Intestinal Ultrasonographic Measurements in Cats Diagnosed with Lymphoplasmacytic Enteritis and Low-Grade T-Cell Lymphoma Based on Either Histology/Immunohistochemistry or Clonality Testing—And Assessment of the Effects of Therapy on Wall Layering After 3 and 6 Months of Treatment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Ultrasonography and Analyses
2.3. Sample Collection and Histopathologic Assessment
2.4. Clonality Testing
2.5. Treatment
2.6. Statistical Evaluation
3. Results
3.1. Study Population
3.2. Results of Histology/IHC and Clonality Testing
3.2.1. Overview of the Course of Disease in Cats with Discordant Results
3.2.2. Histology/IHC and Clonality Testing of Sampled Lymph Nodes
3.3. Ultrasonographic Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paulin, M.V.; Couronne, L.; Beguin, J.; Le Poder, S.; Delverdier, M.; Semin, M.-O.; Bruneau, J.; Cerf-Bensussan, N.; Malamut, G.; Cellier, C.; et al. Feline low-grade alimentary lymphoma: An emerging entity and a potential animal model for human disease. BMC Vet. Res. 2018, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Marsilio, S.; Freiche, V.; Johnson, E.; Leo, C.; Langerak, A.W.; Peters, I.; Ackermann, M.R. ACVIM consensus statement guidelines on diagnosing and distinguishing low-grade neoplastic from inflammatory lymphocytic chronic enteropathies in cats. J. Vet. Intern. Med. 2023, 37, 794–816. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.; Penninck, D.G.; Keating, J.H.; Webster, C.R. Clinicopathological and ultrasonographic features of cats with eosinophilic enteritis. J. Feline Med. Surg. 2014, 16, 950–956. [Google Scholar] [CrossRef]
- Freiche, V.; Paulin, M.V.; Cordonnier, N.; Huet, H.; Turba, M.E.; Macintyre, E.; Molina, T.J.; Hermine, O.; Couronné, L.; Bruneau, J. Histopathologic, phenotypic, and molecular criteria to discriminate low-grade intestinal T-cell lymphoma in cats from lymphoplasmacytic enteritis. J. Vet. Intern. Med. 2021, 35, 2673–2684. [Google Scholar] [CrossRef]
- Moore, P.F.; Rodriguez-Bertos, A.; Kass, P.H. Feline gastrointestinal lymphoma: Mucosal architecture, immunophenotype, and molecular clonality. Vet. Pathol. 2012, 49, 658–668. [Google Scholar] [CrossRef]
- Jergens, A.E. Feline idiopathic inflammatory bowel disease: What we know and what remains to be unraveled. J. Feline Med. Surg. 2012, 14, 445–458. [Google Scholar] [CrossRef]
- Guttin, T.; Walsh, A.; Durham, A.C.; Reetz, J.A.; Brown, D.C.; Rondeau, M.P. Ability of ultrasonography to predict the presence and location of histologic lesions in the small intestine of cats. J. Vet. Intern. Med. 2019, 33, 1278–1285. [Google Scholar] [CrossRef]
- Norsworthy, G.D.; Estep, J.E.; Kiupel, M.; Olson, J.C.; Gassler, L.N. Diagnosis of chronic small bowel disease in cats: 100 cases (2008–2012). J. Am. Vet. Med. Assoc. 2013, 243, 1455–1461. [Google Scholar] [CrossRef]
- Griffin, S. Feline abdominal ultrasonography: What’s normal? What’s abnormal? The diseased gastrointestinal tract. J. Feline Med. Surg. 2019, 21, 1047–1060. [Google Scholar] [CrossRef]
- Norsworthy, G.D.; Estep, J.E.; Hollinger, C.; Steiner, J.M.; Lavallee, J.O.; Gassler, L.N.; Restine, L.M.; Kiupel, M. Prevalence and underlying causes of histologic abnormalities in cats suspected to have chronic small bowel disease: 300 cases (2008–2013). J. Am. Vet. Med. Assoc. 2015, 247, 629–635. [Google Scholar] [CrossRef]
- Zwingenberger, A.L.; Marks, S.L.; Baker, T.W.; Moore, P.F. Ultrasonographic evaluation of the muscularis propria in cats with diffuse small intestinal lymphoma or inflammatory bowel disease. J. Vet. Intern. Med. 2010, 24, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Daniaux, L.A.; Laurenson, M.P.; Marks, S.L.; Moore, P.F.; Taylor, S.L.; Chen, R.X.; Zwingenberger, A.L. Ultrasonographic thickening of the muscularis propria in feline small intestinal small cell T-cell lymphoma and inflammatory bowel disease. J. Feline Med. Surg. 2014, 16, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Freiche, V.; Fages, J.; Paulin, M.V.; Bruneau, J.; Couronné, L.; German, A.J.; Penninck, D.; Hermine, O. Clinical, laboratory and ultrasonographic findings differentiating low-grade intestinal T-cell lymphoma from lymphoplasmacytic enteritis in cats. J. Vet. Intern. Med. 2021, 35, 2685–2696. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.M.; Vernau, W.; Moore, P.F. Clonality testing in veterinary medicine: A review with diagnostic guidelines. Vet. Pathol. 2016, 53, 711–725. [Google Scholar] [CrossRef]
- Sabattini, S.; Bottero, E.; Turba, M.E.; Vicchi, F.; Bo, S.; Bettini, G. Differentiating feline inflammatory bowel disease from alimentary lymphoma in duodenal endoscopic biopsies. J. Small Anim. Pract. 2016, 57, 396–401. [Google Scholar] [CrossRef]
- Marsilio, S.; Ackermann, M.R.; Lidbury, J.A.; Suchodolski, J.S.; Steiner, J.M. Results of histopathology, immunohistochemistry, and molecular clonality testing of small intestinal biopsy specimens from clinically healthy client-owned cats. J. Vet. Intern. Med. 2019, 33, 551–558. [Google Scholar] [CrossRef]
- Kokovic, I.; Novakovic, B.J.; Cerkovnik, P.; Novakovic, S. Clonality analysis of lymphoid proliferations using the BIOMED-2 clonality assays: A single institution experience. Radiol. Oncol. 2014, 48, 155–162. [Google Scholar] [CrossRef]
- Oppliger, S.; Hilbe, M.; Hartnack, S.; Zini, E.; Reusch, C.E.; Kook, P.H. Comparison of Serum Spec fPL and 1,2-o-Dilauryl-Rac-Glycero-3-Glutaric Acid-(6′-Methylresorufin) Ester Assay in 60 Cats Using Standardized Assessment of Pancreatic Histology. J. Vet. Intern. Med. 2016, 30, 764–770. [Google Scholar] [CrossRef]
- Mattoon, J.S.; Auld, D.M.; Nyland, T.G. Small Animal Diagnostic Ultrasound, 2nd ed.; WB Saunders: Philadelphia, PA, USA, 2002; p. 75e8. [Google Scholar]
- Day, M.J.; Bilzer, T.; Mansell, J.; Wilcock, B.; Hall, E.; Jergens, A.; Minami, T.; Willard, M.; Washabau, R. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 2008, 138 (Suppl. 1), S1–S43. [Google Scholar] [CrossRef]
- Washabau, R.J.; Day, M.J.; Willard, M.D.; Hall, E.J.; Jergens, A.E.; Mansell, J.; Minami, T.; Bilzer, T.W.; WSAVA International Gastrointestinal Standardization Group. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 2010, 24, 10–26. [Google Scholar]
- Valli, V.E.; Jacobs, R.M.; Parodi, A.L.; Vernau, W.; Moore, P.F. Histological classification of hematopoietic tumors of domestic animals. In World Health Organization International Histological Classification of Tumors of Domestic Animals, 2nd ed.; Schulman, F.Y., Ed.; Armed Forces Institute of Pathology, American Registry of Pathology: Washington, DC, USA, 2002. [Google Scholar]
- Adams, H.; Liebisch, P.; Schmid, P.M.; Dirnhofer, S.; Tzankov, A. Diagnostic utility of the B-cell lineage markers CD20, CD79a, PAX5, and CD19 in paraffin-embedded tissues from lymphoid neoplasms. Appl. Immunohistochem. Mol. Morphol. 2009, 17, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Nakamura, K.; Sato, H.; Goto-Koshino, Y.; Sato, M.; Takahashi, M.; Fujino, Y.; Ohno, K.; Uchida, K.; Nakayama, H.; et al. Multiplex PCR and Genescan analysis to detect immunoglobulin heavy chain gene rearrangement in feline B-cell neoplasms. Vet. Immunol. Immunopathol. 2011, 143, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Nakamura, K.; Sato, H.; Goto-Koshino, Y.; Sato, M.; Takahashi, M.; Fukushima, K.; Nakashima, K.; Fujino, Y.; Ohno, K.; et al. GeneScan analysis to detect clonality of T-cell receptor gamma gene rearrangement in feline lymphoid neoplasms. Vet. Immunol. Immunopathol. 2012, 145, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, P.; Penninck, D.; Pietra, M.; Cipone, M.; Diana, A. Ultrasonographic measurement of the relative thickness of intestinal wall layers in clinically healthy cats. J. Feline Med. Surg. 2014, 16, 333–339. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 8 February 2025).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lingard, A.E.; Briscoe, K.; Beatty, J.A.; Moore, A.S.; Crowley, A.M.; Krockenberger, M.; Churcher, R.K.; Canfield, P.J.; Barrs, V.R. Low-grade alimentary lymphoma: Clinicopathological findings and response to treatment in 17 cases. J. Feline Med. Surg. 2009, 11, 692–700. [Google Scholar] [CrossRef]
- Schreurs, E.; Vermote, K.; Barberet, V.; Daminet, S.; Rudorf, H.; Saunders, J.H. Ultrasonographic anatomy of abdominal lymph nodes in the normal cat. Vet. Radiol. Ultrasoun 2008, 49, 68–72. [Google Scholar] [CrossRef]
- Kiupel, M.; Smedley, R.C.; Pfent, C.; Xie, Y.; Xue, Y.; Wise, A.G.; DeVaul, J.M.; Maes, R.K. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet. Pathol. 2011, 48, 212–222. [Google Scholar] [CrossRef]
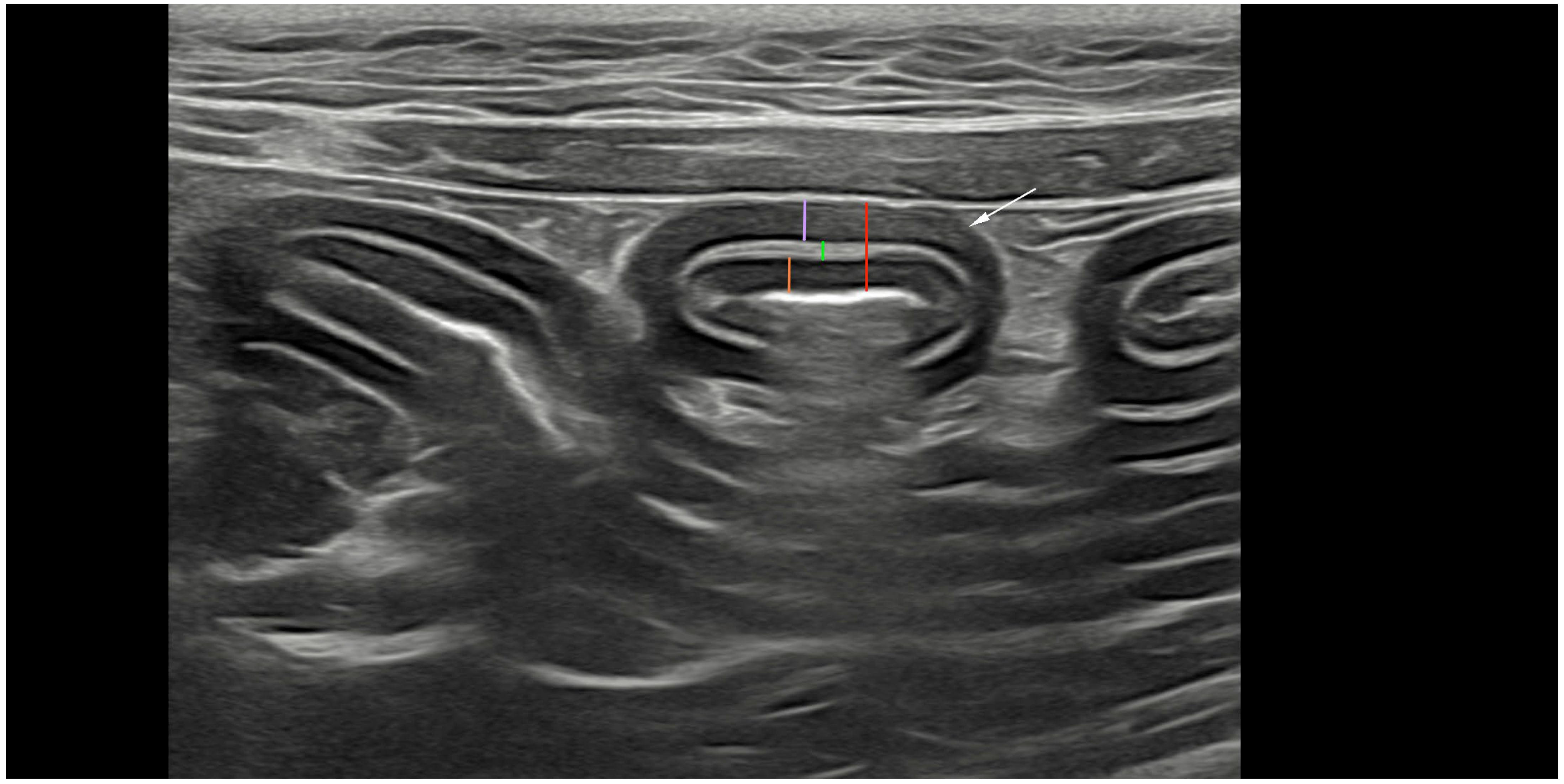
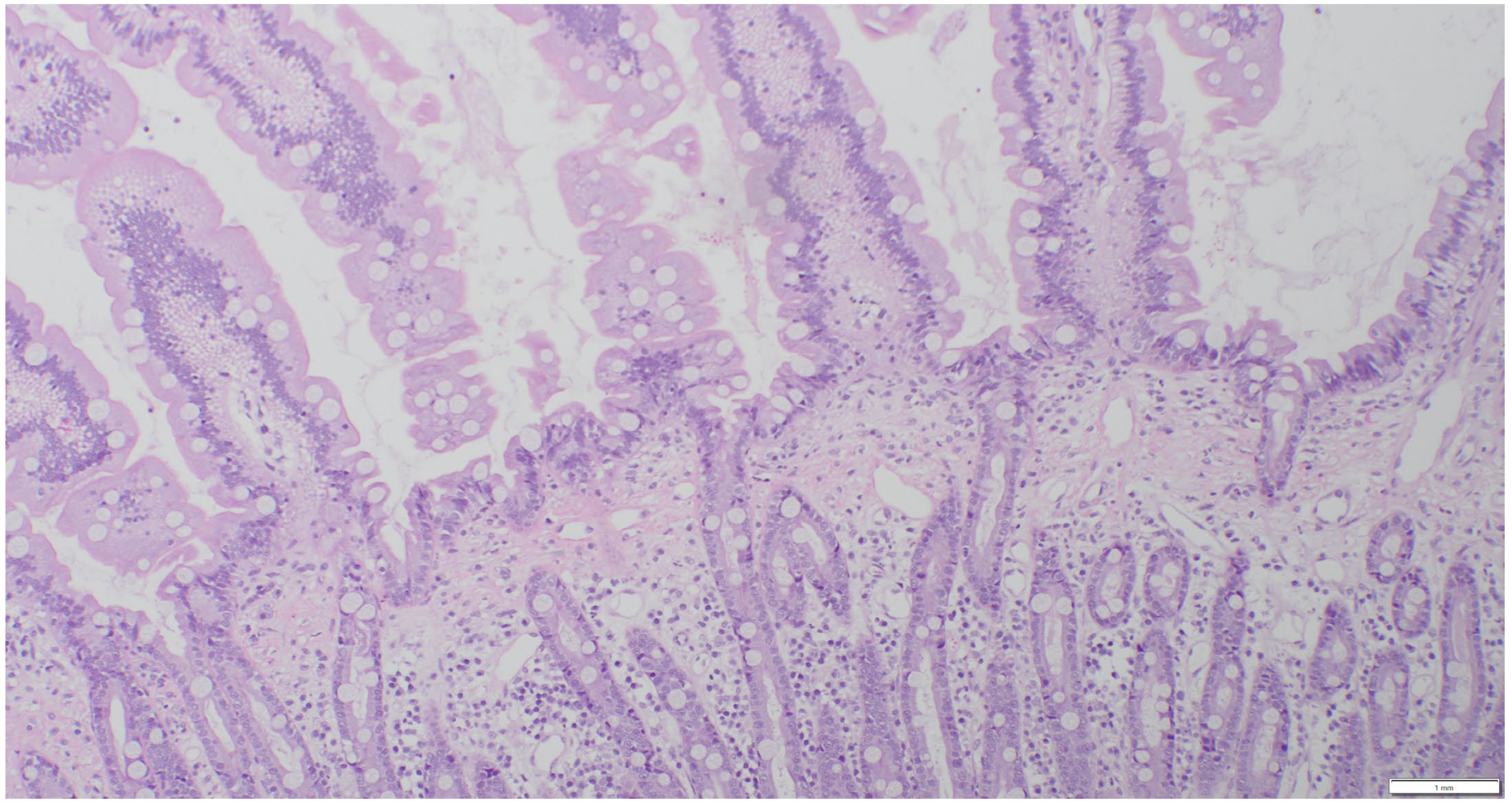
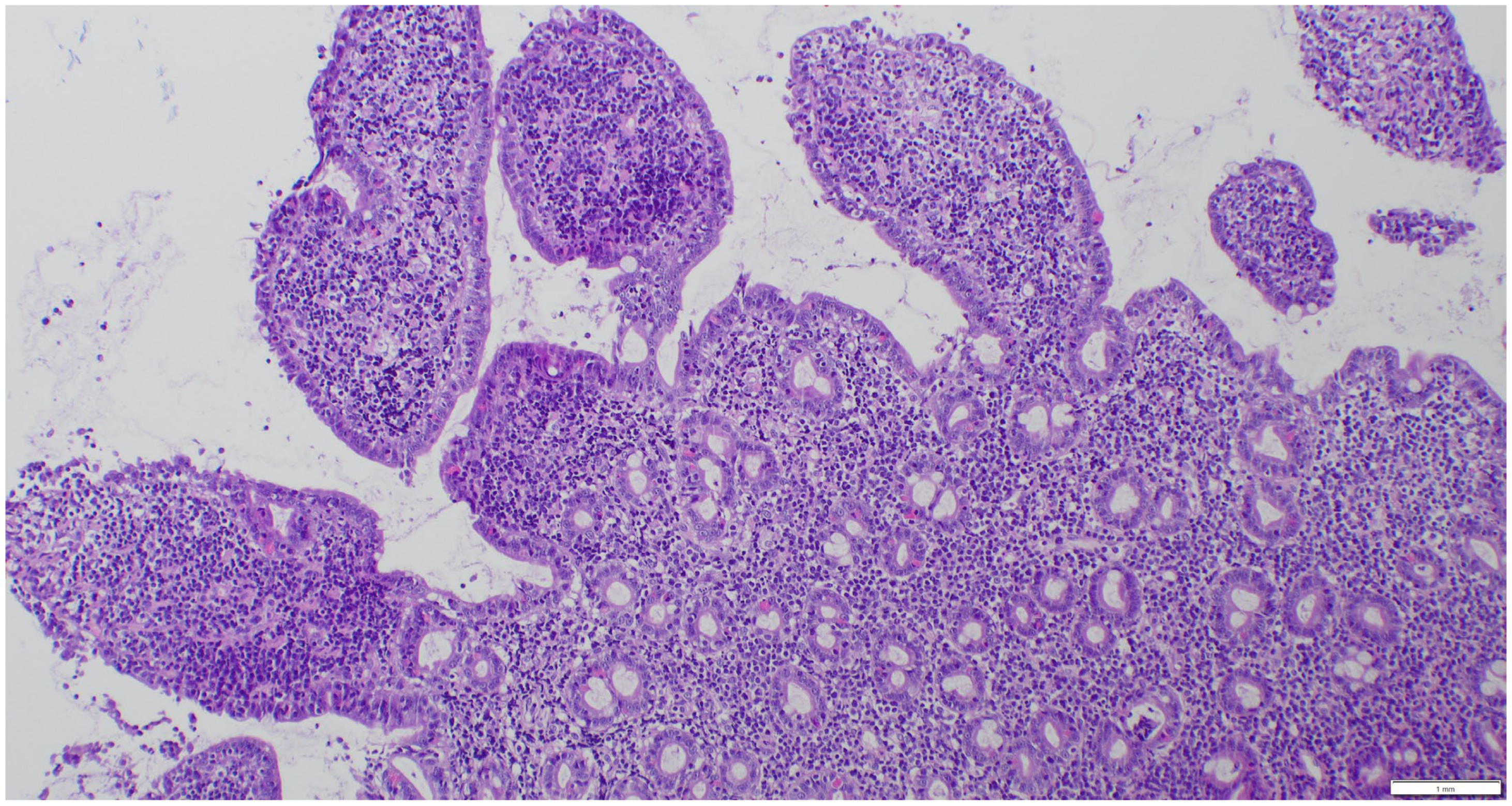
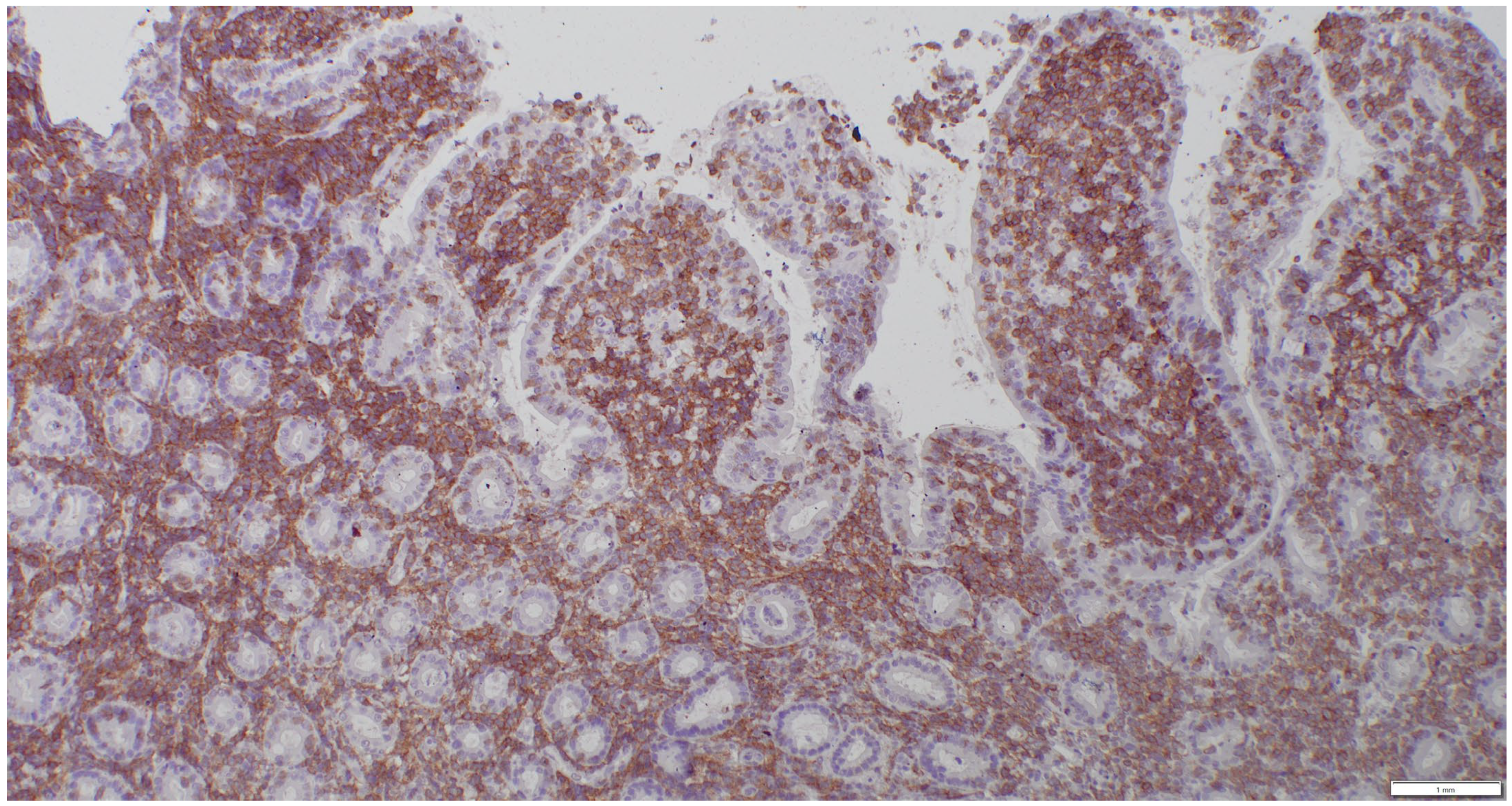


| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Duodenum and Jejunum | ||||||||
| Diagnostic Method | Histo + IHC | Clonality Testing | ||||||
| Diagnosis | n | LPE | n | LGITL | n | LPE | n | LGITL |
| Total Wall thickness | 21 | 2.52 ± 0.50 | 10 | 2.65 ± 0.41 | 19 | 2.47 ± 0.44 | 12 | 2.68 ± 0.51 |
| 2.50 (1.70–3.50) | 2.75 (2.00–3.20) | 2.40 (1.70–3.50) | 2.75 (1.80–3.30) | |||||
| Mucosa | 21 | 1.34 ± 0.36 | 10 | 1.29 ± 0.40 | 19 | 1.34 ± 0.39 | 12 | 1.30 ± 0.35 |
| 1.30 (0.60–2.10) | 1.30 (0.80–2.00) | 1.30 (0.60–2.00) | 1.30 (0.80–2.10) | |||||
| Submucosa | 21 | 0.40 ± 0.12 | 10 | 0.53 ± 0.12 | 19 | 0.43 ± 0.13 | 12 | 0.44 ± 0.14 |
| 0.40 (0.30–0.70) | 0.55 (0.30–0.70) | 0.40 (0.30–0.70) | 0.40 (0.30–0.70) | |||||
| Muscularis | 21 | 0.72 ± 0.44 | 10 | 0.73 ± 0.33 | 19 | 0.66 ± 0.36 | 12 | 0.80 ± 0.45 |
| 0.60 (0.30–1.80) | 0.65 (0.40–1.40) | 0.50 (0.30–1.70) | 0.70 (0.30–1.80) | |||||
| Muscularis: submucosa | 21 | 1.80 ± 1.01 | 10 | 1.44 ± 0.74 | 19 | 1.66 ± 0.97 | 12 | 1.82 ± 0.98 |
| 1.40 (1.00–4.50) | 1.20 (0.67–3.00) | 1.33 (0.67–4.25) | 1.37 (1.00–4.50) | |||||
| Muscularis: total wall thickness | 21 | 0.27 ± 0.13 | 10 | 0.28 ± 0.12 | 19 | 0.26 ± 0.12 | 12 | 0.29 ± 0.12 |
| 0.24 (0.13–0.59) | 0.25 (0.14–0.46) | 0.23 (0.13–0.59) | 0.26 (0.13–0.56) | |||||
| (b) | ||||||||
| Ileum | ||||||||
| Diagnostic Method | Histo + IHC | Clonality testing | ||||||
| Diagnosis | n | LPE | n | LGITL | n | LPE | n | LGITL |
| Total Wall thickness | 11 | 3.25 ± 0.68 | 4 | 3.78 ± 0.80 | 10 | 3.31 ± 0.87 | 5 | 3.54 ± 0.26 |
| 3.40 (2.00–4.20) | 3.60 (3.00–4.90) | 3.20 (2.00–4.90) | 3.60 (3.20–3.90) | |||||
| Mucosa | 11 | 1.40 ± 0.26 | 4 | 1.83 ± 0.54 | 10 | 1.48 ± 0.45 | 5 | 1.58 ± 0.24 |
| 1.40 (1.00–1.90) | 1.80 (1.20–2.50) | 1.35 (1.00–2.50) | 1.60 (1.30–1.90) | |||||
| Submucosa | 11 | 0.59 ± 0.14 | 4 | 0.55 ± 0.06 | 10 | 0.61 ± 0.14 | 5 | 0.52 ± 0.08 |
| 0.60 (0.40–0.80) | 0.55 (0.50–0.60) | 0.60 (0.40–0.80) | 0.50 (0.40–0.60) | |||||
| Muscularis | 11 | 1.33 ± 0.56 | 4 | 1.28 ± 0.25 | 10 | 1.26 ± 0.50 | 5 | 1.42 ± 0.50 |
| 1.20 (0.40–2.20) | 1.25 (1.00–1.60) | 1.25 (0.40–2.20) | 1.20 (1.00–2.10) | |||||
| Muscularis: submucosa | 11 | 2.35 ± 1.05 | 4 | 2.31 ± 0.29 | 10 | 2.17 ± 1.06 | 5 | 2.68 ± 0.58 |
| 2.40 (0.57–4.40) | 2.28 (2.00–2.67) | 2.17 (0.57–4.40) | 2.50 (2.00–3.50) | |||||
| Muscularis: total wall thickness | 11 | 0.39 ± 0.10 | 4 | 0.34 ± 0.05 | 10 | 0.36 ± 0.09 | 5 | 0.40 ± 0.12 |
| 0.38 (0.20–0.54) | 0.35 (0.28–0.40) | 0.36 (0.20–0.52) | 0.38 (0.28–0.54) | |||||
| Diagnostic Method | Histo + IHC | Clonality Testing | ||||||
|---|---|---|---|---|---|---|---|---|
| n | LPE | n | LGITL | n | LPE | n | LGITL | |
| Thickness (mm) of mesenteric lymph nodes | 11 | 5.84 ± 2.47 | 5 | 4.70 ± 1.64 | 11 | 5.79 ± 2.64 | 5 | 4.92 ± 1.34 |
| 6.00 (2.00–10.00) | 4.90 (2.50–7.00) | 6.00 (2.00–10.00) | 5.00 (3.00–6.80) | |||||
| Time Point | 0 (Baseline Examination) | 12th Week of Treatment | 24th Week of Treatment | |||||||||
| Diagnosis | n | LPE | n | LGITL | n | LPE | n | LGITL | n | LPE | n | LGITL |
| Total Wall thickness | 10 | 2.60 ± 0.55 2.70 (1.70–3.30) | 4 | 2.60 ± 0.44 2.60 (2.10–3.10) | 10 | 2.63 ± 0.38 2.65 (2.00–3.20) | 4 | 2.70 ± 0.38 2.60 (2.40–3.20) | 6 | 2.52 ± 0.51 2.40 (1.80–3.20) | 4 | 2.68 ± 0.71 2.40 (2.20–3.70) |
| Mucosa | 10 | 1.22 ± 0.41 1.20 (0.60–2.00) | 4 | 1.38 ± 0.61 1.35 (0.80–2.00) | 10 | 1.70 ± 0.52 1.55 (1.10–2.80) | 4 | 1.80 ± 0.39 1.75 (1.40–2.30) | 6 | 1.52 ± 0.21 1.45 (1.30–1.90) | 4 | 1.48 ± 0.55 1.35 (1.00–2.20) |
| Submucosa | 10 | 0.43 ± 0.14 0.40 (0.30–0.70) | 4 | 0.50 ± 0.14 0.55 (0.30–0.60) | 10 | 0.40 ± 0.12 0.45 (0.20–0.50) | 4 | 0.48 ± 0.10 0.45 (0.40–0.60) | 6 | 0.40 ± 0.11 0.40 (0.30–0.60) | 4 | 0.40 ± 0.08 0.40 (0.30–0.50) |
| Muscularis | 10 | 0.94 ± 0.55 0.80 (0.30–1.80) | 4 | 0.75 ± 0.31 0.75 (0.40–1.10) | 10 | 0.51 ± 0.09 0.50 (0.40–0.70) | 4 | 0.55 ± 0.13 0.55 (0.40–0.70) | 6 | 0.48 ± 0.12 0.50 (0.30–0.60) | 4 | 0.60 ± 0.22 0.55 (0.40–0.90) |
| Muscularis: submucosa | 10 | 2.25 ± 1.35 1.67 (1.00–4.50) | 4 | 1.66 ± 1.00 1.42 (0.80–3.00) | 10 | 1.38 ± 0.44 1.33 (0.80–2.00) | 4 | 1.16 ± 0.20 1.13 (1.00–1.40) | 6 | 1.26 ± 0.43 1.13 (0.83–2.00) | 4 | 1.52 ± 0.53 1.42 (1.00–2.25) |
| Muscularis: total wall thickness | 10 | 0.34 ± 0.15 0.32 (0.18–0.59) | 4 | 0.31 ± 0.16 0.31 (0.14–0.46) | 10 | 0.20 ± 0.04 0.20 (0.13–0.25) | 4 | 0.21 ± 0.07 0.21 (0.13–0.29) | 6 | 0.19 ± 0.03 0.18 (0.17–0.24) | 4 | 0.24 ± 0.12 0.19 (0.16–0.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beatrice, L.; Föhr, J.; Grest, P.; Ruetten, M.; Henrich, M.; Vincenti, S.; Campbell, K.; Kook, P.H. Intestinal Ultrasonographic Measurements in Cats Diagnosed with Lymphoplasmacytic Enteritis and Low-Grade T-Cell Lymphoma Based on Either Histology/Immunohistochemistry or Clonality Testing—And Assessment of the Effects of Therapy on Wall Layering After 3 and 6 Months of Treatment. Animals 2025, 15, 1518. https://doi.org/10.3390/ani15111518
Beatrice L, Föhr J, Grest P, Ruetten M, Henrich M, Vincenti S, Campbell K, Kook PH. Intestinal Ultrasonographic Measurements in Cats Diagnosed with Lymphoplasmacytic Enteritis and Low-Grade T-Cell Lymphoma Based on Either Histology/Immunohistochemistry or Clonality Testing—And Assessment of the Effects of Therapy on Wall Layering After 3 and 6 Months of Treatment. Animals. 2025; 15(11):1518. https://doi.org/10.3390/ani15111518
Chicago/Turabian StyleBeatrice, Laura, Junwei Föhr, Paula Grest, Maja Ruetten, Manfred Henrich, Simona Vincenti, Karolin Campbell, and Peter Hendrik Kook. 2025. "Intestinal Ultrasonographic Measurements in Cats Diagnosed with Lymphoplasmacytic Enteritis and Low-Grade T-Cell Lymphoma Based on Either Histology/Immunohistochemistry or Clonality Testing—And Assessment of the Effects of Therapy on Wall Layering After 3 and 6 Months of Treatment" Animals 15, no. 11: 1518. https://doi.org/10.3390/ani15111518
APA StyleBeatrice, L., Föhr, J., Grest, P., Ruetten, M., Henrich, M., Vincenti, S., Campbell, K., & Kook, P. H. (2025). Intestinal Ultrasonographic Measurements in Cats Diagnosed with Lymphoplasmacytic Enteritis and Low-Grade T-Cell Lymphoma Based on Either Histology/Immunohistochemistry or Clonality Testing—And Assessment of the Effects of Therapy on Wall Layering After 3 and 6 Months of Treatment. Animals, 15(11), 1518. https://doi.org/10.3390/ani15111518






