In Vitro Evaluation of Enzymatically Transformed Alfalfa Saponins on Methane Reduction, Rumen Microbes and Metabolomics in Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Antioxidant Activity of AS
2.1.1. Silage Preparation
2.1.2. Chemical Analysis
2.1.3. Saponin Extraction
2.1.4. Antioxidant Activities
2.2. Enzymatic Biotransformation and Determination
2.3. In Vitro Rumen Fermentation of Goat
2.3.1. Rumen Fluid Collection and Processing
2.3.2. In Vitro Incubation
2.3.3. Fermentation Parameters
2.3.4. Quantitative Real-Time Polymerase Chain Reaction (qPCR) of Ruminal Bacteria
2.3.5. Untargeted Metabolomics Analysis
2.4. Statistical Analysis
3. Results
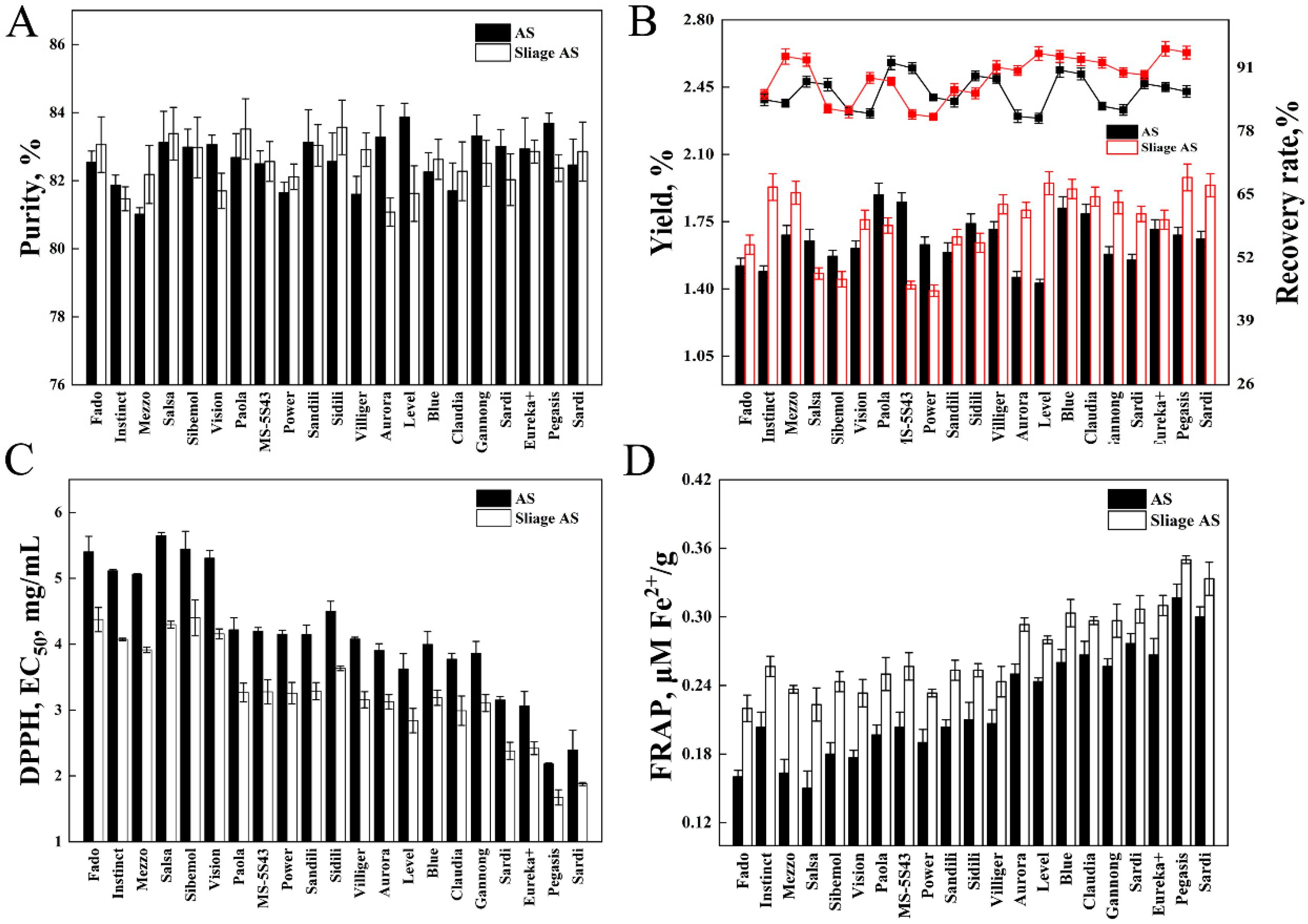
3.1. Chemical Composition of Fresh and Ensilaged Alfalfa
3.2. Antioxidant Activity of Fresh and Silage AS
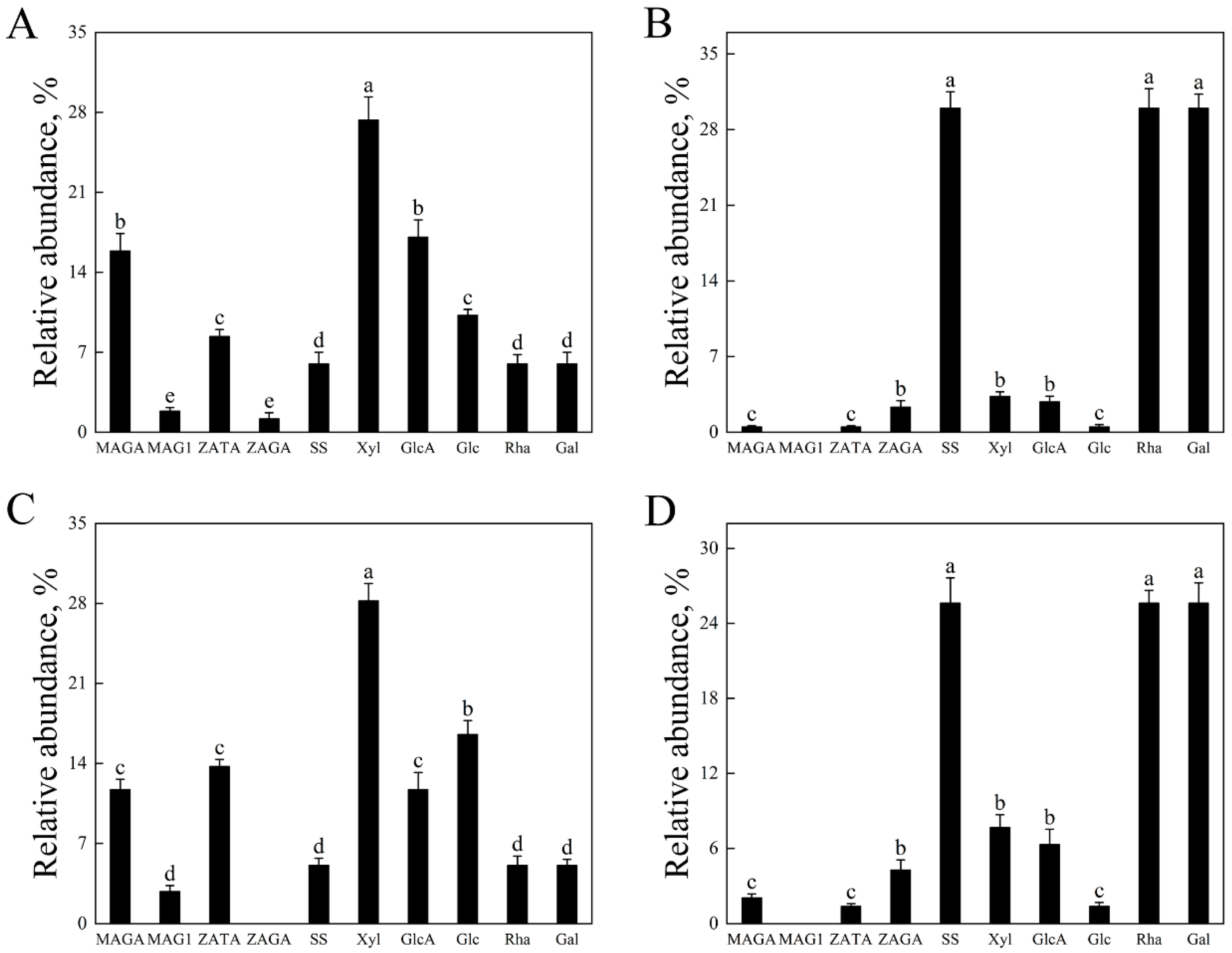
3.3. Saponin Glycosylation Conservation via Mass Spectrometry
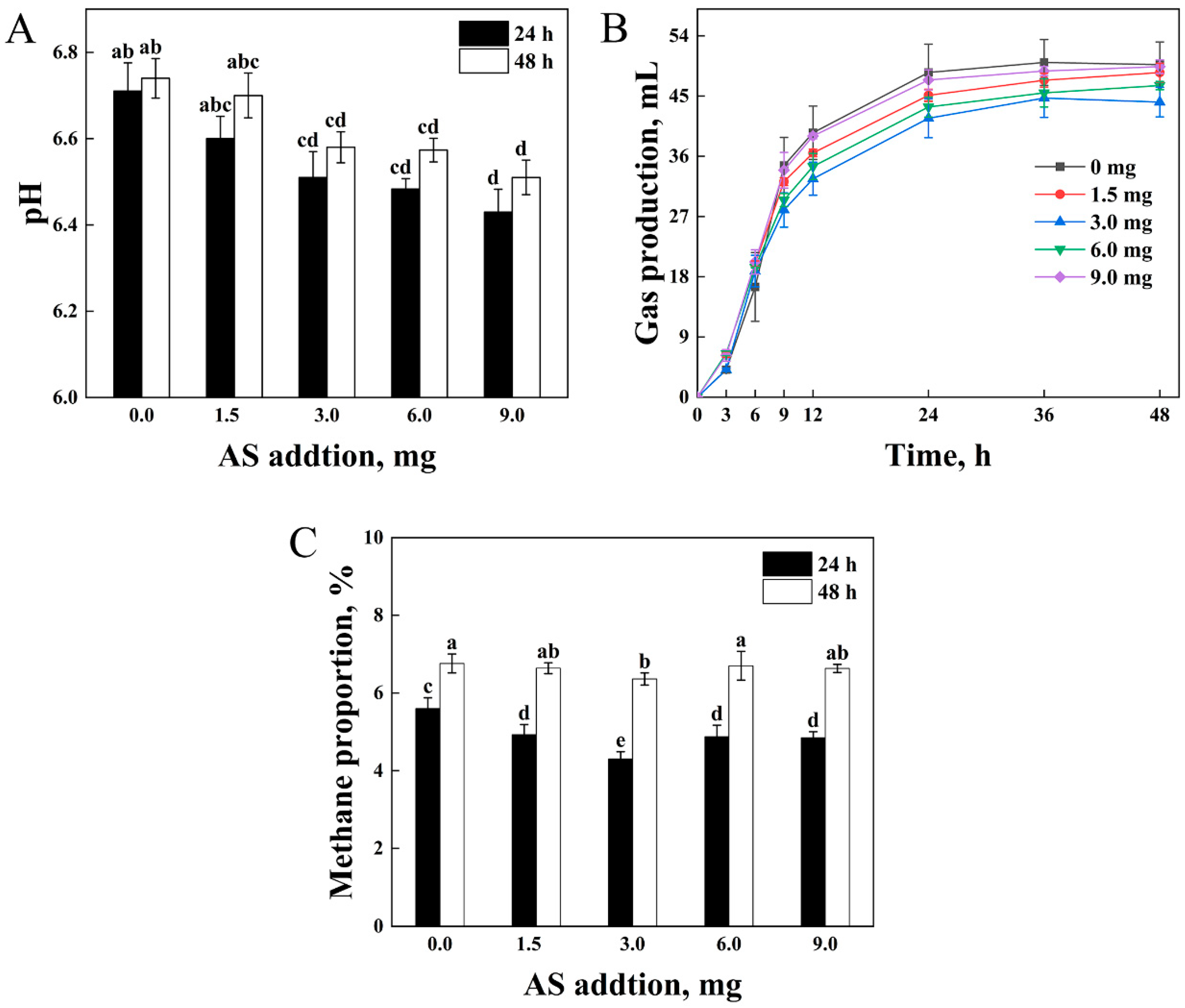
3.4. Fermentation Parameters of the Optimum Addition of AS
3.5. Fermentation Parameters of the AS
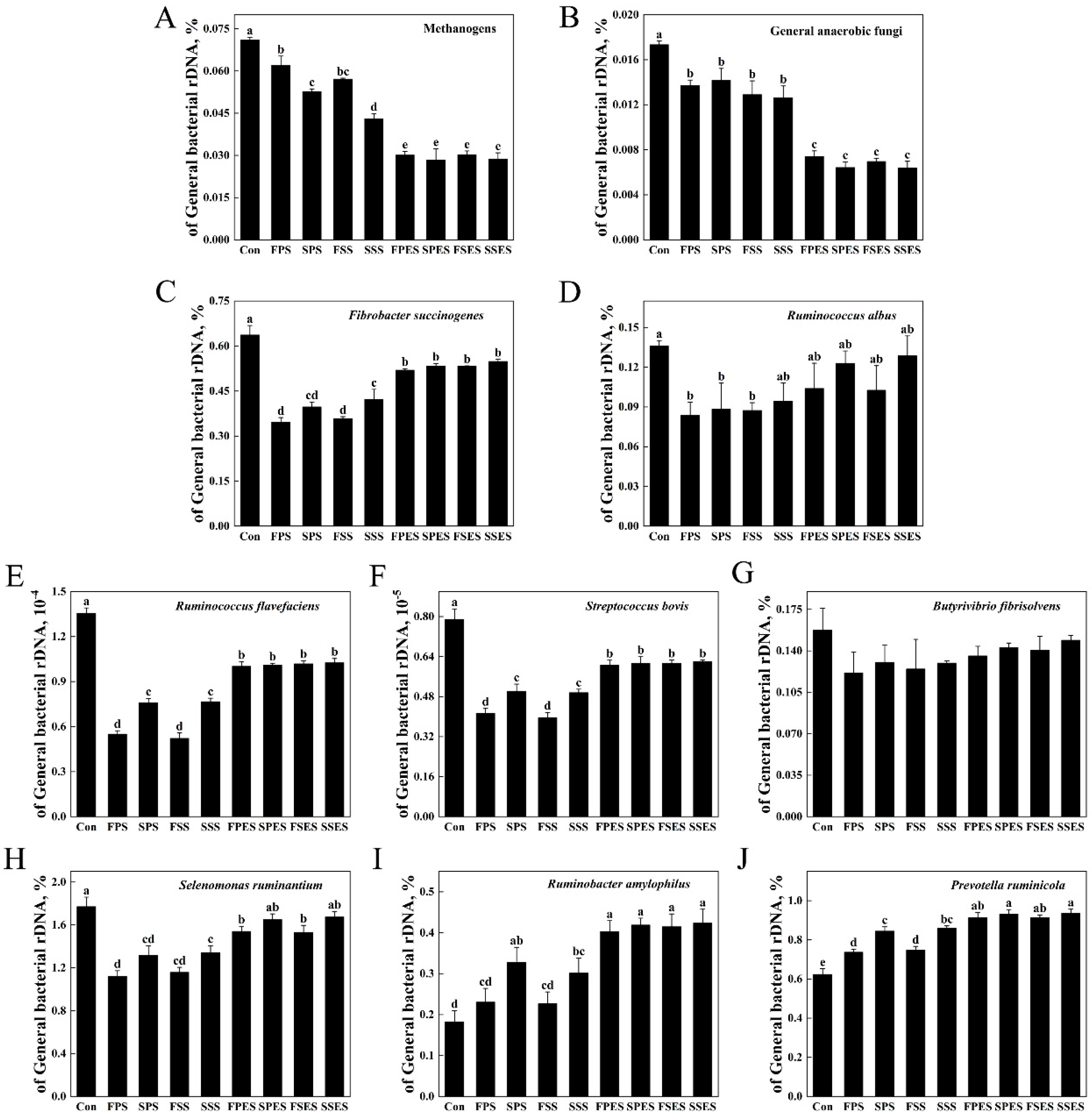
3.6. Microorganisms of the AS
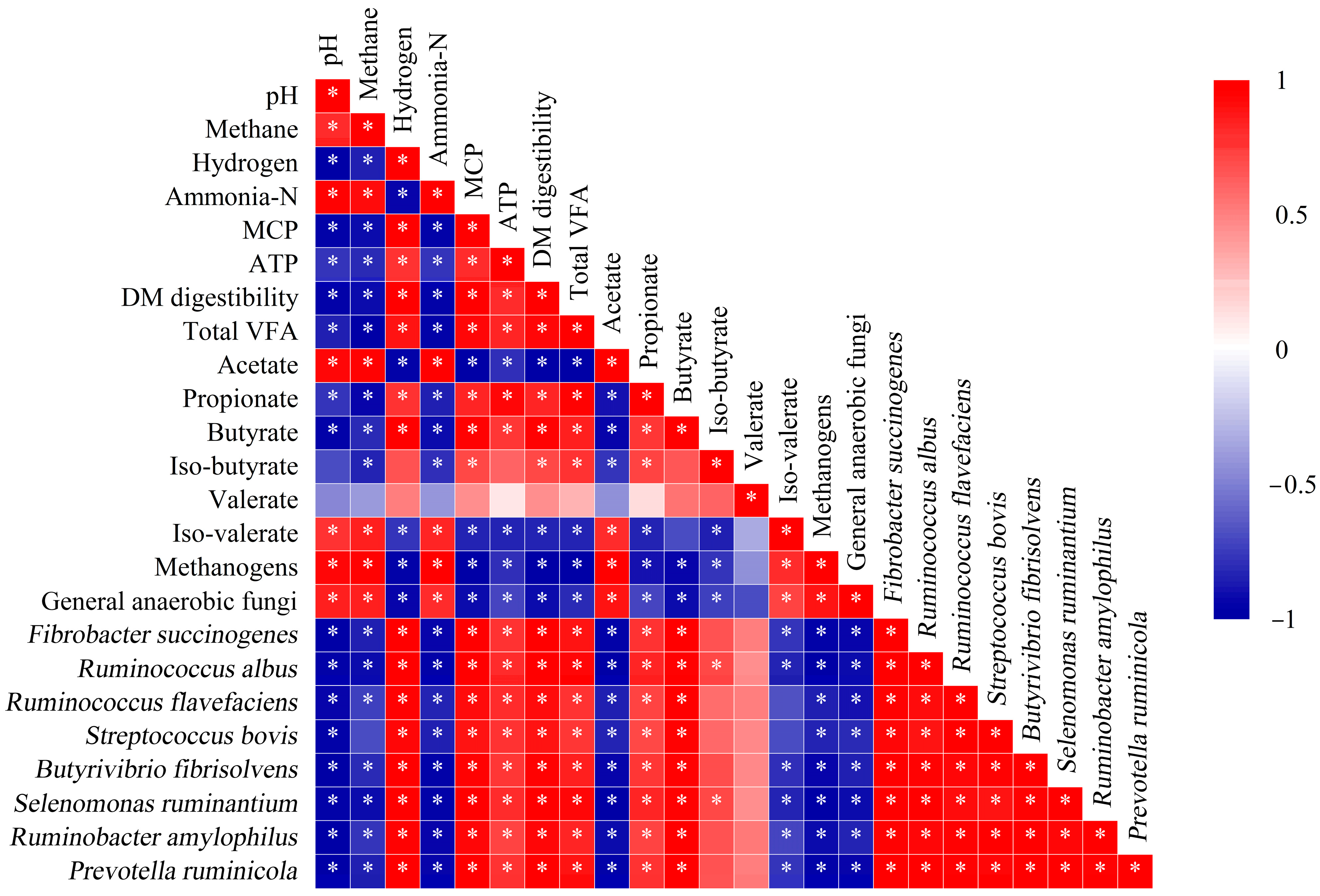
3.7. Correlation Analysis of Fermentation Parameters and Microorganisms
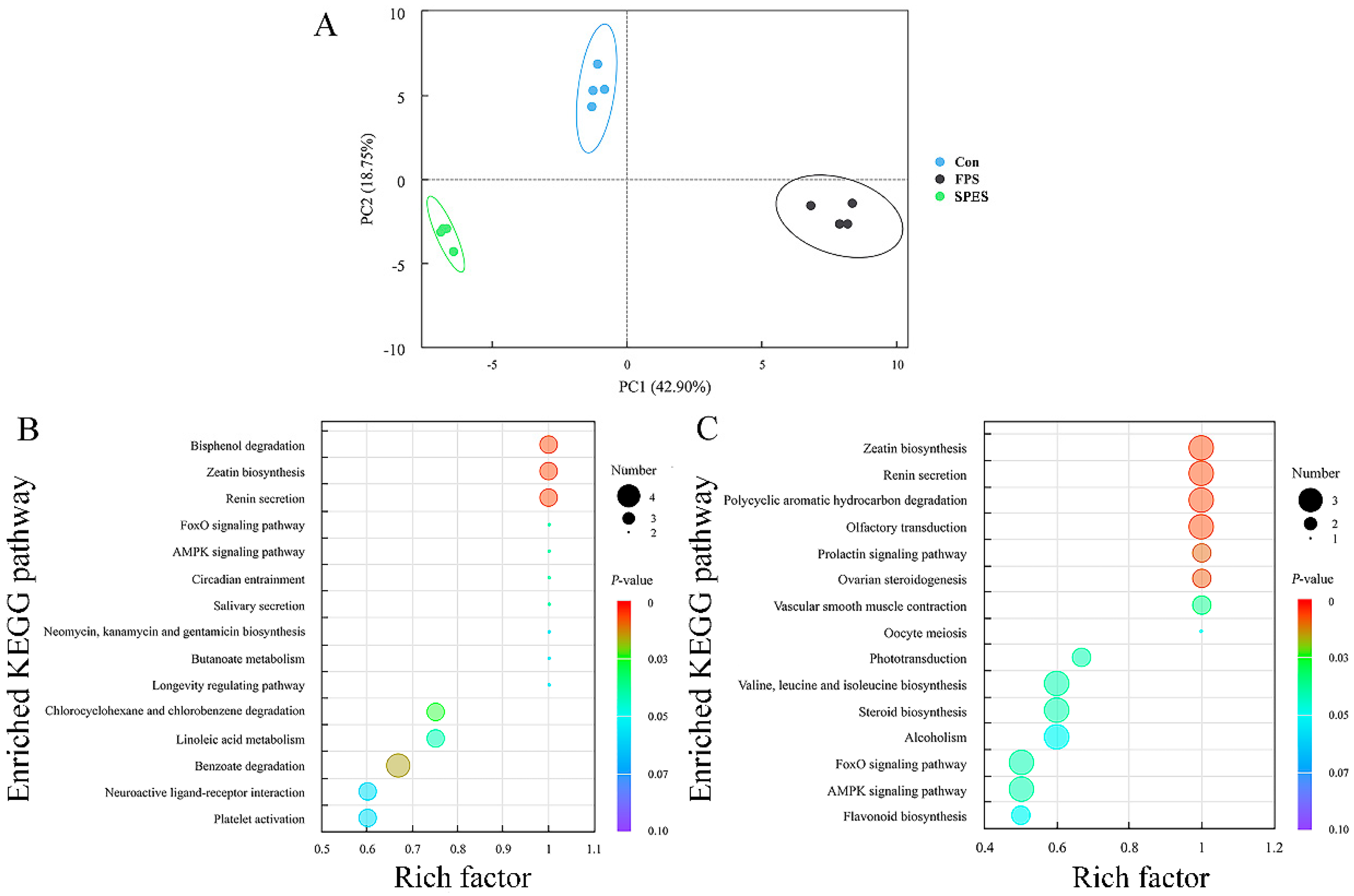
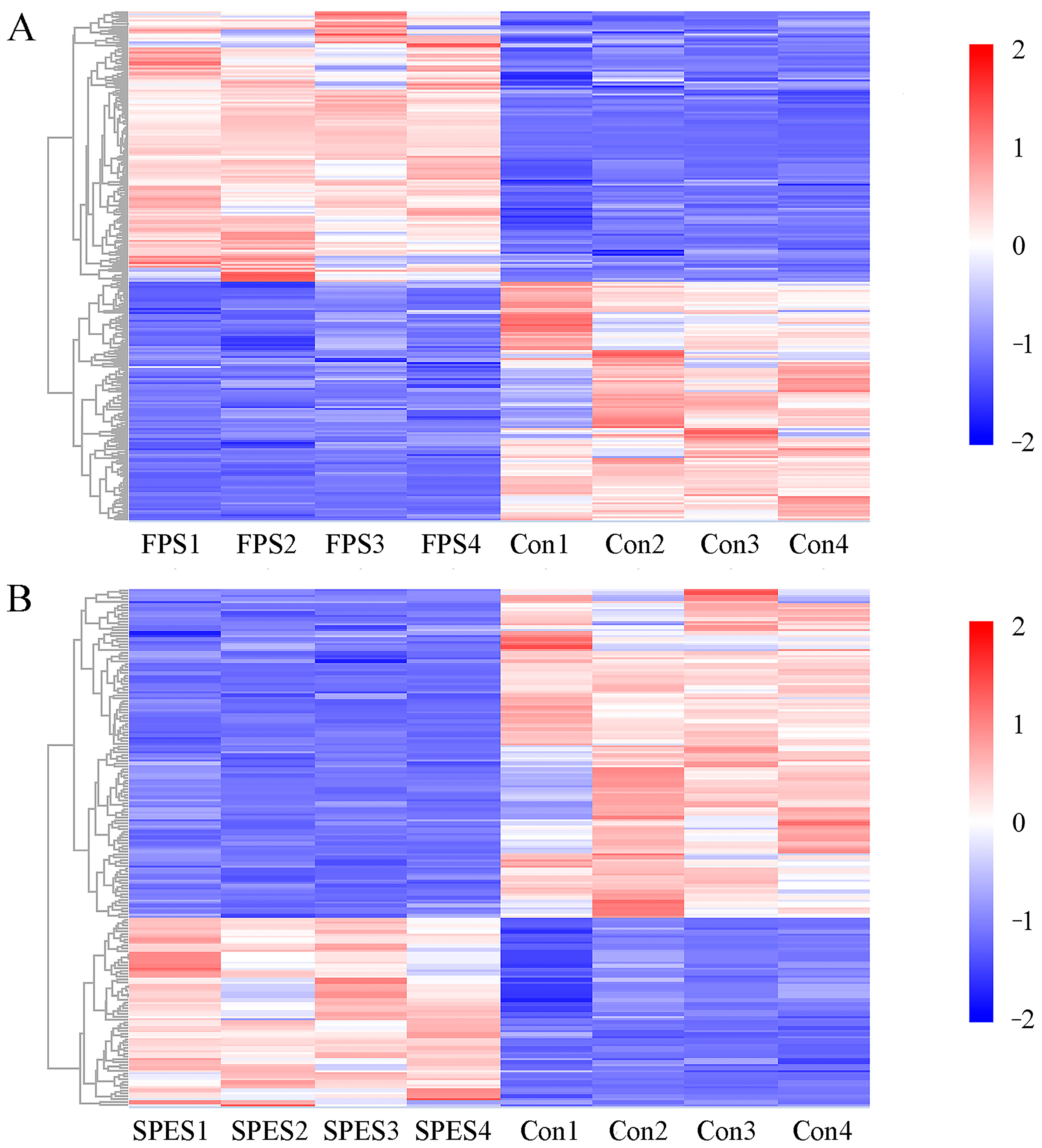
3.8. Rumen Fluid Metabolome
4. Discussion
4.1. The Selection of AS
4.2. Enzymatic AS Modulates Rumen Fermentation
4.3. Rumen Microorganisms and Spearman Correlation
4.4. Rumen Metabolomics Are Influenced by FPS and SPES
4.5. Limitations of in Vitro Models and Translational Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minde, J.J.; Venkataramana, P.B.; Matemu, A.O. Dolichos Lablab-an underutilized crop with future potentials for food and nutrition security: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2249–2261. [Google Scholar] [CrossRef]
- Kozłowska, M.; Cieślak, A.; Jóźwik, A.; El-Sherbiny, M.; Stochmal, A.; Oleszek, W.; Kowalczyk, M.; Filipiak, W.; Szumacher-Strabel, M. The effect of total and individual alfalfa saponins on rumen methane production. J. Sci. Food Agric. 2020, 100, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Brown, H.E.; Teixeira, E.I.; Moot, D.J. Development of a lucerne model in APSIM next generation: 2 canopy expansion and light interception of genotypes with different fall dormancy ratings. Eur. J. Agron. 2022, 139, 126570. [Google Scholar] [CrossRef]
- Szumacher-Strabel, M.; Stochmal, A.; Cieslak, A.; Kozłowska, M.; Kuznicki, D.; Kowalczyk, M.; Oleszek, W. Structural and quantitative changes of saponins in fresh alfalfa compared to alfalfa silage. J. Sci. Food Agric. 2019, 99, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, X.; Ayesha, K.; Khuram, S.; Cui, J.; Wang, G.; Yangzong, Z.; Shi, M.; Jiang, X.; Li, L.; et al. Multi-omics analysis of the mechanism of alfalfa and wheat-induced rumen flatulence in Xizang sheep. Microbiol. Spectr. 2025, 13, e03268-24. [Google Scholar] [CrossRef]
- Doreau, M.; Van Der Werf, H.M.G.; Micol, D.; Dubroeucq, H.; Agabriel, J.; Rochette, Y.; Martin, C. Enteric methane production and greenhouse gases balance of diets differing in concentrate in the fattening phase of a beef production system. J. Anim. Sci. 2011, 89, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Nasehi, M.; Torbatinejad, N.M.; Rezaie, M.; Ghoorchi, T. Effects of partial substitution of alfalfa hay with green tea waste on growth performance and in vitro methane emission of fat-tailed lambs. Small Rumin. Res. 2018, 168, 52–59. [Google Scholar] [CrossRef]
- Kannan, P.; Shafreen, M.M.; Achudhan, A.B.; Gupta, A.; Saleena, L.M. A review on applications of β-glucosidase in food, brewery, pharmaceutical and cosmetic industries. Carbohydr. Res. 2023, 530, 108855. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, D.; Yang, S.; Lin, H.; Chen, H. Identification of an intracellular β-glucosidase in Aspergillus niger with transglycosylation activity. Appl. Microbiol. Biotechnol. 2020, 104, 8367–8380. [Google Scholar] [CrossRef]
- Chang, K.H.; Jo, M.N.; Kim, K.T.; Paik, H.D. Evaluation of glucosidases of Aspergillus niger strain comparing with other glucosidases in transformation of ginsenoside Rb1 to ginsenosides Rg3. J. Ginseng Res. 2014, 38, 47–51. [Google Scholar] [CrossRef]
- Kandar, C.C. Secondary metabolites from plant sources. In Bioactive Natural Products for Pharmaceutical Applications; Pal, D., Nayak, A.K., Eds.; Springer: Cham, Switzerland, 2021; pp. 329–377. [Google Scholar] [CrossRef]
- Thiex, N.J.W. Animal Feed. In Official Methods of Analysis of AOAC International, 22nd, ed.; Latimer, G.W., Ed.; Oxford University Press: Oxford, UK, 2023; p. C4. [Google Scholar] [CrossRef]
- Wan, X.L.; Niu, Y.; Zheng, X.C.; Huang, Q.; Su, W.P.; Zhang, J.F.; Zhang, L.L.; Wang, T. Antioxidant capacities of Artemisia annua L. leaves and enzymatically treated Artemisia annua L. in vitro and in broilers. Anim. Feed Sci. Technol. 2016, 221 Pt A, 27–34. [Google Scholar] [CrossRef]
- Wie, H.J.; Zhao, H.; Chang, J.H.; Kim, Y.S.; Hwang, I.; Ji, G. Enzymatic modification of saponins from Platycodon grandiflorum with Aspergillus niger. J. Agric. Food Chem. 2007, 55, 8908–8913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Du, Z.; Li, Z.; Feng, Y.; Yan, X. Improving the bioactivity of water-soluble alfalfa saponins using biotransformation. J. Food Sci. 2024, 89, 8628–8643. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Hu, W.; Liu, J.; Ye, J.; Wu, Y.; Guo, Y. Effect of tea saponin on rumen fermentation in vitro. Anim. Feed Sci. Technol. 2005, 120, 333–339. [Google Scholar] [CrossRef]
- Cersosimo, L.M.; Bainbridge, M.L.; Kraft, J.; Wright, A.G. Influence of periparturient and postpartum diets on rumen methanogen communities in three breeds of primiparous dairy cows. BMC Microbiol. 2016, 16, 78. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Sharma, O.P.; Dawra, R.K.; Negi, S.S. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef]
- Jolly, A.; Kim, H.; Moon, J.Y.; Mohan, A.; Lee, Y.C. Exploring the imminent trends of saponins in personal care product development: A review. Ind. Crops Prod. 2023, 205, 117489. [Google Scholar] [CrossRef]
- Chen, L.; Qu, H.; Bai, S.; Yan, L.; You, M.; Gou, W.; Li, P.; Gao, F. Effect of wet sea buckthorn pomace utilized as an additive on silage fermentation profile and bacterial community composition of alfalfa. Bioresour. Technol. 2020, 314, 123773. [Google Scholar] [CrossRef] [PubMed]
- Coblentz, W.K.; Akins, M.S. Nutritive value, silage fermentation characteristics, and aerobic stability of round-baled, alfalfa–grass forages ensiled at 2 moisture concentrations with or without a propionic-acid-based preservative. Appl. Anim. Sci. 2021, 37, 89–105. [Google Scholar] [CrossRef]
- Esmaeelian, M.; Jahani, M.; Feizy, J.; Einafshar, S. Foam and emulsion properties of crude saponin extract from saffron (Crocus sativus L.) corm. J. Food Eng. 2024, 370, 111956. [Google Scholar] [CrossRef]
- Chen, B.J.; Liu, Y.; Yang, K.; Li, X.; Dong, X.; Guan, Y.; Ismail, A.; Khoo, H.E. Amylase-assisted extraction alters nutritional and physicochemical properties of polysaccharides and saponins isolated from Ganoderma spp. Food Chem. X 2023, 20, 100913. [Google Scholar] [CrossRef] [PubMed]
- Tippel, J.; Gies, K.; Harbaum-Piayda, B.; Steffen-Heins, A.; Drusch, S. Composition of Quillaja saponin extract affects lipid oxidation in oil-in-water emulsions. Food Chem. 2017, 221, 386–394. [Google Scholar] [CrossRef]
- Lamont, B.B.; Pausas, J.G. Seed dormancy revisited: Dormancy-release pathways and environmental interactions. Funct. Ecol. 2023, 37, 1106–1125. [Google Scholar] [CrossRef]
- Yanza, Y.R.; Szumacher-Strabel, M.; Jayanegara, A.; Kasenta, A.M.; Gao, M.; Huang, H.; Patra, A.; Warzych, E.; Cieślak, A. The effects of dietary medium-chain fatty acids on ruminal methanogenesis and fermentation in vitro and in vivo: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2021, 105, 874–889. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Waldrip, H.M.; Parker, D.; Todd, R.W.; Brauer, D. Dietary mitigation of enteric methane emissions from ruminants: A review of plant tannin mitigation options. Anim. Nutr. 2020, 6, 231–246. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; de Campeneere, S.; Van Ranst, G.; Fievez, V. Interactions between methane mitigation additives and basal substrates on in vitro methane and VFA production. Anim. Feed Sci. Technol. 2012, 176, 47–60. [Google Scholar] [CrossRef]
- Steiner, S.; Linhart, N.; Neidl, A.; Baumgartner, W.; Tichy, A.; Wittek, T. Evaluation of the therapeutic efficacy of rumen transfaunation. J. Anim. Physiol. Anim. Nutr. 2020, 104, 56–63. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Metabolic hydrogen flows in rumen fermentation: Principles and possibilities of interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Wang, R.; Si, H.B.; Wang, M.; Lin, B.; Deng, J.P.; Tan, L.W.; Liu, W.X.; Sun, X.Z.; Teklebrhan, T.; Tan, Z.L. Effects of elemental magnesium and magnesium oxide on hydrogen, methane and volatile fatty acids production in in vitro rumen batch cultures. Anim. Feed Sci. Technol. 2019, 252, 74–82. [Google Scholar] [CrossRef]
- Smith, D.R.; DiLorenzo, N.; Leibovich, J.; May, M.L.; Quinn, M.J.; Homm, J.W.; Galyean, M.L. Effects of sulfur and monensin concentrations on in vitro dry matter disappearance, hydrogen sulfide production, and volatile fatty acid concentrations in batch culture ruminal fermentations. J. Anim. Sci. 2010, 88, 1503–1512. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Hailemariam, S.; Zheng, N.; Wang, M.; Zhao, S.; Wang, J. Biochanin a inhibits ruminal nitrogen-metabolizing bacteria and alleviates the decomposition of amino acids and urea in vitro. Animals 2020, 10, 368. [Google Scholar] [CrossRef]
- Mao, H.; Mao, H.; Wang, J.K.; Liu, J.X.; Yoon, I. Effects of Saccharomyces cerevisiae fermentation product on in vitro fermentation and microbial communities of low-quality forages and mixed diets. J. Anim. Sci. 2013, 91, 3291–3298. [Google Scholar] [CrossRef]
- Tan, P.; Liu, H.; Zhao, J.; Gu, X.; Wei, X.; Zhang, X.; Ma, N.; Johnston, L.J.; Bai, Y.; Zhang, W.; et al. Amino acids metabolism by rumen microorganisms: Nutrition and ecology strategies to reduce nitrogen emissions from the inside to the outside. Sci. Total Environ. 2021, 800, 149596. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effective reduction of enteric methane production by a combination of nitrate and saponin without adverse effect on feed degradability, fermentation, or bacterial and archaeal communities of the rumen. Bioresour. Technol. 2013, 148, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.M.; Badhan, A.K.; Reid, I.D.; Ribeiro, G.; Gruninger, R.; Tsang, A.; Guan, L.L.; McAllister, T. Comparative analysis of functional diversity of rumen microbiome in bison and beef heifers. Appl. Environ. Microbiol. 2023, 89, e01320-23. [Google Scholar] [CrossRef]
- Braga, C.S.; Martins, G.; Soares, O.S.; Pereira, M.F.; Pereira, I.A.; Pereira, L.; Alves, M.M.; Salvador, A. Non-conductive silicon-containing materials improve methane production by pure cultures of methanogens. Bioresour. Technol. 2024, 408, 131144. [Google Scholar] [CrossRef]
- Tamilselvan, R.; Selwynraj, A.I. Enhancing biogas generation from lignocellulosic biomass through biological pretreatment: Exploring the role of ruminant microbes and anaerobic fungi. Anaerobe 2024, 85, 102815. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr. Res. Rev. 2009, 22, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.; Beauchemin, K.; McGinn, S. PSX-22 Feeding condensed and hydrolysable tannins in high protein finishing diets to beef cattle to reduce ammonia emissions from manure. J. Anim. Sci. 2018, 96, 415. [Google Scholar] [CrossRef]
- Wang, K.; Xiong, B.; Zhao, X. Could propionate formation be used to reduce enteric methane emission in ruminants? Sci. Total Environ. 2023, 855, 158867. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Hollmann, M.; Sabitzer, S.; Podstatzky-Lichtenstein, L.; Klein, D.; Zebeli, Q. Epithelial response to high-grain diets involves alteration in nutrient transporters and Na+/K+-ATPase mRNA expression in rumen and colon of goats. J. Anim. Sci. 2013, 91, 4256–4266. [Google Scholar] [CrossRef]
- Ma, Y.; Han, L.; Zhang, S.; Zhang, X.; Hou, S.; Gui, L.; Sun, S.; Yuan, Z.; Wang, Z.; Yang, B. Insight into the differences of meat quality between Qinghai white Tibetan sheep and black Tibetan sheep from the perspective of metabolomics and rumen microbiota. Food Chem. X 2023, 19, 100843. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Bai, H.; Zhang, J.; Mao, S.; Jin, W. Live yeast supplementation altered the bacterial community’s composition and function in rumen and hindgut and alleviated the detrimental effects of heat stress on dairy cows. J. Anim. Sci. 2023, 101, skac410. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, H.; Tang, Y.; Xu, R.; Liu, J.; Yao, R.; Wei, J.; Li, C.; Zhao, X.; Wei, Q.; et al. G protein-coupled estrogen receptor signaling dependent epidermal growth-like factor expression is required for NPR2 inhibition and meiotic resumption in goat oocytes. Theriogenology 2021, 176, 35–42. [Google Scholar] [CrossRef]
- Guyader, J.; Eugène, M.; Doreau, M.; Morgavi, D.P.; Gérard, C.; Loncke, C.; Martin, C. Nitrate but not tea saponin feed additives decreased enteric methane emissions in nonlactating cows. J. Anim. Sci. 2015, 93, 5367–5377. [Google Scholar] [CrossRef]
- Su, F.; Xue, Y.; Ye, S.; Yu, B.; Li, J.; Xu, L.; Yuan, X. Integrative transcriptomic and metabolomic analysis in mice reveals the mechanism by which ginseng stem-leaf saponins enhance mucosal immunity induced by a porcine epidemic diarrhea virus vaccination. Vaccine 2023, 41, 6379–6390. [Google Scholar] [CrossRef]
- Christodoulou, C.; Kliem, K.E.; Auffret, M.D.; Humphries, D.J.; Newbold, J.R.; Davison, N.; Crompton, L.; Dhanoa, M.S.; Smith, L.G.; Stergiadis, S. In vitro rumen degradation, fermentation, and methane production of four agro-industrial protein-rich co-products, compared with soyabean meal. Anim. Feed Sci. Technol. 2025, 319, 116151. [Google Scholar] [CrossRef]
- Gebeyew, K.; Mi, H.; Du, R.; Gao, M.; Diba, D.; Tang, S.; He, Z.; Tan, Z. Wheat straw and alfalfa hay alone or combined in a high-concentrate diet alters microbial-host interaction in the rumen of lambs. Anim. Nutr. 2025, 20, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Ruiz, D.R.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.P.; O’Kiely, P.; Reynolds, C.K.; Schwarm, A.; Shingfield, K.J.; Yu, Z.; et al. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants—A review. Anim. Feed Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]

| No. | Cultivars | Fall Dormancy | Dormancy Category | Origin |
|---|---|---|---|---|
| 1 | Fado | 4 | Semi-dormant | French |
| 2 | Instinct | 4 | Semi-dormant | French |
| 3 | Mezzo | 4 | Semi-dormant | French |
| 4 | Salsa | 4 | Semi-dormant | French |
| 5 | Sibemol | 4 | Semi-dormant | French |
| 6 | Vision | 4 | Semi-dormant | French |
| 7 | Paola | 5 | Moderate | Italian |
| 8 | MS-5S43 | 5 | Moderate | American |
| 9 | Power 5020 | 5 | Moderate | Canadian |
| 10 | Sandili | 5 | Moderate | American |
| 11 | Sidili | 5 | Moderate | American |
| 12 | Villiger | 5 | Moderate | Canadian |
| 13 | Aurora | 6 | Moderate | Australian |
| 14 | Level 6 | 6 | Moderate | American |
| 15 | Blue moon | 7 | Moderate | Italian |
| 16 | Claudia | 7 | Moderate | Italian |
| 17 | Gannong No.5 | 7 | Moderate | Chinese |
| 18 | Sardi 7 | 7 | Moderate | Australian |
| 19 | Eureka+ | 8 | Dormant | Australian |
| 20 | Pegasis | 9 | Dormant | Australian |
| 21 | Sardi 10 | 10 | Non-dormant | Australian |
| Primer Name | Sequences |
|---|---|
| General bacteria-F | CGGCAACGAGCGCAACCC |
| General bacteria-R | CCATTGTAGCACGTGTGTAGCC |
| Methanogens-F | CCGGAGATGGAACCTGAGAC |
| Methanogens-R | CGGTCTTGCCCAGCTCTTATTC |
| General anaerobic fungi-F | GAGGAAGTAAAAGTCGTAACAAGGTTTC |
| General anaerobic fungi-R | CAAATTCACAAAGGGTAGGATGATT |
| Fibrobacter succinogenes-F | GCGGGTAGCAAACAGGATTAGA |
| Fibrobacter succinogenes-R | CCCCCGGACACCCAGTAT |
| Ruminococcus albus-F | CCCTAAAAGCAGTCTTAGTTCG |
| Ruminococcus albus-R | CCTCCTTGCGGTTAGAACA |
| Ruminococcus flavefaciens-F | CGAACGGAGATAATTTGAGTTTACTTAGG |
| Ruminococcus flavefaciens-R | CGGTCTCTGTATGTTATGAGGTATTACC |
| Streptococcus bovis-F | TTCCTAGAGATAGGAAGTTTCTTCGG |
| Streptococcus bovis-R | ATGATGGCAACTAACAATAGGGGT |
| Butyrivibrio fibrisolvens-F | GCCTCAGCGTCAGTAATCG |
| Butyrivibrio fibrisolvens-R | GGAGCGTAGGCGGTTTTAC |
| Selenomonas ruminantium-F | CAATAAGCATTCCGCCTGGG |
| Selenomonas ruminantium-R | TTCACTCAATGTCAAGCCCTGG |
| Ruminobacter amylophilus-F | CTGGGGAGCTGCCTGAAT |
| Ruminobacter amylophilus-R | CATCTGAATGCGACTGGTTG |
| Prevotella ruminicola-F | GAAAGTCGGATTAATGCTCTATGTTG |
| Prevotella ruminicola-R | CATCCTATAGCGGTAAACCTTTGG |
| Cultivar | DM, % | CP, % DM | EE, % DM | NDF, % DM | ADF, % DM |
|---|---|---|---|---|---|
| Fado | 24.52 ± 0.89 | 23.42 ± 1.03 a | 4.02 ± 0.16 a | 37.35 ± 1.25 | 26.91 ± 1.15 d |
| Instinct | 25.44 ± 1.32 | 23.27 ± 1.61 a | 4.03 ± 0.13 a | 37.77 ± 1.15 | 26.93 ± 0.49 d |
| Mezzo | 25.28 ± 0.77 | 23.19 ± 0.82 a | 4.02 ± 0.10 a | 37.53 ± 0.83 | 26.63 ± 0.70 d |
| Salsa | 26.17 ± 1.05 | 23.08 ± 1.27 a | 4.01 ± 0.22 a | 37.23 ± 0.60 | 27.17 ± 0.13 cd |
| Sibemol | 24.09 ± 1.63 | 23.55 ± 1.88 a | 3.97 ± 0.24 a | 37.07 ± 0.68 | 26.99 ± 0.78 d |
| Vision | 25.98 ± 1.41 | 23.24 ± 1.69 a | 3.94 ± 0.18 a | 37.12 ± 0.75 | 26.86 ± 0.39 d |
| Paola | 25.87 ± 0.53 | 22.30 ± 0.53 ab | 3.85 ± 0.37 ab | 37.90 ± 0.56 | 27.93 ± 0.40 bcd |
| MS-5S43 | 24.73 ± 0.81 | 22.64 ± 0.57 ab | 3.83 ± 0.17 ab | 38.02 ± 0.63 | 27.78 ± 0.32 bcd |
| Power 5020 | 25.62 ± 0.92 | 22.15 ± 0.93 ab | 3.80 ± 0.20 ab | 38.04 ± 0.81 | 27.92 ± 0.05 bcd |
| Sandili | 25.51 ± 1.27 | 22.45 ± 1.15 bc | 3.83 ± 0.33 ab | 37.95 ± 0.25 | 27.97 ± 0.02 bcd |
| Sidili | 25.39 ± 0.62 | 22.26 ± 0.52 bc | 3.82 ± 0.20 ab | 37.92 ± 1.13 | 27.85 ± 0.59 bcd |
| Villiger | 24.25 ± 0.88 | 22.46 ± 0.86 bc | 3.83 ± 0.24 ab | 37.81 ± 0.95 | 27.82 ± 0.36 bcd |
| Aurora | 26.14 ± 1.18 | 21.24 ± 1.32 cd | 3.76 ± 0.15 bc | 38.27 ± 0.65 | 28.59 ± 0.21 abc |
| Level 6 | 24.96 ± 0.71 | 21.63 ± 0.63 cd | 3.77 ± 0.07 bc | 38.39 ± 0.69 | 28.51 ± 0.07 abc |
| Blue moon | 24.82 ± 1.35 | 20.37 ± 1.42 cd | 3.67 ± 0.17 bcd | 38.73 ± 0.37 | 28.99 ± 0.63 ab |
| Claudia | 25.67 ± 1.16 | 20.62 ± 1.35 de | 3.68 ± 0.16 bcd | 38.79 ± 0.40 | 29.01 ± 0.36 ab |
| Gannong No.5 | 25.53 ± 0.84 | 20.29 ± 0.97 de | 3.67 ± 0.17 bcd | 39.00 ± 0.62 | 28.78 ± 0.08 abc |
| Sardi 7II | 26.38 ± 1.57 | 20.70 ± 1.52 de | 3.71 ± 0.22 bcd | 38.82 ± 0.46 | 29.04 ± 0.11 ab |
| Eureka+ | 25.24 ± 0.73 | 19.69 ± 0.68 ef | 3.64 ± 0.13 cd | 39.17 ± 0.52 | 29.26 ± 0.07 ab |
| Pegasis | 25.09 ± 1.39 | 18.91 ± 1.31 f | 3.57 ± 0.20 d | 39.46 ± 0.58 | 29.56 ± 0.12 a |
| Sardi 10 | 24.95 ± 1.12 | 18.06 ± 1.05 f | 3.40 ± 0.39 d | 39.99 ± 0.78 | 30.00 ± 0.20 a |
| Cultivar | DM, % | CP, % DM | EE, % DM | NDF, % DM | ADF, % DM | pH | Ammonia-N, % of Total N | Lactic Acid, % DM | Acetic Acid, % DM |
|---|---|---|---|---|---|---|---|---|---|
| Fado | 39.04 ± 1.78 | 23.53 ± 1.11 a | 3.87 ± 0.28 ab | 33.35 ± 1.20 | 23.40 ± 1.35 bc | 4.67 ± 0.03 | 2.77 ± 0.37 | 5.89 ± 0.18 ab | 1.55 ± 0.09 a |
| Instinct | 40.88 ± 2.64 | 23.33 ± 1.75 ab | 3.99 ± 0.38 a | 33.71 ± 1.33 | 23.42 ± 0.69 bc | 4.65 ± 0.04 | 2.81 ± 0.13 | 5.88 ± 0.12 ab | 1.53 ± 0.10 a |
| Mezzo | 40.56 ± 1.54 | 23.37 ± 0.69 ab | 3.75 ± 0.19 abc | 33.50 ± 0.96 | 23.16 ± 1.00 c | 4.63 ± 0.12 | 2.79 ± 0.24 | 5.89 ± 0.20 ab | 1.54 ± 0.09 a |
| Salsa | 42.34 ± 2.10 | 23.25 ± 1.19 ab | 3.99 ± 0.15 a | 33.24 ± 0.70 | 23.63 ± 0.18 bc | 4.68 ± 0.06 | 2.78 ± 0.20 | 5.89 ± 0.10 ab | 1.55 ± 0.09 a |
| Sibemol | 38.18 ± 3.26 | 23.57 ± 1.94 a | 3.67 ± 0.13 abc | 33.10 ± 0.95 | 23.47 ± 1.09 bc | 4.72 ± 0.22 | 2.77 ± 0.18 | 5.88 ± 0.09 ab | 1.55 ± 0.02 a |
| Vision | 41.96 ± 2.82 | 23.41 ± 1.76 ab | 3.79 ± 0.33 abc | 33.15 ± 1.05 | 23.36 ± 0.69 c | 4.71 ± 0.19 | 2.77 ± 0.35 | 5.90 ± 0.07 a | 1.54 ± 0.06 a |
| Paola | 41.74 ± 1.06 | 22.34 ± 0.44 abc | 3.59 ± 0.18 bcd | 33.83 ± 0.78 | 24.29 ± 0.70 abc | 4.70 ± 0.05 | 2.66 ± 0.01 | 5.66 ± 0.15 abc | 1.45 ± 0.07 ab |
| MS-5 S43 | 39.46 ± 1.62 | 22.54 ± 0.68 abc | 3.53 ± 0.11 bcd | 33.93 ± 0.88 | 24.16 ± 0.57 abc | 4.67 ± 0.12 | 2.63 ± 0.04 | 5.62 ± 0.10 abc | 1.45 ± 0.16 ab |
| Power 5020 | 41.24 ± 1.84 | 22.25 ± 0.85 abc | 3.55 ± 0.10 bcd | 33.95 ± 1.13 | 24.28 ± 0.09 abc | 4.69 ± 0.05 | 2.68 ± 0.12 | 5.64 ± 0.19 abc | 1.44 ± 0.14 ab |
| Sandili | 41.02 ± 2.54 | 22.75 ± 1.13 ab | 3.65 ± 0.21 abc | 33.87 ± 0.35 | 24.32 ± 0.03 abc | 4.68 ± 0.06 | 2.66 ± 0.27 | 5.68 ± 0.19 abc | 1.45 ± 0.04 ab |
| Sidili | 40.78 ± 1.24 | 22.46 ± 0.45 abc | 3.51 ± 0.03 bcd | 33.84 ± 1.88 | 24.22 ± 1.06 abc | 4.71 ± 0.20 | 2.65 ± 0.37 | 5.66 ± 0.16 abc | 1.45 ± 0.03 ab |
| Villiger | 38.50 ± 1.76 | 22.54 ± 0.99 abc | 3.53 ± 0.08 bcd | 33.75 ± 1.60 | 24.19 ± 0.64 abc | 4.69 ± 0.06 | 2.66 ± 0.13 | 5.64 ± 0.12 abc | 1.44 ± 0.07 ab |
| Aurora | 42.28 ± 2.36 | 21.36 ± 1.24 bcd | 3.48 ± 0.27 bcd | 34.15 ± 0.90 | 24.86 ± 0.36 abc | 4.71 ± 0.03 | 2.63 ± 0.20 | 5.50 ± 0.02 bcd | 1.43 ± 0.02 ab |
| Level 6 | 39.92 ± 1.42 | 21.53 ± 0.55 bcd | 3.45 ± 0.44 bcd | 34.25 ± 0.96 | 24.79 ± 0.13 abc | 4.69 ± 0.15 | 2.62 ± 0.07 | 5.51 ± 0.01 bcd | 1.42 ± 0.09 ab |
| Blue moon | 39.64 ± 2.70 | 20.56 ± 1.21 bcd | 3.37 ± 0.21 bcd | 34.55 ± 0.52 | 25.21 ± 1.12 abc | 4.68 ± 0.08 | 2.60 ± 0.13 | 5.40 ± 0.14 cd | 1.36 ± 0.08 ab |
| Claudia | 41.34 ± 2.32 | 20.77 ± 1.24 bcd | 3.40 ± 0.16 bcd | 34.60 ± 0.56 | 25.23 ± 0.64 abc | 4.63 ± 0.12 | 2.61 ± 0.06 | 5.42 ± 0.12 cd | 1.36 ± 0.10 ab |
| Gannong No.5 | 41.06 ± 1.68 | 20.41 ± 0.91 bcd | 3.37 ± 0.36 bcd | 34.78 ± 1.03 | 25.03 ± 0.14 abc | 4.63 ± 0.13 | 2.60 ± 0.13 | 5.46 ± 0.08 bcd | 1.35 ± 0.04 ab |
| Sardi 7II | 42.76 ± 3.14 | 20.68 ± 0.44 bcd | 3.31 ± 0.16 bcd | 34.63 ± 0.78 | 25.25 ± 0.19 abc | 4.69 ± 0.06 | 2.59 ± 0.11 | 5.48 ± 0.15 bcd | 1.35 ± 0.03 ab |
| Eureka+ | 40.48 ± 1.46 | 19.74 ± 0.62 cd | 3.15 ± 0.27 bcd | 34.93 ± 0.88 | 25.44 ± 0.12 abc | 4.71 ± 0.13 | 2.57 ± 0.22 | 5.29 ± 0.04 cd | 1.29 ± 0.02 ab |
| Pegasis | 40.18 ± 2.78 | 18.97 ± 0.25 cd | 3.06 ± 0.20 cd | 35.18 ± 0.98 | 25.70 ± 0.21 ab | 4.72 ± 0.06 | 2.55 ± 0.08 | 5.12 ± 0.07 d | 1.21 ± 0.05 b |
| Sardi 10 | 39.90 ± 2.24 | 18.14 ± 1.00 d | 2.96 ± 0.09 d | 35.65 ± 1.30 | 26.09 ± 0.35 a | 4.76 ± 0.03 | 2.55 ± 0.09 | 5.10 ± 0.08 d | 1.22 ± 0.07 b |
| Item | Control | FPS | SPS | FSS | SSS | FPES | SPES | FSES | SSES |
|---|---|---|---|---|---|---|---|---|---|
| Total VFA, mM | 78.39 ± 0.47 d | 84.44 ± 1.00 c | 87.28 ± 0.56 b | 86.45 ± 0.75 c | 87.98 ± 0.74 b | 89.56 ± 0.20 a | 89.61 ± 0.30 a | 89.24 ± 0.09 a | 89.39 ± 0.14 a |
| Acetate, % | 68.32 ± 0.49 a | 65.98 ± 0.33 b | 64.88 ± 0.26 c | 65.89 ± 0.65 b | 64.51 ± 1.02 c | 61.59 ± 0.54 d | 61.25 ± 0.56 d | 61.69 ± 0.07 d | 61.56 ± 0.46 d |
| Propionate, % | 17.99 ± 0.57 d | 20.71 ± 0.61 c | 21.66 ± 0.70 b | 21.20 ± 0.77 c | 21.94 ± 0.58 b | 24.10 ± 0.28 a | 23.84 ± 0.48 a | 23.65 ± 0.64 a | 23.55 ± 0.52 a |
| Butyrate, % | 7.51 ± 0.39 c | 7.05 ± 0.18 d | 7.32 ± 0.21 c | 6.84 ± 0.83 d | 7.37 ± 0.56 c | 8.57 ± 0.19 a | 8.70 ± 0.27 a | 8.68 ± 0.28 a | 8.77 ± 0.61 a |
| Iso-butyrate, % | 2.08 ± 0.05 a | 1.60 ± 0.05 c | 1.57 ± 0.02 c | 1.64 ± 0.04 c | 1.57 ± 0.05 c | 1.93 ± 0.03 a | 2.05 ± 0.08 a | 1.90 ± 0.07 b | 1.93 ± 0.06 b |
| Valerate, % | 1.74 ± 0.03 | 1.53 ± 0.02 | 1.50 ± 0.03 | 1.53 ± 0.03 | 1.51 ± 0.05 | 1.51 ± 0.03 | 1.60 ± 0.05 | 1.53 ± 0.03 | 1.65 ± 0.02 |
| Iso-valerate, % | 2.36 ± 0.32 | 3.13 ± 0.36 | 3.07 ± 0.27 | 2.91 ± 0.49 | 3.11 ± 0.44 | 2.29 ± 0.26 | 2.55 ± 0.17 | 2.56 ± 0.40 | 2.54 ± 0.21 |
| Acetate to propionate ratio | 3.80 ± 0.09 a | 3.19 ± 0.11 b | 3.00 ± 0.11 c | 3.11 ± 0.09 b | 2.95 ± 0.12 c | 2.56 ± 0.05 d | 2.57 ± 0.07 d | 2.61 ± 0.07 d | 2.62 ± 0.05 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Bao, X.; Shi, X.; Jin, S.; Meng, Y.; Li, Z.; Du, Z.; Yan, X. In Vitro Evaluation of Enzymatically Transformed Alfalfa Saponins on Methane Reduction, Rumen Microbes and Metabolomics in Goats. Animals 2025, 15, 1516. https://doi.org/10.3390/ani15111516
Zhang R, Bao X, Shi X, Jin S, Meng Y, Li Z, Du Z, Yan X. In Vitro Evaluation of Enzymatically Transformed Alfalfa Saponins on Methane Reduction, Rumen Microbes and Metabolomics in Goats. Animals. 2025; 15(11):1516. https://doi.org/10.3390/ani15111516
Chicago/Turabian StyleZhang, Ran, Xinran Bao, Xingqi Shi, Shixuan Jin, Ying Meng, Zhiwei Li, Zhumei Du, and Xuebing Yan. 2025. "In Vitro Evaluation of Enzymatically Transformed Alfalfa Saponins on Methane Reduction, Rumen Microbes and Metabolomics in Goats" Animals 15, no. 11: 1516. https://doi.org/10.3390/ani15111516
APA StyleZhang, R., Bao, X., Shi, X., Jin, S., Meng, Y., Li, Z., Du, Z., & Yan, X. (2025). In Vitro Evaluation of Enzymatically Transformed Alfalfa Saponins on Methane Reduction, Rumen Microbes and Metabolomics in Goats. Animals, 15(11), 1516. https://doi.org/10.3390/ani15111516






