, 14, 816. [Google Scholar] [CrossRef]
- Gross, J.J.; Schwarz, F.J.; Eder, K.; van Dorland, H.A.; Bruckmaier, R.M. Liver fat content and lipid metabolism in dairy cows during early lactation and during a mid-lactation feed restriction. J. Dairy Sci. 2013, 96, 5008–5017. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Vels, L.; Rontved, C.M.; Bjerring, M.; Ingvartsen, K.L. Cytokine and acute phase protein gene expression in repeated liver biopsies of dairy cows with a lipopolysaccharide-induced mastitis. J. Dairy Sci. 2009, 92, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Zebeli, Q.; Metzler-Zebeli, B.U.; Ametaj, B.N. Meta-analysis reveals threshold level of rapidly fermentable dietary concentrate that triggers systemic inflammation in cattle. J. Dairy Sci. 2012, 95, 2662–2672. [Google Scholar] [CrossRef]
- Bionaz, M.; Trevisi, E.; Calamari, L.; Librandi, F.; Ferrari, A.; Bertoni, G. Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. J. Dairy Sci. 2007, 90, 1740–1750. [Google Scholar] [CrossRef]
- Gessner, D.K.; Schlegel, G.; Keller, J.; Schwarz, F.J.; Ringseis, R.; Eder, K. Expression of target genes of nuclear factor E2-related factor 2 in the liver of dairy cows in the transition period and at different stages of lactation. J. Dairy Sci. 2013, 96, 1038–1043. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. Redox biology in transition periods of dairy cattle: Role in the health of periparturient and neonatal animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef]
- Yonekura, S. The role of endoplasmic reticulum stress in metabolic diseases and mammary epithelial cell homeostasis in dairy cows. Anim. Sci. J. 2024, 95, e13935. [Google Scholar] [CrossRef]
- Gessner, D.K.; Koch, C.; Romberg, F.J.; Winkler, A.; Dusel, G.; Herzog, E.; Most, E.; Eder, K. The effect of grape seed and grape marc meal extract on milk performance and the expression of genes of endoplasmic reticulum stress and inflammation in the liver of dairy cows in early lactation. J. Dairy Sci. 2015, 98, 8856–8868. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, M.S. FGF21 as a stress hormone: The roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab. J. 2014, 38, 245–251. [Google Scholar] [CrossRef]
- Gessner, D.K.; Winkler, A.; Koch, C.; Dusel, G.; Liebisch, G.; Ringseis, R.; Eder, K. Analysis of hepatic transcript profile and plasma lipid profile in early lactating dairy cows fed grape seed and grape marc meal extract. BMC Genom. 2017, 18, 253. [Google Scholar] [CrossRef]
- Gobert, M.; Martin, B.; Ferlay, A.; Chilliard, Y.; Graulet, B.; Pradel, P.; Bauchart, D.; Durand, D. Plant polyphenols associated with vitamin E can reduce plasma lipoperoxidation in dairy cows given n-3 polyunsaturated fatty acids. J. Dairy Sci. 2009, 92, 6095–6104. [Google Scholar] [CrossRef] [PubMed]
- Signor, M.H.; de Freitas Dos Santos, A.L.; de Vitt, M.G.; Nora, L.; Lago, R.V.P.; Wolschick, G.J.; Correa, N.G.; Klein, B.; Xavier, A.C.H.; Wagner, R.; et al. Grape seed oil in the diet of primiparous Jersey cows before and after parturition: Effects on performance, health, rumen environment, and milk quality. Trop. Anim. Health Prod. 2024, 56, 202. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Oikonomou, G.; Hu, J.; Li, Y.; Du, X.; Du, Y.; Liu, Y.; Zhang, P.; Wang, P.; Yu, H.; et al. Effect of feeding grape seed Proanthocyanidin extract on production performance, metabolic and anti-oxidative status of dairy cattle. Arq. Bras. Med. Vet. Zootec. 2019, 71, 1207–1216. [Google Scholar] [CrossRef]
- Urkmez, E.; Biricik, H. Grape seed extract supplementation in heat-stressed preweaning dairy calves: I. Effects on antioxidant status, inflammatory response and physiological parameters. Anim. Feed Sci. Technol. 2022, 292, 115421. [Google Scholar] [CrossRef]
- Ma, J.; Fan, X.; Zhang, W.; Zhou, G.; Yin, F.; Zhao, Z.; Gan, S. Grape seed extract as a feed additive improves the growth performance, ruminal fermentation and immunity of weaned beef calves. Animals 2023, 13, 1876. [Google Scholar] [CrossRef]
- Iannaccone, M.; Elgendy, R.; Giantin, M.; Martino, C.; Giansante, D.; Ianni, A.; Dacasto, M.; Martino, G. RNA sequencing-based whole transcriptome analysis of Friesian cattle fed with grape pomace-supplemented diet. Animals 2018, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Molosse, V.L.; Deolindo, G.L.; Lago, R.V.P.; Cecere, B.G.O.; Zotti, C.A.; Vedovato, M.; Copetti, P.M.; Fracasso, M.; Morsch, V.M.; Xavier, A.C.; et al. The effects of the inclusion of ensiled and dehydrated grape pomace in beef cattle diet: Growth performance, health and economic viability. Anim. Sci. Technol. 2023, 302, 115671. [Google Scholar] [CrossRef]
- Li, Y.; Shi, C.; Deng, J.; Qiu, X.; Zhang, S.; Wang, H.; Qin, X.; He, Y.; Cao, B.; Su, H. Effects of grape pomace on growth performance, nitrogen metabolism, antioxidants, and microbial diversity in Angus bulls. Antioxidants 2024, 13, 412. [Google Scholar] [CrossRef]
- Korver, D.R. Review: Current challenges in poultry nutrition, health, and welfare. Animal 2023, 17 (Suppl. 2), 100755. [Google Scholar] [CrossRef]
- Durand, D.; Collin, A.; Merlot, E.; Baeza, E.; Guilloteau, L.A.; Le Floc’h, N.; Thomas, A.; Fontagne-Dicharry, S.; Gondret, F. Review: Implications of redox balance in animal health and performance at critical periodsm insights from different farm animal species. Animal 2022, 16, 100543. [Google Scholar] [CrossRef]
- Horst, E.A.; Mayorga, E.J.; Baumgard, L.H. International Symposium on Ruminant Physiology: Integrating our understanding of stress physiology. J. Dairy Sci. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyans and gut microbiota. J. Agricult. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, Y.; Gu, T.; Yan, Y.; Guo, J.; Zhang, X.; Pang, H.; Chen, J. The metabolic characteristics and bioavailability of resveratrol based on metabolic enzymes. Nutr. Rev. 2025, 83, 749–770. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Rogowska-van der Molen, M.A.; Berasategui-Lopez, A.; Coolen, S.; Jansen, R.S.; Welte, C.U. Microbial degradation of plant toxins. Microbiol. 2023, 25, 2988–3010. [Google Scholar] [CrossRef]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, microbiome interactions and cellular effects on health in humans and animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef]
- Chamorro, S.; Romero, C.; Brenes, A.; Sanchez-Patan, F.; Bartolome, B.; Viveros, A.; Arija, I. Impact of a sustained consumption of grape extract on digestion, gut microbial metabolism and intestinal barrier in broiler chickens. Food Funct. 2019, 10, 1444–1454. [Google Scholar] [CrossRef]
- Anghel, A.C.; Taranu, I.; Ortan, A.; Marcu Spinu, S.; Dragoi Cudalbeanu, M.; Rosu, P.M.; Babeanu, N.E. Polyphenols and microbiota modulation: Insights from swine and other animal models for human therapeutic strategies. Molecules 2024, 29, 6026. [Google Scholar] [CrossRef]
- Beaumont, M.; Lencina, C.; Painteaux, L.; Viemon-Desplanque, J.; Phornlaphat, O.; Lambert, W.; Chalvon-Demersay, T. A mix of functional amino acids and grape polyphenols promotes the growth of piglets, modulates the gut microbiota in vivo and regulates epithelial homeostasis in intestinal organoids. Amino Acids 2022, 54, 1357–1369. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, M.; Mo, J.; Lan, G.; Liang, J. Dietary supplementation ellagic acid on the growth, microbiota, and inflammation in weaned pigs. Front. Vet. Sci. 2022, 8, 980271. [Google Scholar]
- Ringseis, R.; Gessner, D.K.; Eder, K. The gut-liver axis in the control of energy metabolism and food intake in animals. Annu. Rev. Anim. Biosci. 2020, 8, 295–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tong, T.; Yu, C.; Wu, Q. The research progress on the impact of gut microbiota on health and production performance. Front. Vet. Sci. 2025, 12, 1564519. [Google Scholar] [CrossRef] [PubMed]
- Baron, C.P.; Andersen, J.J. Myoglobin-induced lipid peroxidation. J. Agric. Food Chem. 2017, 50, 3887–3897. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R., Jr.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Sevanian, A.; McLeod, L.L. Cholesterol autoxidation in phospholipid membrane bilayers. Lipids 1987, 22, 627–636. [Google Scholar] [CrossRef]
- Brandsch, C.; Ringseis, R.; Eder, K. High dietary iron concentrations enhance the formation of cholesterol oxidation products in the liver of adult rats fed salmon oil with minimal effects on antioxidant status. J. Nutr. 2002, 132, 2263–2269. [Google Scholar] [CrossRef][Green Version]
- Gentile, C.L.; Frye, M.; Pagliassotti, M.J. Endoplasmic reticulum stress and the unfolded protein response in nonalcoholic fatty liver disease. Antioxid. Redox Signal. 2011, 15, 505–521. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Niu, J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ. Res. 2012, 110, 174–189. [Google Scholar] [CrossRef]
- Jefferi, N.E.S.; Shamhari, A.A.; Hamid, Z.A.; Budin, S.B.; Taib, I.S. Interlinkage between inflammation, oxidative stress, and endoplasmic reticulum stress in bisphenol-induced testicular steroidogenesis disturbance: A mini review. Int. J. Reprod. Biomed. 2025, 23, 17–32. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, X.; Cao, T.; Chen, E.; Li, Y.; Lei, W.; Hu, Y.; He, B.; Liu, S. Endoplasmic reticulum stress and oxidative stress in inflammatory diseases. Cell Biol. 2022, 41, 924–934. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Kaplowitz, N.; Lebeaupin, C.; Kroemer, G.; Kaufman, R.J.; Malhi, H.; Ren, J. Endoplasmic reticulum stress in liver diseases. Hepatology 2023, 77, 619–639. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, Q.; Wang, Y.; Zhao, B.; Zhang, L.; Yang, X.; Wang, Z. The role of endoplasmic reticulum stress in type 2 diabetes mellitus mechanisms and impact of islet function. PeerJ 2025, 13, e19192. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, B.; Wang, J.; Zhang, H.; Yang, Y.; Song, S.; Psifidi, A.; Wu, W.; Loor, J.J.; Xu, C. Caveolin 1 in bovine liver is associated with fatty acid-induced lipid accumulation and the endoplasmic reticulum unfolded protein response: Role in fatty liver development. J. Dairy Sci. 2025, 108, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, M.M.; Mizusawa, M.; Hayashi, S.; Arai, W.; Sakata, S.; Yonekura, S. Effects of fatty acids on inducing endoplasmic reticulum stress in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 8643–8654. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Chen, X.; Yan, Y.; Li, L.; Zhao, W. Transcriptome analysis reveals that NEFA and β-hydroxybutyrate induce oxidative stress and inflammatory response in bovine mammary epithelial cells. Metabolites 2022, 12, 1060. [Google Scholar] [CrossRef]
- Shen, T.; Xia, S.; Usman, M.; Xu, X.; Loor, J.J.; Xu, C. Nuclear factor erythroid 2-related factor 1 regulates the expression of proteosomal genes in ketotic kows and protects mammary cells against free fatty acid-induced endoplasmic reticulum stress. J. Dairy Sci. 2025, 108, 1050–1061. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Li, Y.; Jia, P.; Ji, S.; Chen, Y.; Wang, T. Protective effects of pterostilbene against damage, redox imbalance, mitochondrial dysfunction, and endoplasmic reticulum stress in weanling pigs. J. Anim. Sci. 2020, 98, skaa328. [Google Scholar] [CrossRef]
- Gessner, D.K.; Gröne, B.; Rosenbaum, S.; Most, E.; Hillen, S.; Becker, S.; Erhardt, G.; Reiner, G.; Ringseis, R.; Eder, K. Effect of a negative energy balance induced by feed restriction on pro-inflammatory and endoplasmic reticulum stress signalling pathways in the liver and skeletal muscle of lactating sows. Arch. Anim. Nutr. 2015, 69, 411–423. [Google Scholar] [CrossRef]
- Gessner, D.K.; Gröne, B.; Couturier, A.; Rosenbaum, S.; Hillen, S.; Becker, S.; Erhardt, G.; Reiner, G.; Ringseis, R.; Eder, K. Dietary Fish Oil Inhibits Pro-Inflammatory and ER Stress Signalling Pathways in the Liver of Sows during Lactation. PLoS ONE 2015, 10, e0137684. [Google Scholar] [CrossRef]
- Jing, J.; Zeng, H.; Shao, Q.; Tang, J.; Wang, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; et al. Selenomethionine alleviates environmental heat stress induced hepatic lipid accumulation and glycogen infiltration of broilers via maintaining mitochondrial and endoplasmic reticulum homeostasis. Redox Biol. 2023, 67, 102912. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.L.; Coutinho-Wolino, K.S.; Almeida, P.P.; Trigueira, P.C.; Alves, A.P.P.; Magliano, D.C.; Stockler-Pinto, M.B. Unstressing the reticulum: Nutritional strategies for modulating endoplasmic reticulum stress in obesity. Mol. Nutr. Food Res. 2024, 68, e2400361. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, H.; Miao, D.; Wang, H.; Liu, Y.; Xing, L.; Bao, J.; Li, J. Dietary resveratrol supplementation alleviates cold exposure-induced pyroptosis and inflammation in broiler heart by modulating oxidative stress and endoplasmic reticulum stress. Poult Sci. 2024, 103, 104203. [Google Scholar] [CrossRef]
- Fu, K.; Chen, L.; Miao, L.; Guo, Y.; Zhang, W.; Bai, Y. Grape seed proanthocyanidins protect N2a cells against ischemic injury via endoplasmic reticulum stress and mitochondrial-associated pathways. Neurol. Disord. Drug Targets 2019, 18, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Viveros, A.; Centeno, C.; Romero, C.; Arija, I.; Brenes, A. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal 2013, 7, 555–561. [Google Scholar] [CrossRef]
- Grosu, I.A.; Pistol, G.C.; Marin, D.E.; Cismileanu, A.; Palade, L.M.; Taranu, I. Effects of dietary grape seed meal bioactive compounds on the colonic microbiota of weaned piglets with dextran sodium sulfate-induced colitis used as an inflammatory model. Front. Vet. Sci. 2020, 7, 31. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Rebolé, A.; Rica, B.D.; Arija, I.; Brenes, A. Influence of dietary enzyme addition on polyphenol utilization and meat lipid oxidation of chicks fed grape pomace. Int. Food Res. J. 2015, 73, 197–203. [Google Scholar] [CrossRef]
- Ebrahimzadeh, S.; Navidshad, B.; Farhoomand, P.; Aghjehgheshlagh, F.M. Effects of exogenous tannase enzyme on growth performance, antioxidant status, immune response, gut morphology and intestinal microflora of chicks fed grape pomace. S. Afr. J. Anim. Sci. 2018, 48, 2–18. [Google Scholar] [CrossRef]
- Jonathan, O.; Mnisi, C.M. Effect of dietary red grape pomace on growth performance, hematology, serum biochemistry, and meat quality parameters in Hy-line Silver Brown cockerels. PLoS ONE 2021, 16, e0259630. [Google Scholar] [CrossRef]
- Pascariu, S.; Pop, I.; Simeanu, D.; Pavel, G.; Solcan, C. Effects of wine by-products on growth performance, complete blood count and total antioxidant status in broilers. Braz. J. Poult. Sci. 2017, 19, 191–202. [Google Scholar] [CrossRef]
- Afsana, K.; Shiga, K.; Ishizuka, S.; Hara, H. Reducing effect of ingesting tannic acid on the absorption of iron, but not of zinc, copper and manganese by rats. Biosci. Biotechnol. Biochem. 2004, 68, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, S.; Williams, V.; Flynn, P.; Carlino, R.; Mowry, C.; Dierenfeld, E.; Babb, C.; Fan, J.; Tramontano, W.A. Tannin/Polyphenol effects on iron solubilization in vitro. Bios 2004, 75, 43–52. [Google Scholar] [CrossRef]
- Fiesel, A.; Ehrmann, M.; Gessner, D.K.; Most, E.; Eder, K. Effects of polyphenol-rich plant products from grape or hop as feed supplements on iron, zinc and copper status in piglets. Arch. Anim. Nutr. 2015, 69, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Thanh, L.P.; Kha, P.T.T.; Loor, J.J.; Hang, T.T.T. Grape seed tannin extract and polyunsaturated fatty acids affect in vitro ruminal fermentation and methane production. J. Anim. Sci. 2022, 100, skac039. [Google Scholar] [CrossRef]

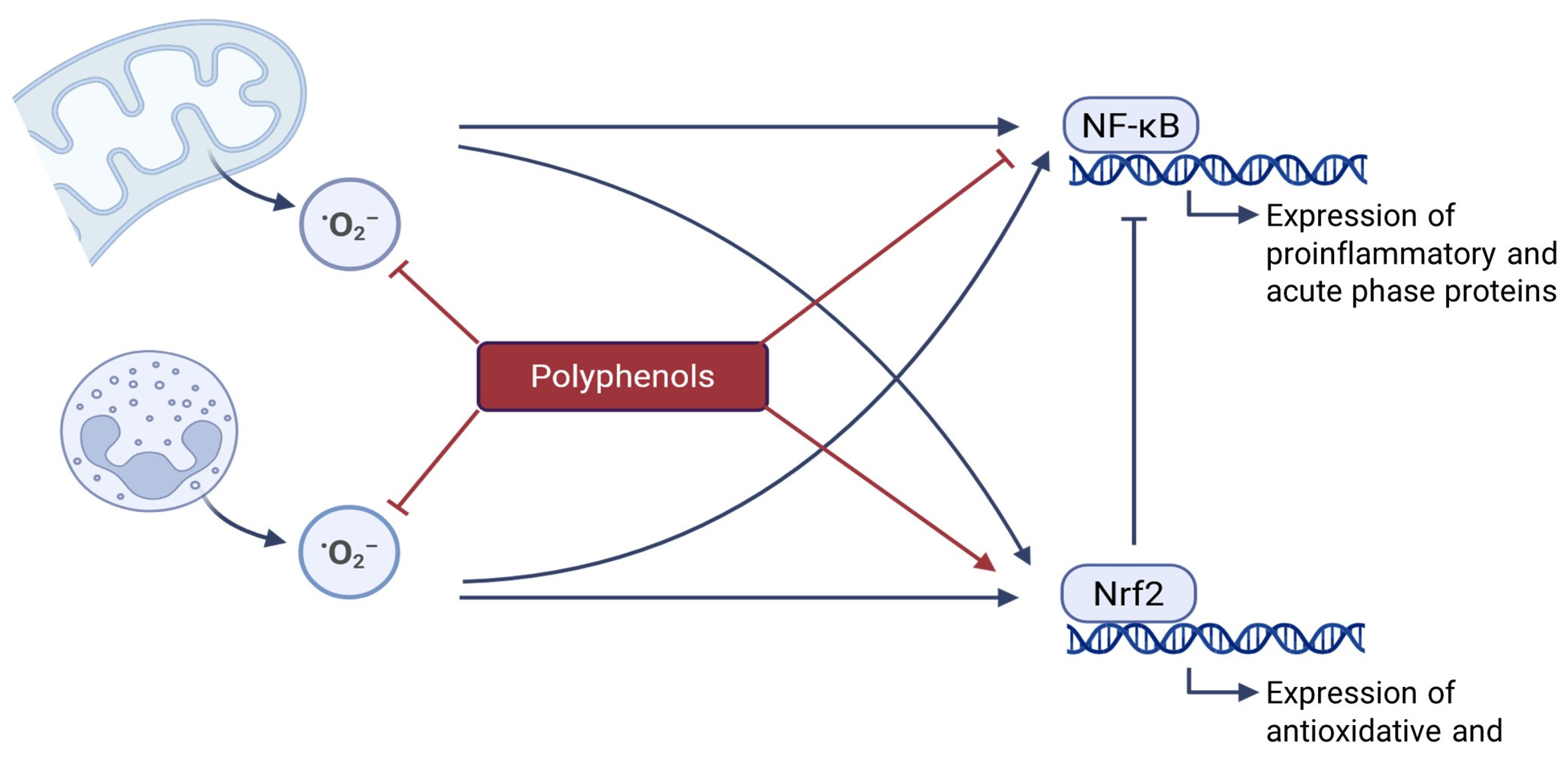
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eder, K.; Ringseis, R.; Gessner, D.K. Effects of Grape By-Products on Oxidative Stress and Inflammation in Farm Animals: An Overview of Studies Performed in Pigs, Chickens, and Cattle. Animals 2025, 15, 1536. https://doi.org/10.3390/ani15111536
Eder K, Ringseis R, Gessner DK. Effects of Grape By-Products on Oxidative Stress and Inflammation in Farm Animals: An Overview of Studies Performed in Pigs, Chickens, and Cattle. Animals. 2025; 15(11):1536. https://doi.org/10.3390/ani15111536
Chicago/Turabian StyleEder, Klaus, Robert Ringseis, and Denise K. Gessner. 2025. "Effects of Grape By-Products on Oxidative Stress and Inflammation in Farm Animals: An Overview of Studies Performed in Pigs, Chickens, and Cattle" Animals 15, no. 11: 1536. https://doi.org/10.3390/ani15111536
APA StyleEder, K., Ringseis, R., & Gessner, D. K. (2025). Effects of Grape By-Products on Oxidative Stress and Inflammation in Farm Animals: An Overview of Studies Performed in Pigs, Chickens, and Cattle. Animals, 15(11), 1536. https://doi.org/10.3390/ani15111536






