Protective Effects of Astaxanthin on Thioacetamide-Induced Hepatopancreatic Damage in Procambarus clarkii: Insights from Biochemical, Histological, and Metabolomic Analyses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design
2.3. Hepatopancreatic Injury Markers Detection
2.4. Hepatopancreatic Oxidative Stress Markers Detection
2.5. Histological Examination of Hepatopancreas
2.6. Hepatopancreatic Fibrosis Detection
2.7. Metabolomic Analysis
2.8. Quantitative Real-Time PCR (qPCR)
2.9. Data Analysis
3. Results
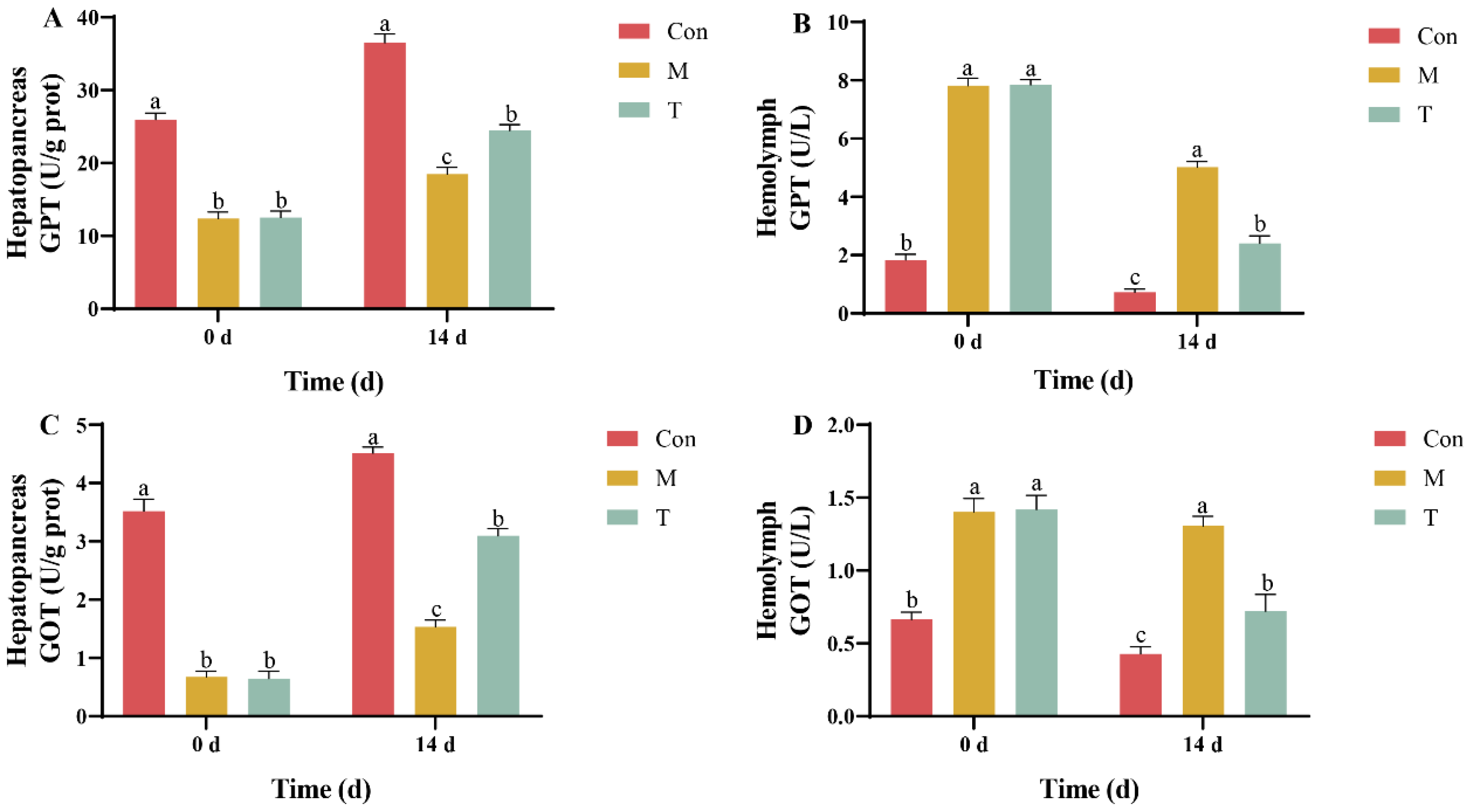
3.1. Analysis of Hepatopancreatic Injury Markers
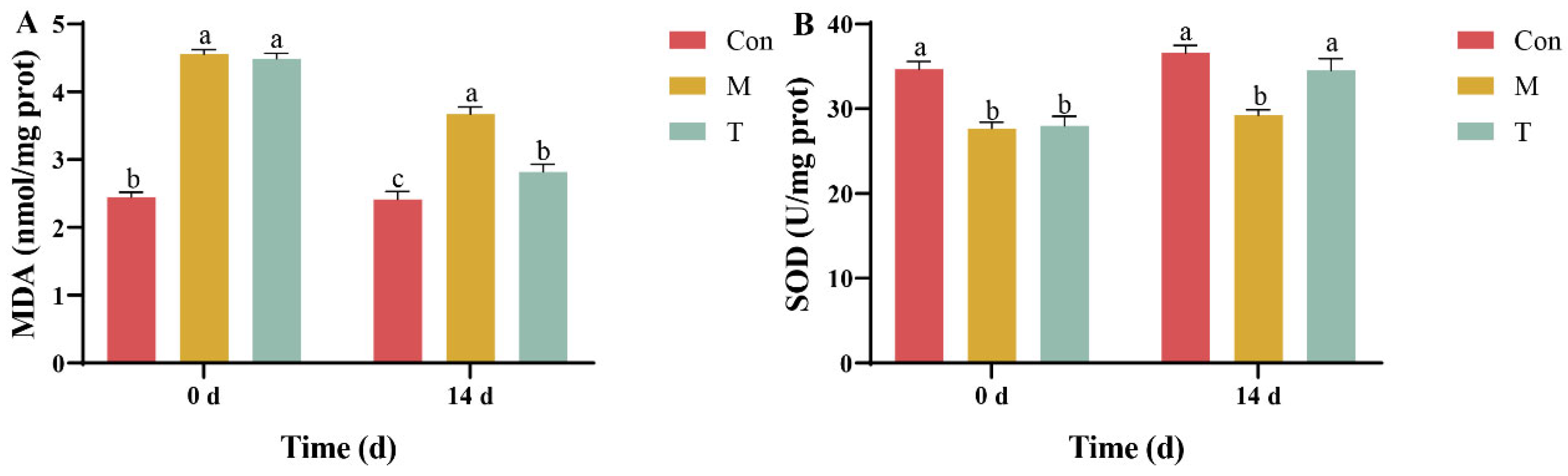
3.2. Analysis of Oxidative Stress Markers in the Hepatopancreas
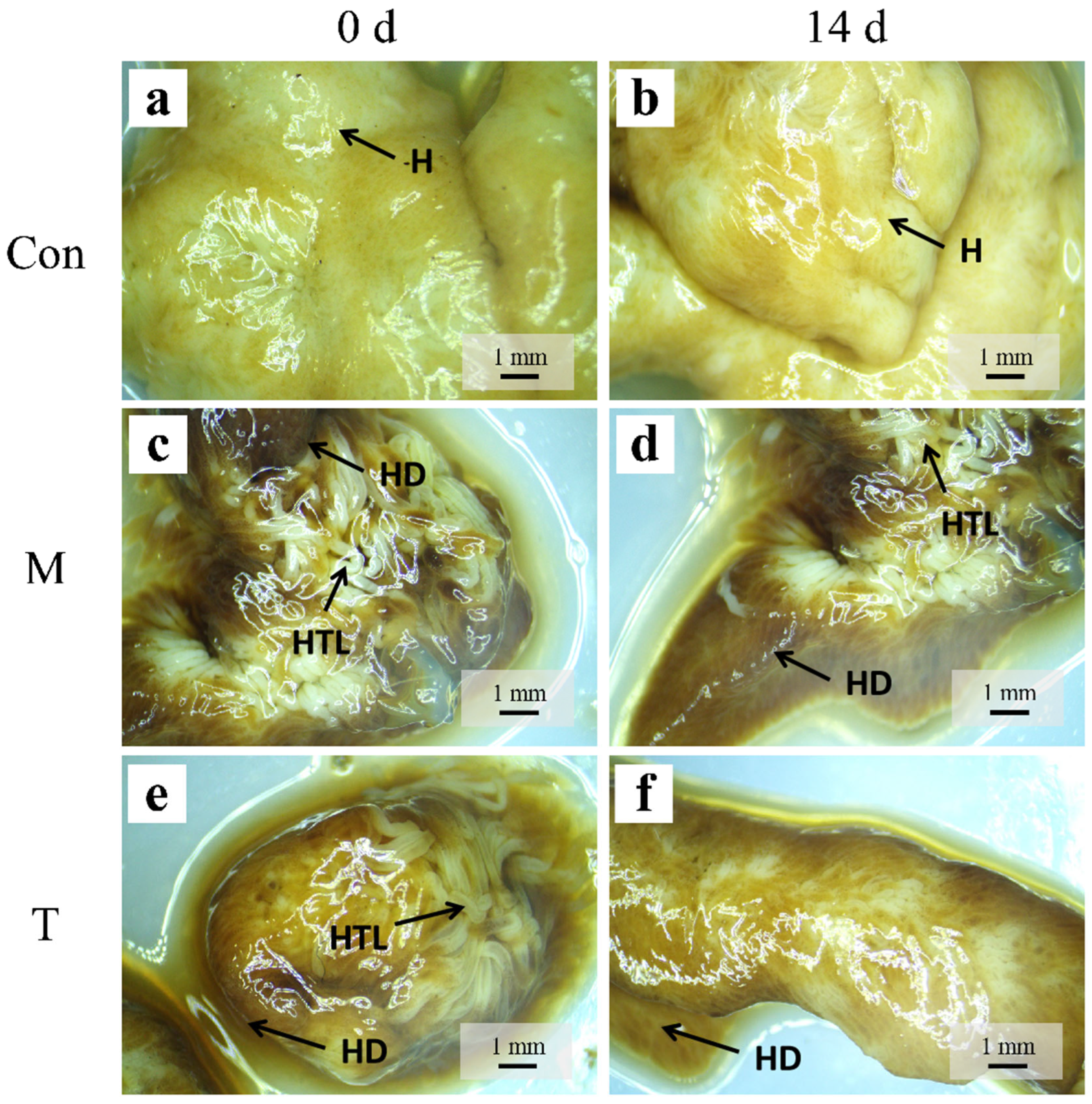
3.3. Histological Examination
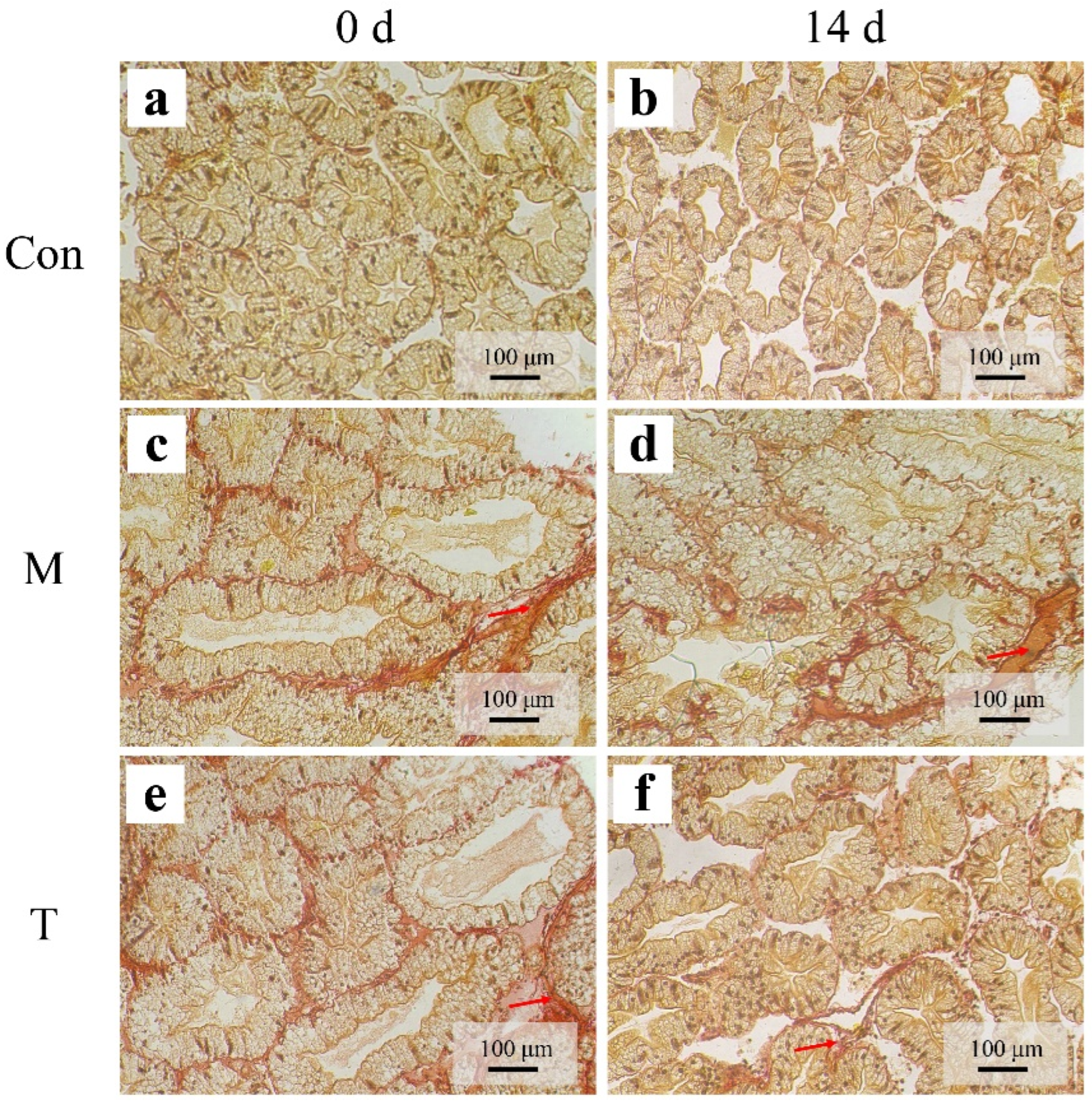
3.4. Sirius Red Staining
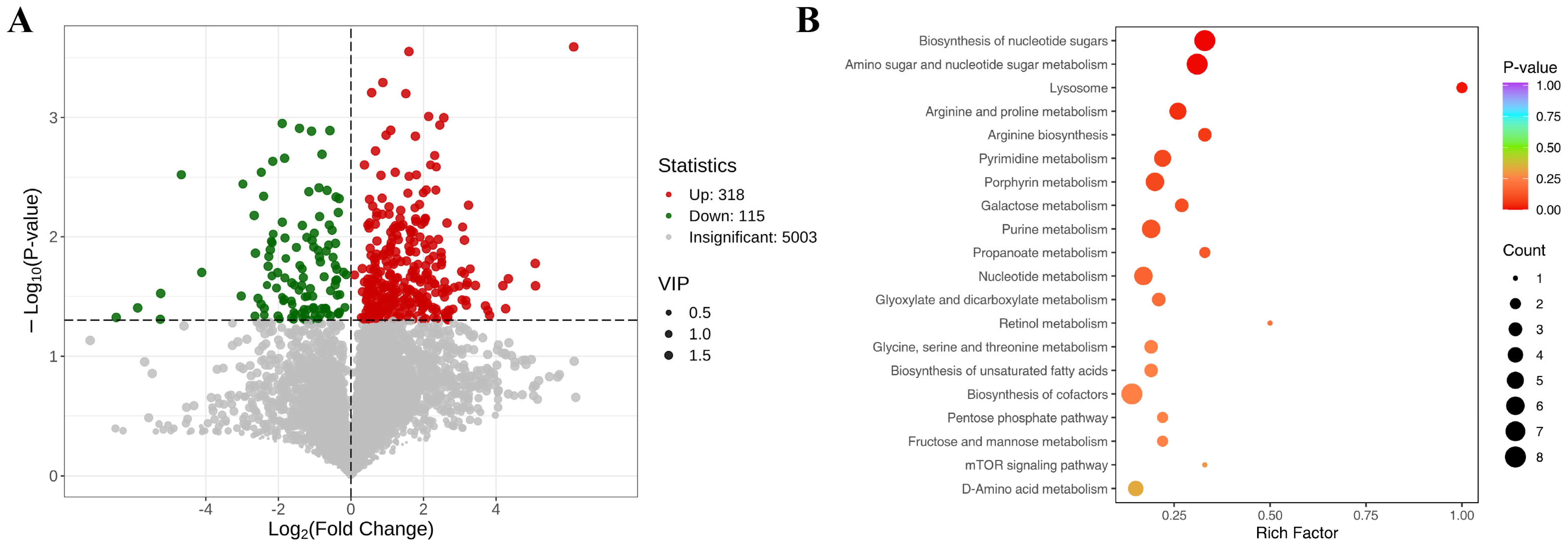
3.5. Metabolomic Analysis Results
3.6. qPCR Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.Q.; Shen, C.C.; Tang, D.; Bai, Y.Z.; Wu, L.; Zhang, Y.P.; Wu, Y.; Wang, Z.F. The effects of ammonia exposure on immune response, oxidative stress, and apoptosis in Procambarus clarkii. Aquac. Int. 2022, 30, 533–546. [Google Scholar] [CrossRef]
- Holdich, D.M. A review of astaciculture: Freshwater crayfish farming. Aquat. Living Kesour. 1993, 6, 307–317. [Google Scholar] [CrossRef]
- Vogt, G. Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphol. 2019, 280, 1405–1444. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.Y.; Kholodkevich, S.; Sharov, A.; Feng, Y.J.; Ren, N.Q.; Sun, K. Cadmium-induced oxidative stress, histopathology, and transcriptome changes in the hepatopancreas of freshwater crayfish (Procambarus clarkii). Sci. Total Environ. 2019, 666, 944–955. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Feng, Y.; Ren, N.; Sun, K. Microcystin-LR-induced changes of hepatopancreatic transcriptome, intestinal microbiota, and histopathology of freshwater crayfish (Procambarus clarkii). Sci. Total Environ. 2020, 711, 134549. [Google Scholar] [CrossRef]
- Zhang, Y.; Mi, K.; Xue, W.; Wei, W.; Yang, H. Acute BPA exposure-induced oxidative stress, depressed immune genes expression and damage of hepatopancreas in red swamp crayfish Procambarus clarkii. Fish Shellfish Immun. 2020, 103, 95–102. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Deng, H.; Li, L.; Huang, X.; Chen, D.; Ouyang, P.; Geng, Y.; Yang, S.; Yin, L.; et al. The Damage of the Crayfish (Procambarus clarkii) Digestive Organs Caused by Citrobacter freundii Is Associated With the Disturbance of Intestinal Microbiota and Disruption of Intestinal-Liver Axis Homeostasis. Front. Cell Infect. Microbiol. 2022, 12, 940576. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Zhang, M.; Chen, Q.; Fan, W.; Zhao, Y. Effect of dietary vitamin E on growth, immunity and regulation of hepatopancreas nutrition in male oriental river prawn, Macrobrachium nipponense. Aquac. Res. 2019, 50, 1741–1751. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Li, H.; Lu, B.; Du, Y.; Chen, J.; Biswas, G. Investigation of the Protective Effect of Probiotic Lactobacillus plantarum Ep-M17 on the Hepatopancreas of Penaeus vannamei. Aquac. Nutr. 2024, 2024, 1–27. [Google Scholar] [CrossRef]
- Zheng, Z.; Aweya, J.J.; Bao, S.; Yao, D.; Li, S.; Tran, N.T.; Ma, H.; Zhang, Y. The Microbial Composition of Penaeid Shrimps’ Hepatopancreas Is Modulated by Hemocyanin. J. Immunol. 2021, 207, 2733–2743. [Google Scholar] [CrossRef]
- Jun, J.W.; Han, J.E.; Giri, S.S.; Tang, K.F.J.; Zhou, X.; Aranguren, L.F.; Kim, H.J.; Yun, S.; Chi, C.; Kim, S.G.; et al. Phage Application for the Protection from Acute Hepatopancreatic Necrosis Disease (AHPND) in Penaeus vannamei. Indian J. Microbiol. 2017, 58, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xie, Y.D.; Xie, M.X.; Liang, H.; Li, M.; Zhou, B.F.; Ran, C.; Zhou, Z.G. The effect of dietary supplementation of medium-chain fatty acids products on gut and hepatopancreas health, and disease resistance in white shrimp (Litopenaeus vannamei). Aquac. Rep. 2023, 29, 101565. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, G.; Liu, J.; Chen, I.T.; Wei, Y.; Liang, M.; Xu, H. Effects of a phytobiotic-based additive on the growth, hepatopancreas health, intestinal microbiota, and Vibrio parahaemolyticus resistance of Pacific white shrimp (Litopenaeus vannamei). Front. Immunol. 2024, 15, 1368444. [Google Scholar] [CrossRef]
- Chen, S.; Zhuang, Z.; Yin, P.; Chen, X.; Zhang, Y.; Tian, L.; Liu, Y. Changes in growth performance, haematological parameters, hepatopancreas histopathology and antioxidant status of Pacific white shrimp (Litopenaeus vannamei) fed oxidized fish oil: Regulation by dietary myo-inositol. Fish Shellfish Immunol. 2019, 88, 53–64. [Google Scholar] [CrossRef]
- Yang, H.; Du, D.; Zhang, Q.; Teame, T.; Wang, A.; Hao, Q.; Zhou, Z. Dietary Bacillus velezensis T23 fermented products supplementation improves growth, hepatopancreas and intestine health of Litopenaeus vannamei. Fish Shellfish Immunol. 2024, 149, 109595. [Google Scholar] [CrossRef]
- Chen, A.Q.; Chen, B.Y.; Zhong, J.; Liao, Z.H.; He, X.S.; Lin, S.H.; Niu, J. Effects of lysophospholipid on growth performance, hepatopancreas health, and intestinal microbiome of Litopenaeus vannamei in low-fishmeal diet. Aquac. Nutr. 2024, 2024, 8883996. [Google Scholar] [CrossRef]
- Elbahnaswy, S.; Elshopakey, G.E. Recent progress in practical applications of a potential carotenoid astaxanthin in aquaculture industry: A review. Fish Physiol. Biochem. 2024, 50, 97–126. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, S. Effect of dietary astaxanthin on the growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkii. Aquaculture 2019, 512, 734341. [Google Scholar] [CrossRef]
- An, Z.; Yang, H.; Liu, X.; Zhang, Y. Effects of astaxanthin on the immune response and reproduction of Procambarus clarkii stressed with microcystin-leucine-arginine. Fish. Sci. 2020, 86, 759–766. [Google Scholar] [CrossRef]
- An, Z.; Zhang, Y.; Sun, L. Effects of Dietary Astaxanthin Supplementation on Energy Budget and Bioaccumulation in Procambarus clarkii (Girard, 1852) Crayfish under Microcystin-LR Stress. Toxins 2018, 10, 277. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Z.; Li, Q.; Yang, H. Current Challenges and Issues in the Application of Astaxanthin. Fishes 2025, 10, 159. [Google Scholar] [CrossRef]
- Gao, C.H.; Gong, N.Y.; Chen, F.T.; Hu, S.R.; Zhou, Q.X.; Gao, X. The Effects of Astaxanthin on Metabolic Syndrome: A Comprehensive Review. Mar. Drugs 2025, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Zhao, W.; Lu, D.Q.; Xie, J.J.; He, X.S.; Fang, H.H.; Liao, S.Y. Dual-Function Analysis of Astaxanthin on Golden Pompano (Trachinotus ovatus) and Its Role in the Regulation of Gastrointestinal Immunity and Retinal Mitochondrial Dysfunction Under Hypoxia Conditions. Front. Physiol. 2020, 11, 568462. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs. 2020, 18, 406. [Google Scholar] [CrossRef]
- Ju, J.; Xu, J.W.; Xu, X.R.; Zhao, H.Y.; Zhang, Y.Y.; Yang, H. Establishment and mechanism of thioacetamide-induced hepatopancreas injury model in red swamp crayfish Procambarus clarkii. Aquaculture 2025, 598, 741942. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wallace, M.; Hamesch, K.; Lunova, M.; Kim, Y.; Weiskirchen, R.; Strnad, P.; Friedman, S. Standard Operating Procedures in Experimental Liver Research: Thioacetamide model in mice and rats. Lab. Anim. 2015, 49 (Suppl. 1), 21–29. [Google Scholar] [CrossRef]
- Junming, C.A.O.; Jing, Y.A.N.; Guoxia, W.; Yanhua, H.; Rongbin, Z.; Tingting, Z.; Qunfang, L.I.U.; Sun, Z. Effects of replacement of fish meal with housefly maggot meal on digestive enzymes, transaminases activities and hepatopancreas histological structure of Litopenaeus vannamei. South China Fish. Sci. 2012, 8, 72–79. [Google Scholar]
- Qiu, M.; Wang, Y.; Wang, X.; Sun, L.; Ye, R.; Xu, D.; Dai, Z.; Liu, Y.; Bi, S.; Yao, Y.; et al. Effects of T-2 toxin on growth, immune function and hepatopancreas microstructure of shrimp (Litopenaeus vannamei). Aquaculture 2016, 462, 35–39. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, S.; Dai, J.; Wang, C.; Chen, S.; Qian, Y.; Gong, Y.; Han, T. Effects of Synthetic Astaxanthin on the Growth Performance, Pigmentation, Antioxidant Capacity, and Immune Response in Black Tiger Prawn (Penaeus monodon). Aquac. Nutr. 2023, 2023, 6632067. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, Y.B.; Choi, J.H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.N.; Aurore, Z.V.; Poirier, L.; Kamari, A.; Châtel, A.; Mouneyrac, C.; Lagarde, F. Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ. Pollut. 2016, 211, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J. Oxidative damage of hepatopancreas induced by pollution depresses humoral immunity response in the freshwater crayfish Procambarus clarkii. Fish. Shellfish Immun. 2015, 43, 510–519. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Li, J.; Liu, P.; Li, J.; Zhao, F. Antioxidant, Transcriptomic and Metabonomic Analysis of Hepatopancreatic Stress Resistance in Exopalaemon carinicauda Following Astaxanthin Feeding. Front. Mar. Sci. 2022, 9, 906963. [Google Scholar] [CrossRef]
- Yang, W.; Liu, D.; Gao, P.; Wu, Q.; Li, Z.; Li, S.; Zhu, L. Oxidative stress and metabolic process responses of Chlorella pyrenoidosa to nanoplastic exposure: Insights from integrated analysis of transcriptomics and metabolomics. Environ. Pollut. 2024, 357, 124466. [Google Scholar] [CrossRef]
- Miranda, M.R.; Canepa, G.E.; Bouvier, L.A.; Pereira, C.A. Trypanosoma cruzi: Oxidative stress induces arginine kinase expression. Exp. Parasitol. 2006, 114, 341–344. [Google Scholar] [CrossRef]
- Zhang, L.; Alfano, J.R.; Becker, D.F. Proline metabolism increases katG expression and oxidative stress resistance in Escherichia coli. J. Bacteriol. 2015, 197, 431–440. [Google Scholar] [CrossRef]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Mercurio, S.; Tolosano, E. Heme in pathophysiology: A matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Bankaitis, V.A.; Garcia-Mata, R.; Mousley, C.J. Golgi Membrane Dynamics and Lipid Metabolism. Curr. Biol. 2012, 22, R414–R424. [Google Scholar] [CrossRef]
- Wang, W.; Cui, J.; Ma, H.; Lu, W.; Huang, J. Targeting pyrimidine metabolism in the era of precision cancer medicine. Front. Oncol. 2021, 11, 684961. [Google Scholar] [CrossRef]


| Gene Name | Accession Number | Primer Name | Primer Sequence 5′ to 3′ | Tm (°C) | Product (bp) |
|---|---|---|---|---|---|
| 18S | XM_045748331 | F | TCGGCATGGCATGGTTAA | 58.6 | 203 |
| R | ACGGCAAGAGCCTTGGAT | 57.6 | |||
| CASP2 | XM_045739219 | F | CCCTTGGCATCTTTACCTTACA | 58.0 | 186 |
| R | AATACTAGGGAAGATCAGAGCAGG | 59.1 | |||
| NDUFA7 | XM_045751651 | F | TGCGTCAAGCAGACATTA | 56.2 | 174 |
| R | CAGATAACAGTTTGGTGGG | 56.2 | |||
| Lysozyme | XM_069326239 | F | GAGGATGTGGTCGTGGGTGA | 60.5 | 271 |
| R | ATTGGTCGTTCTAATGCCGC | 58.9 | |||
| CYTB | MN982313 | F | AAGTTGAAATAAGGGTGAAAGG | 55.3 | 190 |
| R | GGATTTGAGGTGGCTTCG | 55.8 |
| Index | Compounds | p-Value | Type |
|---|---|---|---|
| MW0141633 | 17-phenyl trinor PGF2 diethyl amide | 2.88 × 10−6 | down |
| MW0141691 | 1-Amino-1-deoxy-scyllo-inositol 4-phosphate | 9.81 × 10−6 | down |
| MW0015058 | (R)-Sulcatol | 2.72 × 10−5 | down |
| MW0112744 | Acetamidopropanal | 3.44 × 10−5 | up |
| MW0159233 | Val-Val-Asn-Trp-Asp | 1.19 × 10−4 | up |
| MW0015307 | 8-Deoxy-11,13-dihydroxygrosheimin | 2.13 × 10−4 | up |
| MW0002106 | 2,3′,4,6-Tetrahydroxybenzophenone | 2.18 × 10−4 | down |
| MW0145457 | Arg-Phe-Ala | 2.27 × 10−4 | up |
| FDATN00822 | Propacetamol hydrochloride | 2.63 × 10−4 | up |
| MW0052901 | Desferal-iron(III) | 2.71 × 10−4 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Ju, J.; Jiang, Q.; Zhao, H.; Zhang, Y.; Yang, H. Protective Effects of Astaxanthin on Thioacetamide-Induced Hepatopancreatic Damage in Procambarus clarkii: Insights from Biochemical, Histological, and Metabolomic Analyses. Animals 2025, 15, 1537. https://doi.org/10.3390/ani15111537
He J, Ju J, Jiang Q, Zhao H, Zhang Y, Yang H. Protective Effects of Astaxanthin on Thioacetamide-Induced Hepatopancreatic Damage in Procambarus clarkii: Insights from Biochemical, Histological, and Metabolomic Analyses. Animals. 2025; 15(11):1537. https://doi.org/10.3390/ani15111537
Chicago/Turabian StyleHe, Jiawen, Jian Ju, Qingliang Jiang, Haiyong Zhao, Yingying Zhang, and Hui Yang. 2025. "Protective Effects of Astaxanthin on Thioacetamide-Induced Hepatopancreatic Damage in Procambarus clarkii: Insights from Biochemical, Histological, and Metabolomic Analyses" Animals 15, no. 11: 1537. https://doi.org/10.3390/ani15111537
APA StyleHe, J., Ju, J., Jiang, Q., Zhao, H., Zhang, Y., & Yang, H. (2025). Protective Effects of Astaxanthin on Thioacetamide-Induced Hepatopancreatic Damage in Procambarus clarkii: Insights from Biochemical, Histological, and Metabolomic Analyses. Animals, 15(11), 1537. https://doi.org/10.3390/ani15111537





