The Beneficial Use of Artemisia annua, Artemisinin, and Other Compounds in Animal Health
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
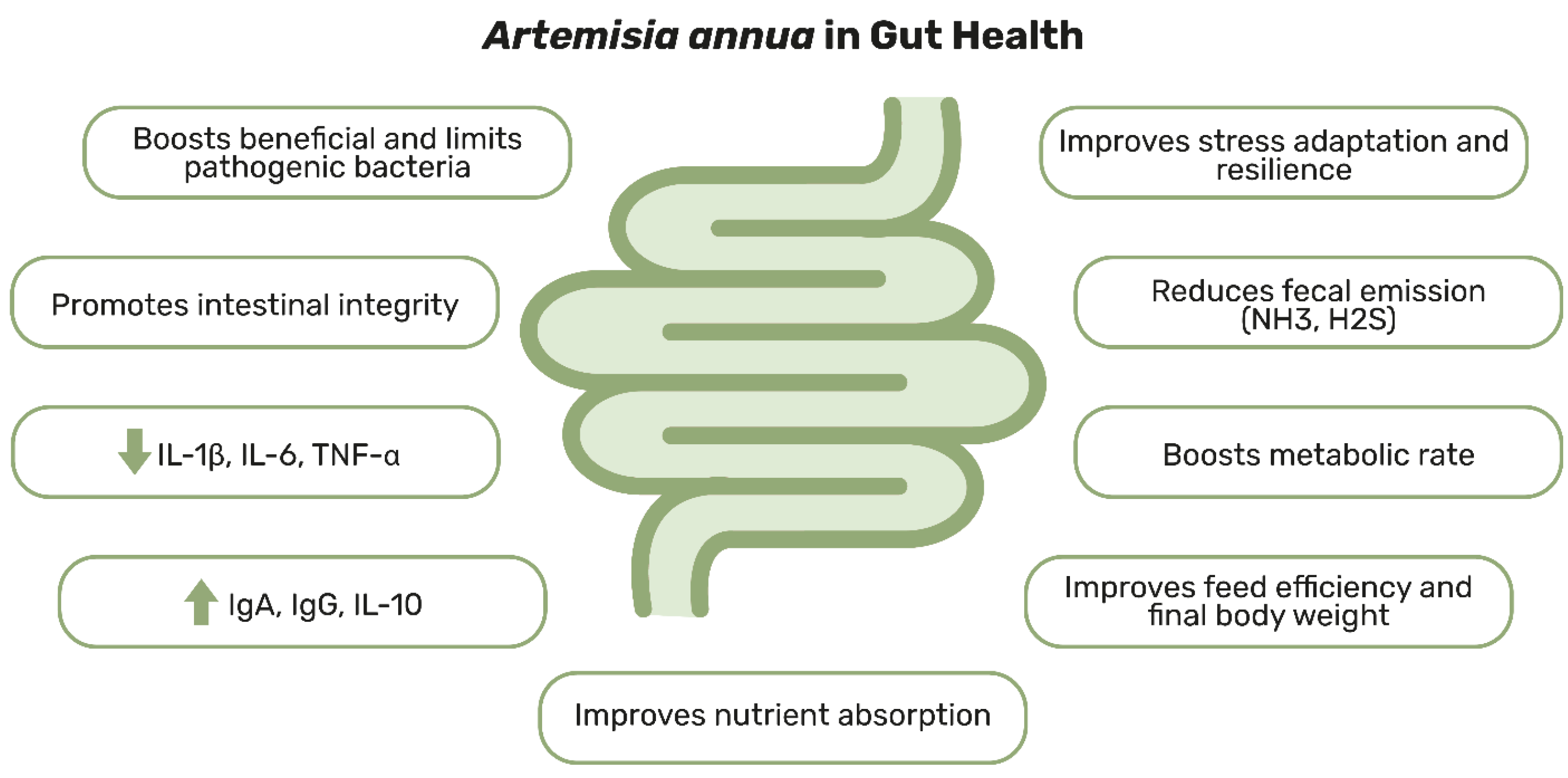
3. Artemisia annua and Its Role in Supporting Gut Health
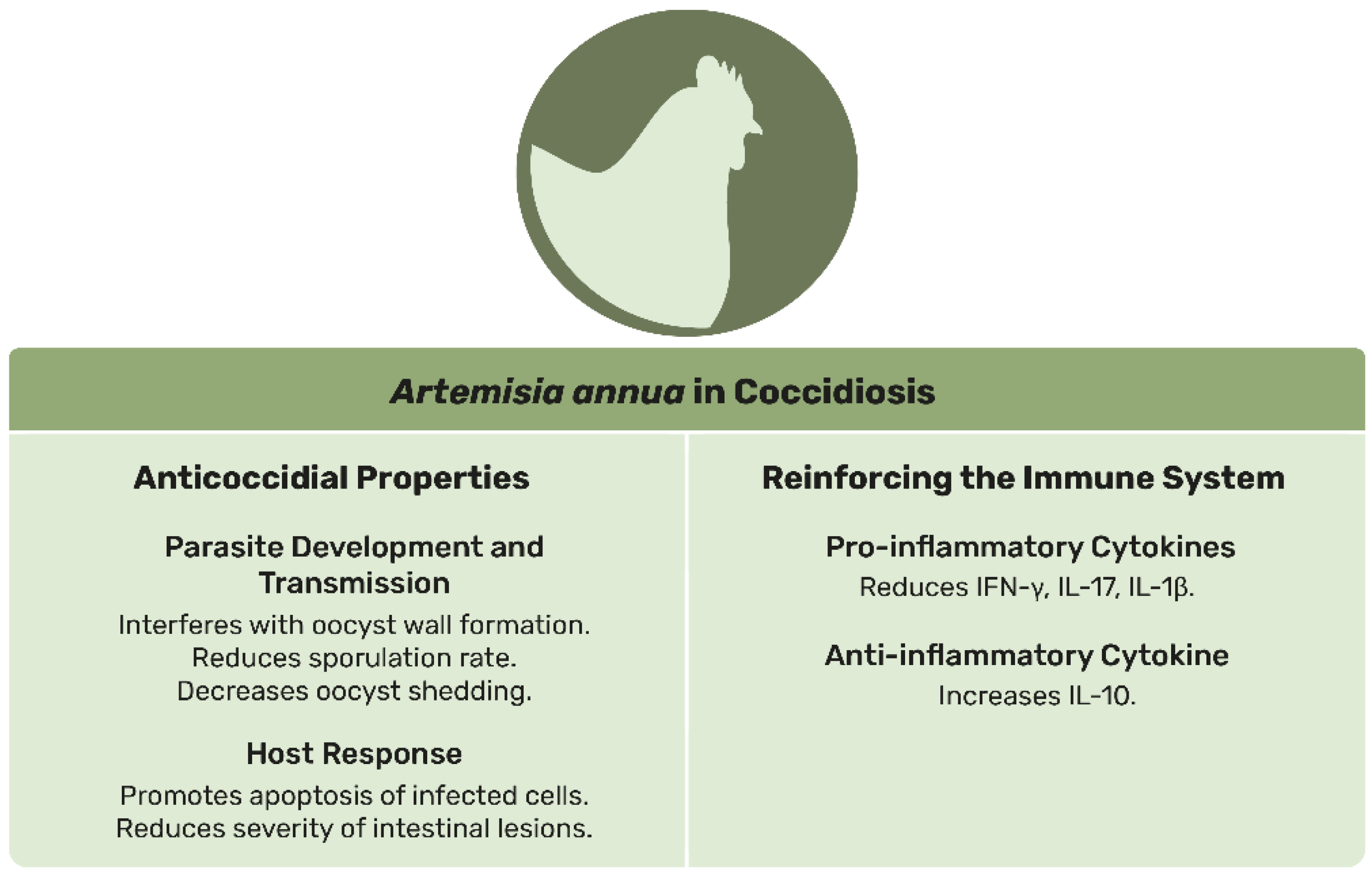
4. The Antiparasitic Potential of Artemisia annua
4.1. Coccidiosis
4.2. Leishmaniasis
4.3. Other Parasites
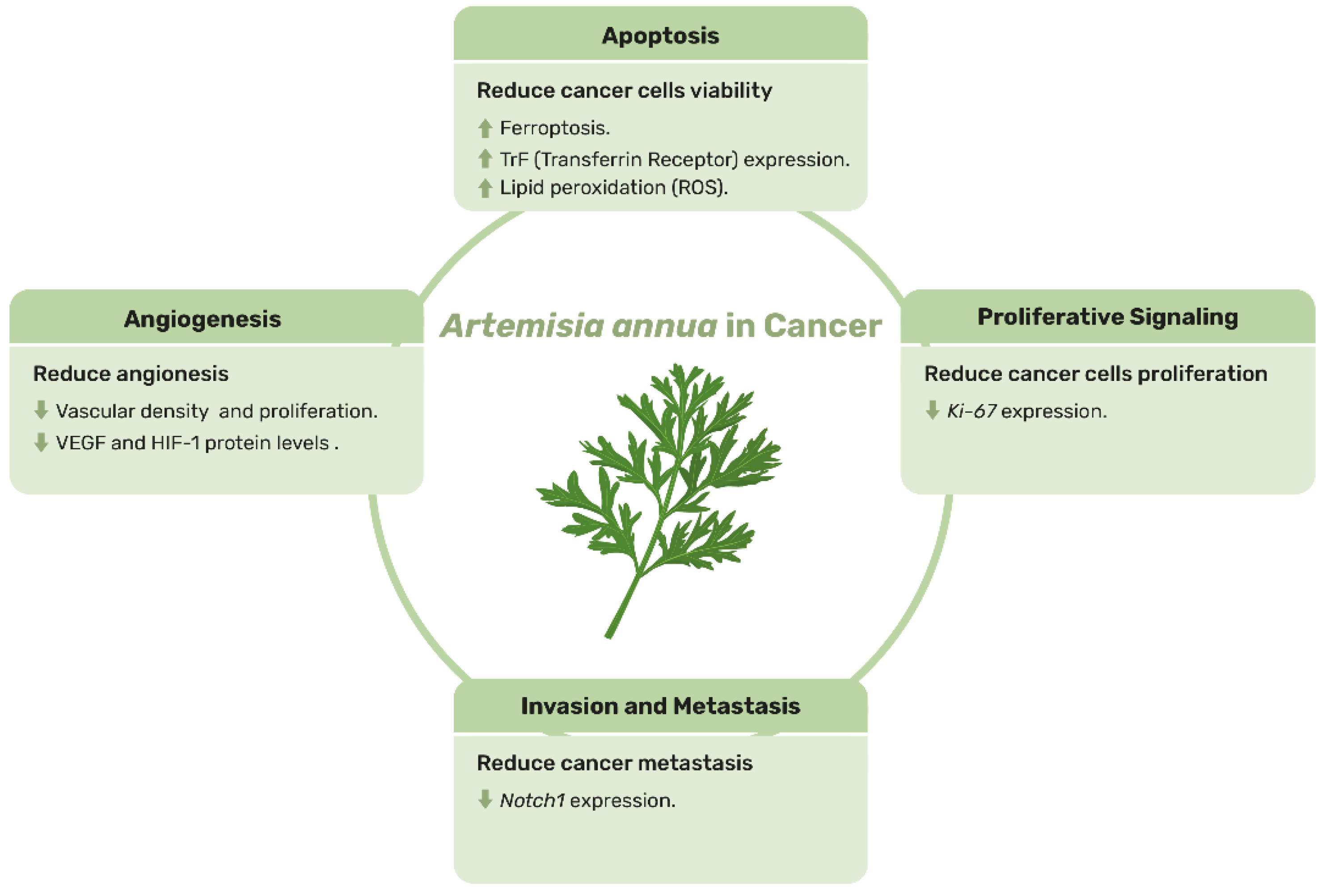
5. Artemisia annua: A Green Hope in the War on Cancer
6. Boosting the Effect: How Artemisinin Works in Synergy with Other Molecules
7. The Key to Quality: The Role of Standardization in Medicinal Plants Like Artemisia annua
8. Future Perspectives
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in Traditional Medicine and Current State of Knowledge on the Chemistry, Biological Activity and Possible Applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Hong Kong Baptist University; Zhao, Z. The Original Source of Modern Research on Chinese Medicinal Materials: Bencao Texts. Altern. Complement. Integr. Med. 2017, 3, 45. [Google Scholar] [CrossRef]
- Miller, L.H.; Su, X. Artemisinin: Discovery from the Chinese Herbal Garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-Z.; Miller, L.H. The Discovery of Artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef]
- Soni, R.; Shankar, G.; Mukhopadhyay, P.; Gupta, V. A Concise Review on Artemisia annua L.: A Major Source of Diverse Medicinal Compounds. Ind. Crops Prod. 2022, 184, 115072. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef]
- Pitt, S.J.; Gunn, A. The One Health Concept. Br. J. Biomed. Sci. 2024, 81, 12366. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Zhang, H.; Rehman, M.U.; Chang, Y.-F.; Zhaoxin, T. Editorial: The Potential Role of Gut Microbiome in Animal Gut-Linked Diseases. Front. Microbiol. 2023, 14, 1179481. [Google Scholar] [CrossRef]
- Fortun-Lamothe, L.; Boullier, S. A Review on the Interactions between Gut Microflora and Digestive Mucosal Immunity. Possible Ways to Improve the Health of Rabbits. Livest. Sci. 2007, 107, 1–18. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining Dysbiosis and Its Influence on Host Immunity and Disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Batool, A.; Arif, S. Healthy Cattle Microbiome and Dysbiosis in Diseased Phenotypes. Ruminants 2022, 2, 134–156. [Google Scholar] [CrossRef]
- Mondo, E.; Marliani, G.; Accorsi, P.A.; Cocchi, M.; Di Leone, A. Role of Gut Microbiota in Dog and Cat’s Health and Diseases. Open Vet. J. 2019, 9, 253. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Analysis of the Gut Microbiome in Dogs and Cats. Vet. Clin. Pathol. 2022, 50, 6–17. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van De Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Kobek-Kjeldager, C.; Schönherz, A.A.; Canibe, N.; Pedersen, L.J. Diet and Microbiota-Gut-Brain Axis in Relation to Tail Biting in Pigs: A Review. Appl. Anim. Behav. Sci. 2022, 246, 105514. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef] [PubMed]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and Prebiotics in Animal Feeding for Safe Food Production. Int. J. Food Microbiol. 2010, 141, S15–S28. [Google Scholar] [CrossRef]
- Bedford, M.R. Exogenous Enzymes in Monogastric Nutrition—Their Current Value and Future Benefits. Anim. Feed Sci. Technol. 2000, 86, 1–13. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R. The Role of Exogenous Enzymes in Promoting Growth and Improving Nutrient Digestibility in Poultry. Iran. J. Vet. Res. 2018, 19, 157–164. [Google Scholar]
- Meineri, G.; Martello, E.; Radice, E.; Bruni, N.; Saettone, V.; Atuahene, D.; Armandi, A.; Testa, G.; Ribaldone, D.G. Chronic Intestinal Disorders in Humans and Pets: Current Management and the Potential of Nutraceutical Antioxidants as Alternatives. Animals 2022, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Zeineldin, M.; Abdelmegeid, M.; Barakat, R.; Ghanem, M. A Review: Herbal Medicine as an Effective Therapeutic Approach for Treating Digestive Disorders in Small Ruminants. Alex. J. Vet. Sci. 2018, 56, 33. [Google Scholar] [CrossRef]
- Kuralkar, P.; Kuralkar, S.V. Role of Herbal Products in Animal Production—An Updated Review. J. Ethnopharmacol. 2021, 278, 114246. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Watanabe, E.; Kawashima, Y.; Plichta, D.R.; Wang, Z.; Ujike, M.; Ang, Q.Y.; Wu, R.; Furuichi, M.; Takeshita, K.; et al. Identification of Trypsin-Degrading Commensals in the Large Intestine. Nature 2022, 609, 582–589. [Google Scholar] [CrossRef]
- Tufail, M.A.; Schmitz, R.A. Exploring the Probiotic Potential of Bacteroides spp. Within One Health Paradigm. Probiotics Antimicrob. Proteins 2025, 17, 681–704. [Google Scholar] [CrossRef]
- Martín, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermúdez-Humarán, L.G.; Sokol, H.; Chatel, J.-M.; Langella, P. Faecalibacterium: A Bacterial Genus with Promising Human Health Applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef]
- Cui, Y.; Leng, X.; Zhao, Y.; Zhao, Y.; Wang, Q. Effects of Dietary Artemisia annua Supplementation on Growth Performance, Antioxidant Capacity, Immune Function, and Gut Microbiota of Geese. Poult. Sci. 2024, 103, 103594. [Google Scholar] [CrossRef] [PubMed]
- Panaite, T.; Criste, R.; Vlaicu, P.; Saracila, M.; Tabuc, C.; Olteanu, M.; Turcu, R.; Buleandră, M. Influence of Artemisia annua on Broiler Performance and Intestinal Microflora. Braz. J. Poult. Sci. 2019, 21, eRBCA-2019-1092. [Google Scholar] [CrossRef]
- El-Demerdash, A.S.; Mohamady, S.N.; Megahed, H.M.; Ali, N.M. Evaluation of Gene Expression Related to Immunity, Apoptosis, and Gut Integrity That Underlies Artemisia’s Therapeutic Effects in Necrotic Enteritis-Challenged Broilers. 3 Biotech 2023, 13, 181. [Google Scholar] [CrossRef]
- Engberg, R.M.; Grevsen, K.; Ivarsen, E.; Fretté, X.; Christensen, L.P.; Højberg, O.; Jensen, B.B.; Canibe, N. The Effect of Artemisia annua on Broiler Performance, on Intestinal Microbiota and on the Course of a Clostridium perfringens Infection Applying a Necrotic Enteritis Disease Model. Avian Pathol. 2012, 41, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Shi, B.; Xing, Y.; Xu, Y.; Jin, X.; Hong, L.; Zhang, S.; Qiao, M.; Yan, S. Artemisia annua L. Polysaccharide Improves the Growth Performance and Intestinal Barrier Function of Broilers Challenged with Escherichia Coli. Front. Microbiol. 2024, 15, 1390815. [Google Scholar] [CrossRef]
- Adil, S.; Magray, S.N. Impact and Manipulation of Gut Microflora in Poultry: A Review. J. Anim. Vet. Adv. 2012, 11, 873–877. [Google Scholar] [CrossRef]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Modulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets1–3. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Hu, J.; Bai, M.; Xing, Y.; Liu, J.; Xu, K.; Xiong, X.; Liu, H.; Yin, Y. Artemisia annua Residue Regulates Immunity, Antioxidant Ability, Intestinal Barrier Function, and Microbial Structure in Weaned Piglets. Animals 2024, 14, 3569. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Song, X.; Yang, M.; Wang, H.; Wu, Y. Feeding Dietary Fermentable Fiber Improved Fecal Microbial Composition and Increased Acetic Acid Production in a Nursery Pig Model. J. Anim. Sci. 2023, 101, skad260. [Google Scholar] [CrossRef]
- Hu, Y.X.; Van Baal, J.; Hendriks, W.H.; Duijster, M.; Van Krimpen, M.M.; Bikker, P. Mucosal Expression of Ca and P Transporters and Claudins in the Small Intestine of Broilers Is Altered by Dietary Ca:P in a Limestone Particle Size Dependent Manner. PLoS ONE 2022, 17, e0273852. [Google Scholar] [CrossRef]
- Kuo, W.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight Junction Proteins Occludin and ZO-1 as Regulators of Epithelial Proliferation and Survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, J.; Wang, Q.; Hu, D.; Zhao, Q.; Zhu, S.; Qiao, Y.; Zhao, F.; Wang, Z.; Wang, J.; et al. Comparative Efficacy of Plant Extracts and Probiotics on Growth and Gut Health in Chickens with Necrotic Enteritis. Animals 2024, 14, 3312. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, Y.; He, J.; Yun, Y.; Shi, Y.; Zhang, L.; Wang, T. Effect of Diet Supplemented with Enzymatically Treated Artemisia annua L. on Intestinal Digestive Function and Immunity in Weaned Pigs. Ital. J. Anim. Sci. 2020, 19, 1170–1179. [Google Scholar] [CrossRef]
- Gholamrezaie Sani, L.; Mohammadi, M.; Jalali Sendi, J.; Abolghasemi, S.A.; Roostaie Ali Mehr, M. Extract and Leaf Powder Effect of Artemisia annua on Performance, Cellular and Humoral Immunity in Broilers. Iran. J. Vet. Res. 2013, 14, 15–20. [Google Scholar] [CrossRef]
- Zarantonello, A.; Revel, M.; Grunenwald, A.; Roumenina, L.T. C3-dependent Effector Functions of Complement. Immunol. Rev. 2023, 313, 120–138. [Google Scholar] [CrossRef]
- Guo, S.; Ma, J.; Xing, Y.; Xu, Y.; Jin, X.; Yan, S.; Shi, L.; Zhang, L.; Shi, B. Effects of Artemisia annua L. Water Extract on Growth Performance and Intestinal Related Indicators in Broilers. J. Poult. Sci. 2023, 60, 2023024. [Google Scholar] [CrossRef]
- Saracila, M.; Criste, R.; Panaite, T.; Vlaicu, P.; Tabuc, C.; Turcu, R.; Olteanu, M. Artemisia annua as Phytogenic Feed Additive in the Diet of Broilers (14-35 Days) Reared under Heat Stress (32 °C). Braz. J. Poult. Sci. 2018, 20, 825–832. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, L.; Cui, C.; Zhao, H.; Zhang, Y.; Tian, Z.; Guan, W.; Chen, F. Maternal Supplementation with Artemisia annua L. Ameliorates Intestinal Inflammation via Inhibiting the TLR4/NF-κB and MAPK Pathways and Improves the Oxidative Stability of Offspring. Food Funct. 2022, 13, 9311–9323. [Google Scholar] [CrossRef]
- Xiong, L.; Zhang, W.; Zhao, H.; Tian, Z.; Ren, M.; Chen, F.; Guan, W.; Zhang, S. Dietary Supplementation of Enzymatically Treated Artemisia annua L. Improves Lactation Performance, Alleviates Inflammatory Response of Sows Reared Under Heat Stress, and Promotes Gut Development in Preweaning Offspring. Front. Vet. Sci. 2022, 9, 843673. [Google Scholar] [CrossRef]
- Gang, G.; Gao, R.; Zhao, H.; Xu, Y.; Xing, Y.; Jin, X.; Hong, L.; Yan, S.; Shi, B. Effects of Water Extracts of Artemisia annua L. on Rumen Immune and Antioxidative Indexes, Fermentation Parameters and Microbials Diversity in Lambs. Front. Microbiol. 2024, 15, 1485882. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xiong, A.; Wang, Y.; Pan, Y.; Zhang, Y.; Jiang, L.; Xiong, B. Effects of Artemisia annua L. Extract on Lactation Performance, Plasma Immune and Antioxidant Indexes of Dairy Cows. Chin. J. Anim. Nutr. 2021, 33, 3896–3903. [Google Scholar]
- Yu, S.; Xiong, A.; Pan, Y.; Wang, Y.; Zhang, Y.; Jiang, L.; Xiong, B. Effects of Artemisia annua L. Extracts on Rumen Fermentation Parameters and Microflora of Lactating Dairy Cows. Chin. J. Anim. Nutr. 2021, 33, 6431–6444. [Google Scholar]
- Tian, R.; Li, Y.; Wang, X.; Li, J.; Li, Y.; Bei, S.; Li, H. A Pharmacoinformatics Analysis of Artemisinin Targets and de Novo Design of Hits for Treating Ulcerative Colitis. Front. Pharmacol. 2022, 13, 843043. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; Chen, Z.; Ma, Z.; You, Q.; Zhang, X.; Zhou, T.; Xiao, Y.; Liang, Q.; Tan, H.; et al. Artemisinin Protects against Dextran Sulfate-Sodium-Induced Inflammatory Bowel Disease, Which Is Associated with Activation of the Pregnane X Receptor. Eur. J. Pharmacol. 2014, 738, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Hur, H.J.; Sung, M.J. The Effect of Artemisinin on Inflammation-Associated Lymphangiogenesis in Experimental Acute Colitis. Int. J. Mol. Sci. 2020, 21, 8068. [Google Scholar] [CrossRef]
- Huai, M.; Zeng, J.; Ge, W. Artemisinin Ameliorates Intestinal Inflammation by Skewing Macrophages to the M2 Phenotype and Inhibiting Epithelial–Mesenchymal Transition. Int. Immunopharmacol. 2021, 91, 107284. [Google Scholar] [CrossRef]
- Kavishe, R.A.; Koenderink, J.B.; Alifrangis, M. Oxidative Stress in Malaria and Artemisinin Combination Therapy: Pros and Cons. FEBS J. 2017, 284, 2579–2591. [Google Scholar] [CrossRef]
- Coccidiosis in Livestock, Poultry, Companion Animals, and Humans, 1st ed.; Dubey, J.P., Ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-29410-5. [Google Scholar]
- Fitzgerald, P.R. The Economic Impact of Coccidiosis in Domestic Animals. Adv. Vet. Sci. Comp. Med. 1980, 24, 121–143. [Google Scholar]
- Lee, Y.; Lu, M.; Lillehoj, H.S. Coccidiosis: Recent Progress in Host Immunity and Alternatives to Antibiotic Strategies. Vaccines 2022, 10, 215. [Google Scholar] [CrossRef]
- Long, P.L. Coccidiosis of Man and Domestic Animals, 1st ed.; Long, P.L., Ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-1-351-07074-4. [Google Scholar]
- Fayer, R. Epidemiology of Protozoan Infections: The Coccidia. Vet. Parasitol. 1980, 6, 75–103. [Google Scholar] [CrossRef]
- Burrell, A.; Tomley, F.M.; Vaughan, S.; Marugan-Hernandez, V. Life Cycle Stages, Specific Organelles and Invasion Mechanisms of Eimeria Species. Parasitology 2020, 147, 263–278. [Google Scholar] [CrossRef]
- Saif, Y.M. Diseases of Poultry, 12th ed.; Blackwell Publ: Ames, IA, USA, 2008; ISBN 978-0-8138-0718-8. [Google Scholar]
- Mesa-Pineda, C.; Navarro-Ruíz, J.L.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front. Vet. Sci. 2021, 8, 787653. [Google Scholar] [CrossRef]
- Daugschies, A.; Najdrowski, M. Eimeriosis in Cattle: Current Understanding. J. Vet. Med. Ser. B 2005, 52, 417–427. [Google Scholar] [CrossRef]
- Keeton, S.T.N.; Navarre, C.B. Coccidiosis in Large and Small Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chartier, C.; Paraud, C. Coccidiosis Due to Eimeria in Sheep and Goats, a Review. Small Rumin. Res. 2012, 103, 84–92. [Google Scholar] [CrossRef]
- Mohammed, N.H.; Alobaidii, W.A.; Hasan, M.H. COCCIDIOSIS IN SHEEP AND GOATS (REVIEW). Assiut Vet. Med. J. 2021, 67, 33–39. [Google Scholar] [CrossRef]
- Peek, H.W.; Landman, W.J.M. Coccidiosis in Poultry: Anticoccidial Products, Vaccines and Other Prevention Strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef]
- Usman, J.G.; Gadzama, U.N.; Kwaghe, A.V.; Madziga, H.A. Anticoccidial Resistance In Poultry: A Review. N. Y. Sci. J. 2011, 4, 102–109. [Google Scholar]
- Saeed, Z.; Alkheraije, K.A. Botanicals: A Promising Approach for Controlling Cecal Coccidiosis in Poultry. Front. Vet. Sci. 2023, 10, 1157633. [Google Scholar] [CrossRef]
- Millet, S.; Maertens, L. The European Ban on Antibiotic Growth Promoters in Animal Feed: From Challenges to Opportunities. Vet. J. 2011, 187, 143–144. [Google Scholar] [CrossRef]
- Sharma, M.K.; Liu, G.; Choppa, V.S.R.; Rafieian-Naeini, H.R.; Mahdavi, F.S.; Marshall, B.; Gogal, R.M.; Kim, W.K. Effects of Artemisia annua Supplementation on the Performance and Gut Health of Laying Hens Challenged with Mixed Eimeria Species. Front. Physiol. 2024, 15, 1381548. [Google Scholar] [CrossRef]
- Drăgan, L.; Györke, A.; Ferreira, J.F.S.; Pop, I.A.; Dunca, I.; Drăgan, M.; Mircean, V.; Dan, I.; Cozma, V. Effects of Artemisia annua and Foeniculum Vulgare on Chickens Highly Infected with Eimeria Tenella (Phylum Apicomplexa). Acta Vet. Scand. 2014, 56, 22. [Google Scholar] [CrossRef]
- Hady, M.M.; Zaki, M.M. Efficacy of Some Herbal Feed Additives on Performance and Control of Cecal Coccidiosis in Broilers. APCBEE Procedia 2012, 4, 163–168. [Google Scholar] [CrossRef]
- Allen, P.; Lydon, J.; Danforth, H. Effects of Components of Artemisia annua on Coccidia Infections in Chickens. Poult. Sci. 1997, 76, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, W.; Haq, S.U.; Guo, Z.; Cui, D.; Yang, F.; Cheng, F.; Wei, X.; Lv, J. Anticoccidial Activity of Qinghao Powder Against Eimeria Tenella in Broiler Chickens. Front. Vet. Sci. 2021, 8, 709046. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Saxena, V.K.; Singh, M.K. Genetic Analysis of Residual Feed Intake, Feed Conversion Ratio and Related Growth Parameters in Broiler Chicken: A Review. Worlds Poult. Sci. J. 2020, 76, 304–317. [Google Scholar] [CrossRef]
- Coroian, M.; Pop, L.M.; Popa, V.; Friss, Z.; Oprea, O.; Kalmár, Z.; Pintea, A.; Borșan, S.-D.; Mircean, V.; Lobonțiu, I.; et al. Efficacy of Artemisia annua against Coccidiosis in Broiler Chickens: A Field Trial. Microorganisms 2022, 10, 2277. [Google Scholar] [CrossRef]
- Almeida, G.F.D.; Horsted, K.; Thamsborg, S.M.; Kyvsgaard, N.C.; Ferreira, J.F.S.; Hermansen, J.E. Use of Artemisia annua as a Natural Coccidiostat in Free-Range Broilers and Its Effects on Infection Dynamics and Performance. Vet. Parasitol. 2012, 186, 178–187. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Cheng, S.; Liu, M.; Li, J.; Li, S.; Li, X.; Zhang, L.; Jian, F. Effect of Artemisia annua on Anticoccidial Action, Intestinal Microbiota and Metabolites of Hu Lambs. BMC Vet. Res. 2025, 21, 41. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Lu, S.; Yang, M.; Liu, M.; Li, J.; Li, S.; Jian, F. Effects of Fermented Artemisia annua on the Intestinal Microbiota and Metabolites of Hu Lambs with Naturally Infected with Eimeria spp. Front. Cell. Infect. Microbiol. 2025, 14, 1448516. [Google Scholar] [CrossRef] [PubMed]
- Abousekken, M.S.; Azazy, M.F.; El-Khtam, A.O.; Zaglool, W.K.S. Impact of Artemisia annua L. Supplementation On Growth Performance And Control Of Coccidiosis in Rabbits. J. Am. Sci. 2015, 11, 159–169. [Google Scholar]
- Varga, M. Rabbit Basic Science. In Textbook of Rabbit Medicine; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–108. ISBN 978-0-7020-4979-8. [Google Scholar]
- Del Cacho, E.; Gallego, M.; Francesch, M.; Quílez, J.; Sánchez-Acedo, C. Effect of Artemisinin on Oocyst Wall Formation and Sporulation during Eimeria Tenella Infection. Parasitol. Int. 2010, 59, 506–511. [Google Scholar] [CrossRef]
- Del Cacho, E.; Gallego, M.; López-Bernad, F.; Quílez, J.; Sánchez-Acedo, C. Expression of Anti-Apoptotic Factors in Cells Parasitized by Second-Generation Schizonts of Eimeria Tenella and Eimeria Necatrix. Vet. Parasitol. 2004, 125, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Mo, P.; Ma, Q.; Zhao, X.; Cheng, N.; Tao, J.; Li, J. Apoptotic Effects of Antimalarial Artemisinin on the Second Generation Merozoites of Eimeria Tenella and Parasitized Host Cells. Vet. Parasitol. 2014, 206, 297–303. [Google Scholar] [CrossRef]
- Jiao, J.; Yang, Y.; Liu, M.; Li, J.; Cui, Y.; Yin, S.; Tao, J. Artemisinin and Artemisia annua Leaves Alleviate Eimeria Tenella Infection by Facilitating Apoptosis of Host Cells and Suppressing Inflammatory Response. Vet. Parasitol. 2018, 254, 172–177. [Google Scholar] [CrossRef]
- Fatemi, A.; Razavi, S.M.; Asasi, K.; Torabi Goudarzi, M. Effects of Artemisia annua Extracts on Sporulation of Eimeria Oocysts. Parasitol. Res. 2015, 114, 1207–1211. [Google Scholar] [CrossRef]
- Pop, L.; Györke, A.; Tǎbǎran, A.F.; Dumitrache, M.O.; Kalmár, Z.; Magdaş, C.; Mircean, V.; Zagon, D.; Balea, A.; Cozma, V. Effects of Artemisinin in Broiler Chickens Challenged with Eimeria acervulina, E. maxima and E. tenella in Battery Trials. Vet. Parasitol. 2015, 214, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Brisibe, E.A.; Umoren, U.E.U.E.; Owai, P.U.O.; Brisibe, F. Dietary Inclusion of Dried Artemisia annua Leaves for Management of Coccidiosis and Growth Enhancement in Chickens. Afr. J. Biotechnol. 2008, 7, 4083–4092. [Google Scholar]
- Brisibe, E.A.; Umoren, U.E.; Brisibe, F.; Magalhäes, P.M.; Ferreira, J.F.S.; Luthria, D.; Wu, X.; Prior, R.L. Nutritional Characterisation and Antioxidant Capacity of Different Tissues of Artemisia annua L. Food Chem. 2009, 115, 1240–1246. [Google Scholar] [CrossRef]
- Vilas-Boas, D.F.; Nakasone, E.K.N.; Gonçalves, A.A.M.; Lair, D.F.; de Oliveira, D.S.; Pereira, D.F.S.; Silva, G.G.; Conrado, I.D.S.S.; Resende, L.A.; Zaldívar, M.F.; et al. Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease. Pathogens 2024, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Priolo, V.; Ippolito, D.; Rivas-Estanga, K.; De Waure, C.; Martínez-Orellana, P. Canine Leishmaniosis Global Prevalence over the Last Three Decades: A Meta-Analysis and Systematic Review. Comp. Immunol. Microbiol. Infect. Dis. 2024, 112, 102211. [Google Scholar] [CrossRef]
- LeishVet Practical Management of Canine Leishmaniosis. 2024. Available online: https://www.leishvet.org/publications/canine-leishmaniosis-guidelines/ (accessed on 27 March 2025).
- Ribeiro, R.R.; Michalick, M.S.M.; Da Silva, M.E.; Dos Santos, C.C.P.; Frézard, F.J.G.; Da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. BioMed Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Montserrat-Sangrà, S.; Ordeix, L.; Martínez-Orellana, P. Leishmania infantum-Specific Production of IFN-γ and IL-10 in Stimulated Blood from Dogs with Clinical Leishmaniosis. Parasit. Vectors 2016, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.-G.; Cardoso, L.; Baneth, G.; Bourdeau, P.; Koutinas, A.; Miró, G.; Oliva, G.; Solano-Gallego, L. LeishVet Update and Recommendations on Feline Leishmaniosis. Parasit. Vectors 2015, 8, 302. [Google Scholar] [CrossRef]
- Ahuir-Baraja, A.E.; Ruiz, M.P.; Garijo, M.M.; Llobat, L. Feline Leishmaniosis: An Emerging Public Health Problem. Vet. Sci. 2021, 8, 173. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Cardoso, L.; Pennisi, M.G.; Petersen, C.; Bourdeau, P.; Oliva, G.; Miró, G.; Ferrer, L.; Baneth, G. Diagnostic Challenges in the Era of Canine Leishmania Infantum Vaccines. Trends Parasitol. 2017, 33, 706–717. [Google Scholar] [CrossRef]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine Leishmaniasis: Update on Epidemiology, Diagnosis, Treatment, and Prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef]
- Baxarias, M.; Martínez-Orellana, P.; Baneth, G.; Solano-Gallego, L. Immunotherapy in Clinical Canine Leishmaniosis: A Comparative Update. Res. Vet. Sci. 2019, 125, 218–226. [Google Scholar] [CrossRef]
- Yasur-Landau, D.; Jaffe, C.L.; David, L.; Baneth, G. Allopurinol Resistance in Leishmania Infantum from Dogs with Disease Relapse. PLoS Negl. Trop. Dis. 2016, 10, e0004341. [Google Scholar] [CrossRef]
- Yasur-Landau, D.; Jaffe, C.L.; Doron-Faigenboim, A.; David, L.; Baneth, G. Induction of Allopurinol Resistance in Leishmania Infantum Isolated from Dogs. PLoS Negl. Trop. Dis. 2017, 11, e0005910. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R.; Yang, Y.Z.; Lima, V.; Kuypers, F.; Kamchonwongpaisan, S.; Yuthavong, Y. Iron-Dependent Free Radical Generation from the Antimalarial Agent Artemisinin (Qinghaosu). Antimicrob. Agents Chemother. 1993, 37, 1108–1114. [Google Scholar] [CrossRef]
- Krishna, S.; Uhlemann, A.; Haynes, R. Artemisinins: Mechanisms of Action and Potential for Resistance. Drug Resist. Updat. 2004, 7, 233–244. [Google Scholar] [CrossRef]
- Krauth-Siegel, R.L.; Comini, M.A. Redox Control in Trypanosomatids, Parasitic Protozoa with Trypanothione-Based Thiol Metabolism. Biochim. Biophys. Acta BBA—Gen. Subj. 2008, 1780, 1236–1248. [Google Scholar] [CrossRef]
- Sen, R.; Bandyopadhyay, S.; Dutta, A.; Mandal, G.; Ganguly, S.; Saha, P.; Chatterjee, M. Artemisinin Triggers Induction of Cell-Cycle Arrest and Apoptosis in Leishmania Donovani Promastigotes. J. Med. Microbiol. 2007, 56, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Ganguly, S.; Saha, P.; Chatterjee, M. Efficacy of Artemisinin in Experimental Visceral Leishmaniasis. Int. J. Antimicrob. Agents 2010, 36, 43–49. [Google Scholar] [CrossRef]
- Ghaffarifar, F.; Esavand Heydari, F.; Dalimi, A.; Hassan, Z.M.; Delavari, M.; Mikaeiloo, H. Evaluation of Apoptotic and Antileishmanial Activities of Artemisinin on Promastigotes and BALB/C Mice Infected with Leishmania Major. Iran. J. Parasitol. 2015, 10, 258–267. [Google Scholar] [PubMed]
- De Sarkar, S.; Sarkar, D.; Sarkar, A.; Dighal, A.; Chakrabarti, S.; Staniek, K.; Gille, L.; Chatterjee, M. The Leishmanicidal Activity of Artemisinin Is Mediated by Cleavage of the Endoperoxide Bridge and Mitochondrial Dysfunction. Parasitology 2019, 146, 511–520. [Google Scholar] [CrossRef]
- Dighal, A.; De Sarkar, S.; Gille, L.; Chatterjee, M. Can the Iron Content of Culture Media Impact on the Leishmanicidal Effect of Artemisinin? Free Radic. Res. 2021, 55, 282–295. [Google Scholar] [CrossRef]
- Yang, D.M.; Liew, F.Y. Effects of Qinghaosu (Artemisinin) and Its Derivatives on Experimental Cutaneous Leishmaniasis. Parasitology 1993, 106, 7–11. [Google Scholar] [CrossRef]
- Neamah, S.D.; Ali, H.Z.; Al-Halbosiy, M.M.F. Detection of Artemisinin Effect on Macrophage Inducible Nitric Oxide Gene Expression in Macrophage Infected with Leishmania Donovani. Ann. Parasitol. 2022, 68, 331–338. [Google Scholar] [CrossRef]
- Islamuddin, M.; Chouhan, G.; Tyagi, M.; Abdin, M.Z.; Sahal, D.; Afrin, F. Leishmanicidal Activities of Artemisia annua Leaf Essential Oil against Visceral Leishmaniasis. Front. Microbiol. 2014, 5, 626. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.T.; De Andrade, G.F.; Araújo, A.D.S.; Almeida, A.D.C.; Coimbra, E.S.; De Souza, M.V.N. In Vitro Assessment of Camphor Hydrazone Derivatives as an Agent Against Leishmania Amazonensis. Acta Parasitol. 2020, 65, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Islamuddin, M.; Chouhan, G.; Farooque, A.; Dwarakanath, B.S.; Sahal, D.; Afrin, F. Th1-Biased Immunomodulation and Therapeutic Potential of Artemisia annua in Murine Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e3321. [Google Scholar] [CrossRef]
- Soares, D.C.; Portella, N.A.; Ramos, M.F.D.S.; Siani, A.C.; Saraiva, E.M. Trans-β-Caryophyllene: An Effective Antileishmanial Compound Found in Commercial Copaiba Oil (Copaifera spp.). Evid. Based Complement. Altern. Med. 2013, 2013, 761323. [Google Scholar] [CrossRef] [PubMed]
- Hopke, K.; Meyers, A.; Auckland, L.; Hamer, S.; Florin, D.; Diesel, A.; Patterson, A. Leishmania Mexicana in a Central Texas Cat: Clinical Presentation, Molecular Identification, Sandfly Vector Collection and Novel Management. J. Feline Med. Surg. Open Rep. 2021, 7, 2055116921999595. [Google Scholar] [CrossRef]
- Tejada, R. Tratamiento de Leishmaniosis Canina Con Extracto de Artemisia annua. Argos Inf. Vet. 2019, 213, 40–41. [Google Scholar]
- De Oliveira, T.C.; Silva, D.A.O.; Rostkowska, C.; Béla, S.R.; Ferro, E.A.V.; Magalhães, P.M.; Mineo, J.R. Toxoplasma Gondii: Effects of Artemisia annua L. on Susceptibility to Infection in Experimental Models in Vitro and in Vivo. Exp. Parasitol. 2009, 122, 233–241. [Google Scholar] [CrossRef]
- Jones-Brando, L.; D’Angelo, J.; Posner, G.H.; Yolken, R. In Vitro Inhibition of Toxoplasma Gondii by Four New Derivatives of Artemisinin. Antimicrob. Agents Chemother. 2006, 50, 4206–4208. [Google Scholar] [CrossRef]
- Nagamune, K.; Beatty, W.L.; Sibley, L.D. Artemisinin Induces Calcium-Dependent Protein Secretion in the Protozoan Parasite Toxoplasma Gondii. Eukaryot. Cell 2007, 6, 2147–2156. [Google Scholar] [CrossRef]
- D’Angelo, J.G.; Bordón, C.; Posner, G.H.; Yolken, R.; Jones-Brando, L. Artemisinin Derivatives Inhibit Toxoplasma Gondii in Vitro at Multiple Steps in the Lytic Cycle. J. Antimicrob. Chemother. 2009, 63, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Berrizbeitia De Morgado, M.; Cariaco Sifontes, Y.; Imery Buiza, J.; Lutgen, P. Actividad de infusiones de Artemisia annua sobre epimastigotes de Trypanosoma cruzi. Enfermedades Infecc. Microbiol. Clínica 2017, 35, 390–392. [Google Scholar] [CrossRef]
- Mishina, Y.V.; Krishna, S.; Haynes, R.K.; Meade, J.C. Artemisinins Inhibit Trypanosoma Cruzi and Trypanosoma Brucei Rhodesiense In Vitro Growth. Antimicrob. Agents Chemother. 2007, 51, 1852–1854. [Google Scholar] [CrossRef] [PubMed]
- Weerarathne, P.; Reichard, M.; Miller, C.; Scimeca, R.C. The Establishment of a Novel In Vitro System for Culturing Cytauxzoon Felis. Pathogens 2024, 13, 565. [Google Scholar] [CrossRef]
- Kim, J.-T.; Park, J.-Y.; Seo, H.-S.; Oh, H.-G.; Noh, J.-W.; Kim, J.-H.; Kim, D.-Y.; Youn, H.-J. In Vitro Antiprotozoal Effects of Artemisinin on Neospora Caninum. Vet. Parasitol. 2002, 103, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Enkai, S.; Kouguchi, H.; Inaoka, D.K.; Irie, T.; Yagi, K.; Kita, K. Effect of the Anti-Parasitic Compounds Pyrvinium Pamoate and Artemisinin in Enzymatic and Culture Assays: Data on the Search for New Anti-Echinococcal Drugs. Data Brief 2021, 34, 106629. [Google Scholar] [CrossRef]
- Allam, A.F.; Mostafa, R.A.; Lotfy, W.; Farag, H.F.; Fathi, N.; Moneer, E.A.; Shehab, A.Y. Therapeutic Efficacy of Mebendazole and Artemisinin in Different Phases of Trichinellosis: A Comparative Experimental Study. Parasitology 2021, 148, 630–635. [Google Scholar] [CrossRef]
- Pinello, K.; Amorim, I.; Pires, I.; Canadas-Sousa, A.; Catarino, J.; Faísca, P.; Branco, S.; Peleteiro, M.C.; Silva, D.; Severo, M.; et al. Vet-OncoNet: Malignancy Analysis of Neoplasms in Dogs and Cats. Vet. Sci. 2022, 9, 535. [Google Scholar] [CrossRef]
- Pinello, K.; Pires, I.; Castro, A.F.; Carvalho, P.T.; Santos, A.; De Matos, A.; Queiroga, F.; Canadas-Sousa, A.; Dias-Pereira, P.; Catarino, J.; et al. Cross Species Analysis and Comparison of Tumors in Dogs and Cats, by Age, Sex, Topography and Main Morphologies. Data from Vet-OncoNet. Vet. Sci. 2022, 9, 167. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the Hallmarks of Cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Sarver, A.L.; Makielski, K.M.; DePauw, T.A.; Schulte, A.J.; Modiano, J.F. Increased Risk of Cancer in Dogs and Humans: A Consequence of Recent Extension of Lifespan beyond Evolutionarily-Determined Limitations? Aging Cancer 2022, 3, 3–19. [Google Scholar] [CrossRef]
- Dong, J.; Chen, Y.; Yang, W.; Zhang, X.; Li, L. Antitumor and Anti-Angiogenic Effects of Artemisinin on Breast Tumor Xenografts in Nude Mice. Res. Vet. Sci. 2020, 129, 66–69. [Google Scholar] [CrossRef]
- Jung, E.J.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.-M.; Hong, S.C.; Chung, K.H.; Kim, C.W.; Lee, W.S. Anticancer Effect by Combined Treatment of Artemisia annua L. Polyphenols and Docetaxel in DU145 Prostate Cancer Cells and HCT116 Colorectal Cancer Cells. Curr. Issues Mol. Biol. 2024, 46, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Botrous, S.; Elmaghraby, A.; Achy, S.E.; Mustafa, Y.; Abdel-Rahman, S. Artemisinin Pre-Treatment Fore Cisplatin Dosage Enhances High Grade Urothelial Carcinoma Treatment in Male Albino Mice via Reverse Gene Expression Modulation of FGFR3, HRAS, P53 and KDM6A. BMC Cancer 2024, 24, 971. [Google Scholar] [CrossRef]
- Wen, L.; Chan, B.C.-L.; Qiu, M.-H.; Leung, P.-C.; Wong, C.-K. Artemisinin and Its Derivatives as Potential Anticancer Agents. Molecules 2024, 29, 3886. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Goslinski, T. Searching for the Holy Grail − Highly Potent Bridged Endoperoxides for Targeted Cancer Therapy. Bioorganic Chem. 2024, 153, 107893. [Google Scholar] [CrossRef]
- Waseem, Y.; Hasan, C.A.; Ahmed, F. Artemisinin: A Promising Adjunct for Cancer Therapy. Cureus 2018, 10, e3628. [Google Scholar] [CrossRef]
- Konstat-Korzenny, E.; Ascencio-Aragón, J.; Niezen-Lugo, S.; Vázquez-López, R. Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer. Med. Sci. 2018, 6, 19. [Google Scholar] [CrossRef]
- Gong, R.-H.; Yang, D.-J.; Kwan, H.-Y.; Lyu, A.-P.; Chen, G.-Q.; Bian, Z.-X. Cell Death Mechanisms Induced by Synergistic Effects of Halofuginone and Artemisinin in Colorectal Cancer Cells. Int. J. Med. Sci. 2022, 19, 175–185. [Google Scholar] [CrossRef]
- Zhelyazkova, M.Y.; Hristova-Avakumova, N.G.; Momekov, G.T. Antitumor Activity of the Combination of Artemisinin and Epirubicin in Human Leukemia Cells. Folia Med. 2021, 63, 488–495. [Google Scholar] [CrossRef]
- Isani, G.; Bertocchi, M.; Andreani, G.; Farruggia, G.; Cappadone, C.; Salaroli, R.; Forni, M.; Bernardini, C. Cytotoxic Effects of Artemisia annua L. and Pure Artemisinin on the D-17 Canine Osteosarcoma Cell Line. Oxid. Med. Cell. Longev. 2019, 2019, 1615758. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Breuer, E.; Hegazy, M.; Efferth, T. Retrospective Study of Small Pet Tumors Treated with Artemisia annua and Iron. Int. J. Oncol. 2019, 56, 123–138. [Google Scholar] [CrossRef]
- Salaroli, R.; Andreani, G.; Bernardini, C.; Zannoni, A.; La Mantia, D.; Protti, M.; Forni, M.; Mercolini, L.; Isani, G. Anticancer Activity of an Artemisia annua L. Hydroalcoholic Extract on Canine Osteosarcoma Cell Lines. Res. Vet. Sci. 2022, 152, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zou, T.; Tuo, Q.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and Links with Diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Q.; Huo, C.; Li, Y.; He, L.; Ran, B.; Chen, J.; Li, Y.; Liu, W. Ferroptosis: A Novel Mechanism of Artemisinin and Its Derivatives in Cancer Therapy. Curr. Med. Chem. 2021, 28, 329–345. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Ren, M.; Wang, Z. Ferroptosis: A New Research Direction of Artemisinin and Its Derivatives in Anti-Cancer Treatment. Am. J. Chin. Med. 2024, 52, 161–181. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, N.; Yang, T.; Yan, J.; Wang, W.; Li, X. The Potential Mechanisms by Which Artemisinin and Its Derivatives Induce Ferroptosis in the Treatment of Cancer. Oxid. Med. Cell. Longev. 2022, 2022, 1458143. [Google Scholar] [CrossRef]
- Breuer, E.; Efferth, T. Treatment of Iron-Loaded Veterinary Sarcoma by Artemisia annua. Nat. Prod. Bioprospecting 2014, 4, 113–118. [Google Scholar] [CrossRef]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a Tool to Improve the Profiles of High-Value Secondary Metabolites and Pharmacological Properties of Hypericum Perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Dulloo, A.; Seydoux, J.; Girardier, L.; Chantre, P.; Vandermander, J. Green Tea and Thermogenesis: Interactions between Catechin-Polyphenols, Caffeine and Sympathetic Activity. Int. J. Obes. 2000, 24, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.-X.; Li, J.-X.; Fang, X.; Wang, L.-J.; Hu, W.-L.; Chen, X.-Y.; Yang, C.-Q. Isolation and Characterization of Three New Monoterpene Synthases from Artemisia annua. Front. Plant Sci. 2016, 7, 638. [Google Scholar] [CrossRef]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential Oil of Artemisia annua L.: An Extraordinary Component with Numerous Antimicrobial Properties. Evid. Based Complement. Altern. Med. 2014, 2014, 159819. [Google Scholar] [CrossRef]
- Juteau, F.; Masotti, V.; Bessière, J.M.; Dherbomez, M.; Viano, J. Antibacterial and Antioxidant Activities of Artemisia annua Essential Oil. Fitoterapia 2002, 73, 532–535. [Google Scholar] [CrossRef]
- Tzenkova, R.; Kamenarska, Z.; Draganov, A.; Atanassov, A. Composition of Artemisia annua Essential Oil Obtained from Species Growing Wild in Bulgaria. Biotechnol. Biotechnol. Equip. 2010, 24, 1833–1835. [Google Scholar] [CrossRef]
- Guo, W.; Wang, W.; Lei, F.; Zheng, R.; Zhao, X.; Gu, Y.; Yang, M.; Tong, Y.; Wang, Y. Identifying the Main Components and Mechanisms of Action of Artemisia annua L. in the Treatment of Endometrial Cancer Using Network Pharmacology. ACS Omega 2024, 9, 8055–8066. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhou, H.; Du, Z. Anticancer Effects of Natural Phytochemicals in Anaplastic Thyroid Cancer (Review). Oncol. Rep. 2022, 48, 156. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical Composition of Artemisia annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, M.R.; Mittleman, A.; Weathers, P.J. Dried Leaf Artemisia annua Improves Bioavailability of Artemisinin via Cytochrome P450 Inhibition and Enhances Artemisinin Efficacy Downstream. Biomolecules 2020, 10, 254. [Google Scholar] [CrossRef]
- Weathers, P.J. Artemisinin as a Therapeutic vs. Its More Complex Artemisia Source Material. Nat. Prod. Rep. 2023, 40, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Reddy, N.; Khoo, C.S.; Koyyalamudi, S.R. Structural Characterization and In-Vitro Antioxidant and Immunomodulatory Activities of Polysaccharide Fractions Isolated from Artemisia annua L. Molecules 2022, 27, 3643. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Shinyuy, L.M.; Loe, G.E.; Jansen, O.; Mamede, L.; Ledoux, A.; Noukimi, S.F.; Abenwie, S.N.; Ghogomu, S.M.; Souopgui, J.; Robert, A.; et al. Secondary Metabolites Isolated from Artemisia Afra and Artemisia annua and Their Anti-Malarial, Anti-Inflammatory and Immunomodulating Properties—Pharmacokinetics and Pharmacodynamics: A Review. Metabolites 2023, 13, 613. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Gong, M.; Wang, M. Combination of Artemisinin-based Natural Compounds from Artemisia annua L. for the Treatment of Malaria: Pharmacodynamic and Pharmacokinetic Studies. Phytother. Res. 2018, 32, 1415–1420. [Google Scholar] [CrossRef]
- Xiong, H.-H.; Lin, S.-Y.; Chen, L.-L.; Ouyang, K.-H.; Wang, W.-J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef]
- Kondža, M.; Brizić, I.; Jokić, S. Flavonoids as CYP3A4 Inhibitors In Vitro. Biomedicines 2024, 12, 644. [Google Scholar] [CrossRef]
- Miron, A.; Aprotosoaie, A.C.; Trifan, A.; Xiao, J. Flavonoids as Modulators of Metabolic Enzymes and Drug Transporters. Ann. N. Y. Acad. Sci. 2017, 1398, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Rassias, D.J.; Weathers, P.J. Dried Leaf Artemisia annua Efficacy against Non-Small Cell Lung Cancer. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 52, 247–253. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Tian, F.; Zhang, S.-Q.; Jin, H. Advances in Pharmacokinetic Mechanisms of Transporter-Mediated Herb-Drug Interactions. Pharmaceuticals 2022, 15, 1126. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.; Feng, D.; Qin, H.; Wen, H.; Yin, Z.; Gao, G.; Li, C. Protective Effect of Quercetin against Oxidative Stress and Brain Edema in an Experimental Rat Model of Subarachnoid Hemorrhage. Int. J. Med. Sci. 2014, 11, 282–290. [Google Scholar] [CrossRef]
- Umamaheswari, D.; Muthuraja, R.; Kumar, M.; Venkateswarlu, B.S. Standardization of Herbal Drugs—A Overview. Int. J. Pharm. Sci. Rev. Res. 2021, 68, 213–219. [Google Scholar] [CrossRef]
- Balekundri, A.; Mannur, V. Quality Control of the Traditional Herbs and Herbal Products: A Review. Future J. Pharm. Sci. 2020, 6, 67. [Google Scholar] [CrossRef]
- Sachan, A.K.; Vishnoi, G.; Kumar, R. Need of Standardization of Herbal Medicines in Modern Era. Int. J. Phytomedicine 2016, 8, 300. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.A.; Asif, M.; Akhtar, M.; Saud, I. Physicochemical and Phytochemical Standardization of Berries of Myrtus Communis Linn. J. Pharm. Bioallied Sci. 2012, 4, 322–326. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, B.-E. A Broad Review of Commercially Important Southern African Medicinal Plants. J. Ethnopharmacol. 2008, 119, 342–355. [Google Scholar] [CrossRef]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of Herbal Medicines—A Review. Int. J. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Tandon, N.; Yadav, S.S. Contributions of Indian Council of Medical Research (ICMR) in the Area of Medicinal Plants/Traditional Medicine. J. Ethnopharmacol. 2017, 197, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Guidelines for the Treatment of Malaria; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Herrmann, S.; Jessing, K.K.; Jørgensen, N.O.G.; Cedergreen, N.; Kandeler, E.; Strobel, B.W. Distribution and Ecological Impact of Artemisinin Derived from Artemisia annua L. in an Agricultural Ecosystem. Soil Biol. Biochem. 2013, 57, 164–172. [Google Scholar] [CrossRef]
- Anyinkeng, N.; Tambe Bechem, E.E.; Bizama, F.M. Evaluation of the Artemisinin Content of Artemisia annua L. Grown in Different Agro Ecological Zones of Cameroon. World J. Adv. Res. Rev. 2023, 20, 681–689. [Google Scholar] [CrossRef]
- Numonov, S.; Sharopov, F.; Salimov, A.; Sukhrobov, P.; Atolikshoeva, S.; Safarzoda, R.; Habasi, M.; Aisa, H.A. Assessment of Artemisinin Contents in Selected Artemisia Species from Tajikistan (Central Asia). Medicines 2019, 6, 23. [Google Scholar] [CrossRef]
- Pulice, G.; Pelaz, S.; Matías-Hernández, L. Molecular Farming in Artemisia annua, a Promising Approach to Improve Anti-Malarial Drug Production. Front. Plant Sci. 2016, 7, 329. [Google Scholar] [CrossRef]
- Delabays, N.; Simonnet, X.; Gaudin, M. The Genetics of Artemisinin Content in Artemisia annua L. and the Breeding of High Yielding Cultivars. Curr. Med. Chem. 2001, 8, 1795–1801. [Google Scholar] [CrossRef]
- Berman, A.R.; Birkenheuer, A.J.; Sorah, E.L.; Papich, M.G. Analysis of US Marketed Artemisinin Supplements for Use in Dogs. J. Vet. Pharmacol. Ther. 2025, 48, 56–60. [Google Scholar] [CrossRef]
- Radulović, N.S.; Randjelović, P.J.; Stojanović, N.M.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Ilić, I.R.; Djordjević, V.B. Toxic Essential Oils. Part II: Chemical, Toxicological, Pharmacological and Microbiological Profiles of Artemisia annua L. Volatiles. Food Chem. Toxicol. 2013, 58, 37–49. [Google Scholar] [CrossRef]
- Dnyandeo Chepte, S. Phytochemical Analysis and Acute Toxicity Studies of Artemisia annua in Swiss Albino Mice. J. Pharmacogn. Phytochem. 2018, 7, 1893–1895. [Google Scholar]
- World Health Organization. Artemisinin Derivatives: Summary of Nonclinical Safety Data; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Hien, T.T. An Overview of the Clinical Use of Artemisinin and Its Derivatives in the Treatment of Falciparum Malaria in Viet Nam. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.R.; Charles, D.J.; Simon, J.E. Seasonal Accumulation of Artemisinin of Artemisia annua L. Acta Hortic. 1993, 344, 416–420. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Use of Non-Pharmaceutical Forms of Artemisia; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and Pharmacological Activity of the Genus Artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Kshirsagar, S.G.; Rao, R.V. Antiviral and Immunomodulation Effects of Artemisia. Med. Kaunas Lith. 2021, 57, 217. [Google Scholar] [CrossRef] [PubMed]
- Baggieri, M.; Gioacchini, S.; Borgonovo, G.; Catinella, G.; Marchi, A.; Picone, P.; Vasto, S.; Fioravanti, R.; Bucci, P.; Kojouri, M.; et al. Antiviral, Virucidal and Antioxidant Properties of Artemisia annua against SARS-CoV-2. Biomed. Pharmacother. 2023, 168, 115682. [Google Scholar] [CrossRef]
- Khatoon, N.; Sharma, Y.; Sardar, M.; Manzoor, N. Mode of Action and Anti-Candida Activity of Artemisia annua Mediated-Synthesized Silver Nanoparticles. J. Mycol. Médicale 2019, 29, 201–209. [Google Scholar] [CrossRef]
- Zhu, C.; Liao, B.; Ye, X.; Zhou, Y.; Chen, X.; Liao, M.; Cheng, L.; Zhou, X.; Ren, B. Artemisinin Elevates Ergosterol Levels of Candida Albicans to Synergise with Amphotericin B against Oral Candidiasis. Int. J. Antimicrob. Agents 2021, 58, 106394. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Nanoparticles Formulations of Artemisinin and Derivatives as Potential Therapeutics for the Treatment of Cancer, Leishmaniasis and Malaria. Pharmaceutics 2020, 12, 748. [Google Scholar] [CrossRef]
- Want, M.Y.; Islamuddin, M.; Chouhan, G.; Ozbak, H.A.; Hemeg, H.A.; Dasgupta, A.K.; Chattopadhyay, A.P.; Afrin, F. Therapeutic Efficacy of Artemisinin-Loaded Nanoparticles in Experimental Visceral Leishmaniasis. Colloids Surf. B Biointerfaces 2015, 130, 215–221. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, P.; Li, Y. Biomineralized Apoferritin Nanoparticles Delivering Dihydroartemisinin and Calcium for Synergistic Breast Cancer Therapy. Sci. Rep. 2024, 14, 29402. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morua, E.; Cuyas, L.; Matías-Hernández, L. The Beneficial Use of Artemisia annua, Artemisinin, and Other Compounds in Animal Health. Animals 2025, 15, 1359. https://doi.org/10.3390/ani15101359
Morua E, Cuyas L, Matías-Hernández L. The Beneficial Use of Artemisia annua, Artemisinin, and Other Compounds in Animal Health. Animals. 2025; 15(10):1359. https://doi.org/10.3390/ani15101359
Chicago/Turabian StyleMorua, Estefania, Laura Cuyas, and Luis Matías-Hernández. 2025. "The Beneficial Use of Artemisia annua, Artemisinin, and Other Compounds in Animal Health" Animals 15, no. 10: 1359. https://doi.org/10.3390/ani15101359
APA StyleMorua, E., Cuyas, L., & Matías-Hernández, L. (2025). The Beneficial Use of Artemisia annua, Artemisinin, and Other Compounds in Animal Health. Animals, 15(10), 1359. https://doi.org/10.3390/ani15101359






