Metagenomic Analysis Reveals the Characteristics of Cecal Microbiota in Chickens with Different Levels of Resistance During Recovery from Eimeria tenella Infection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Oocysts
2.2. Sample Collection
2.3. Growth Performance
2.4. Intestinal Morphology
2.5. DNA Extraction and Metagenome Sequencing Analysis
2.6. Functional Annotation Analysis
2.7. Statistical Analysis
3. Results
3.1. Chicken Models of E. tenella Infection
3.2. Effect of Growth Performance in Different Resistance Groups
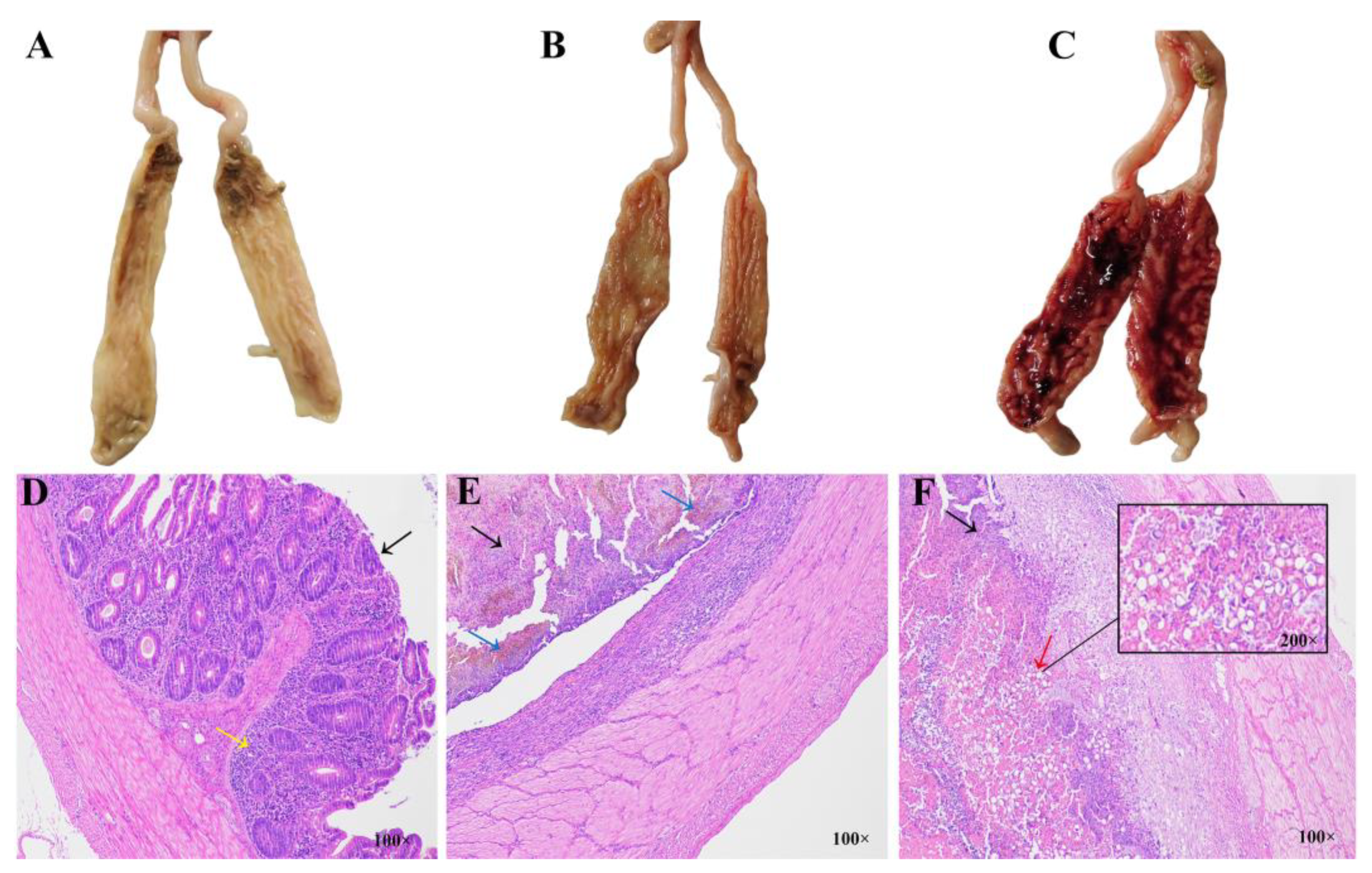
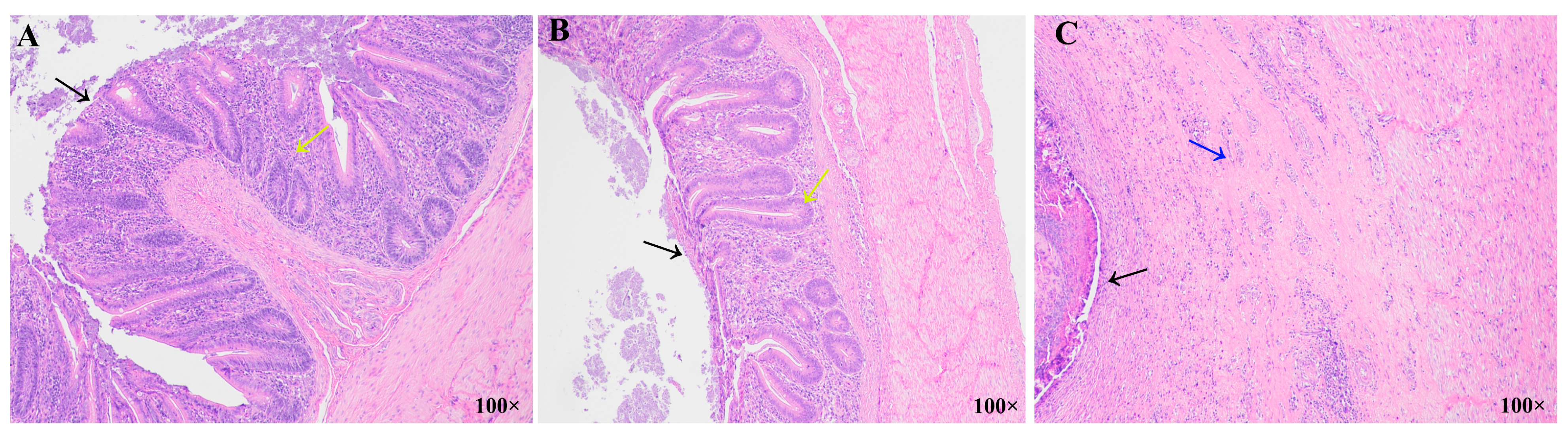
3.3. Histopathological Analysis of the Ceca in Different Resistance Groups During Recovery
3.4. Changes in Cecal Microbial Diversity in Different Resistance Groups During Recovery
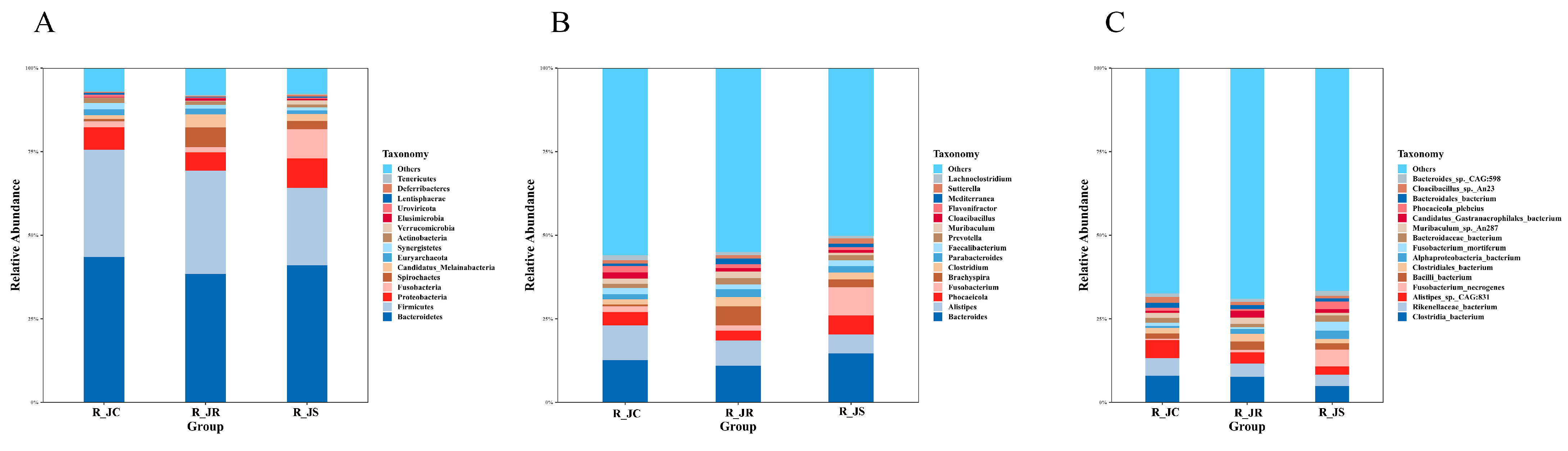
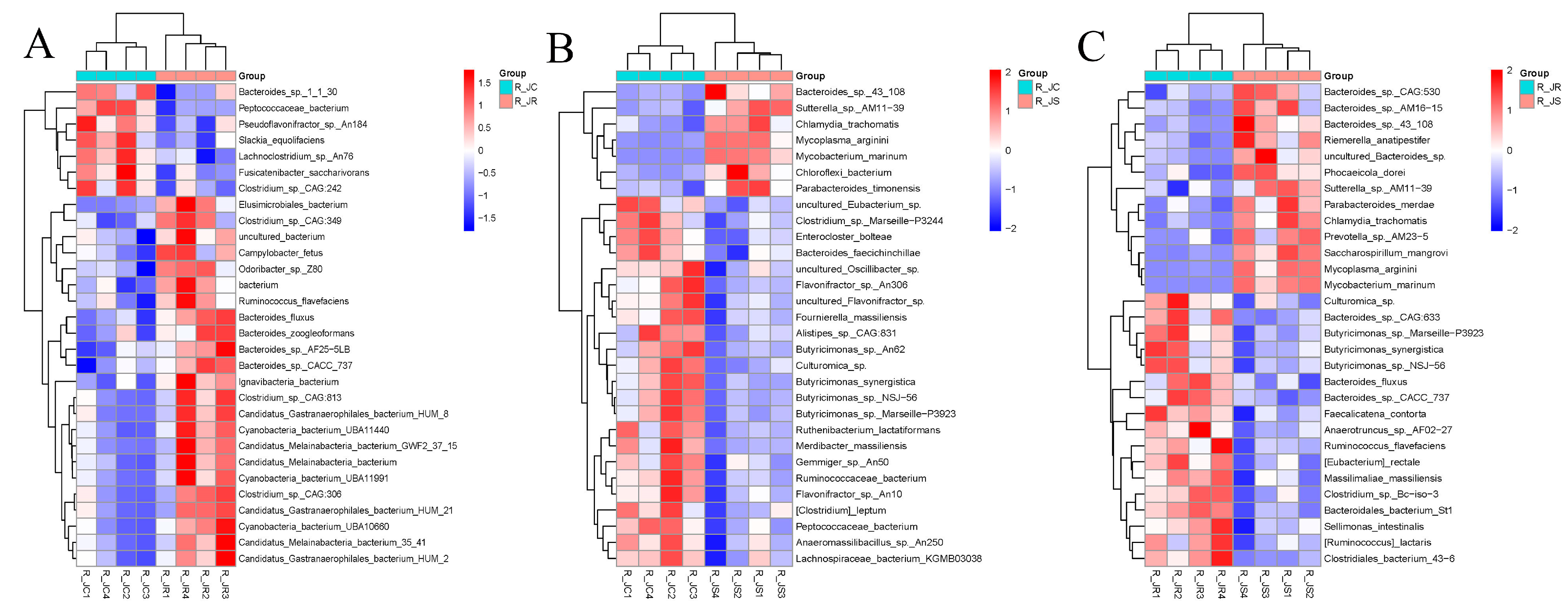
3.5. Composition of Cecal Microbiota in Different Resistance Groups During Recovery
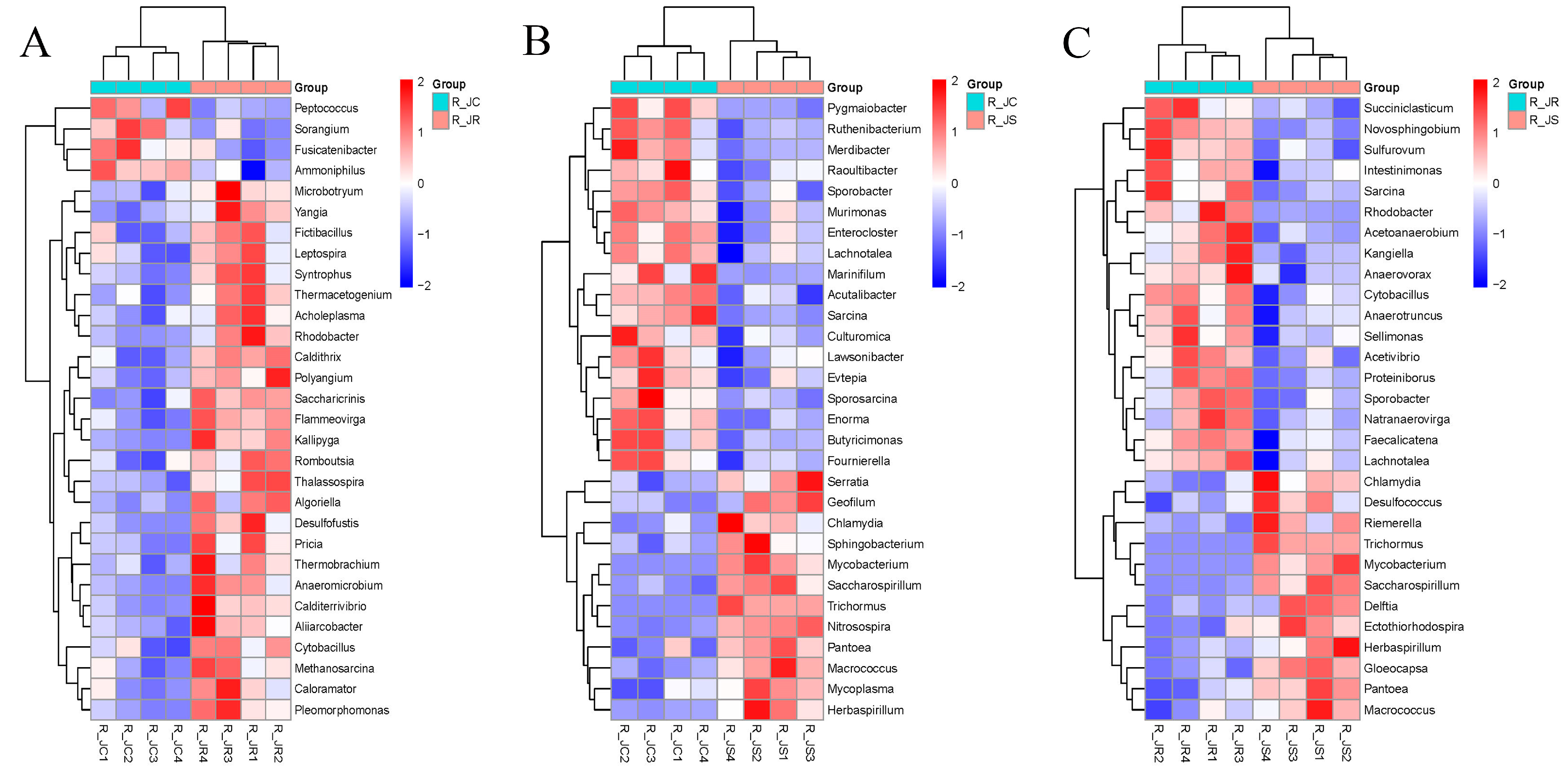
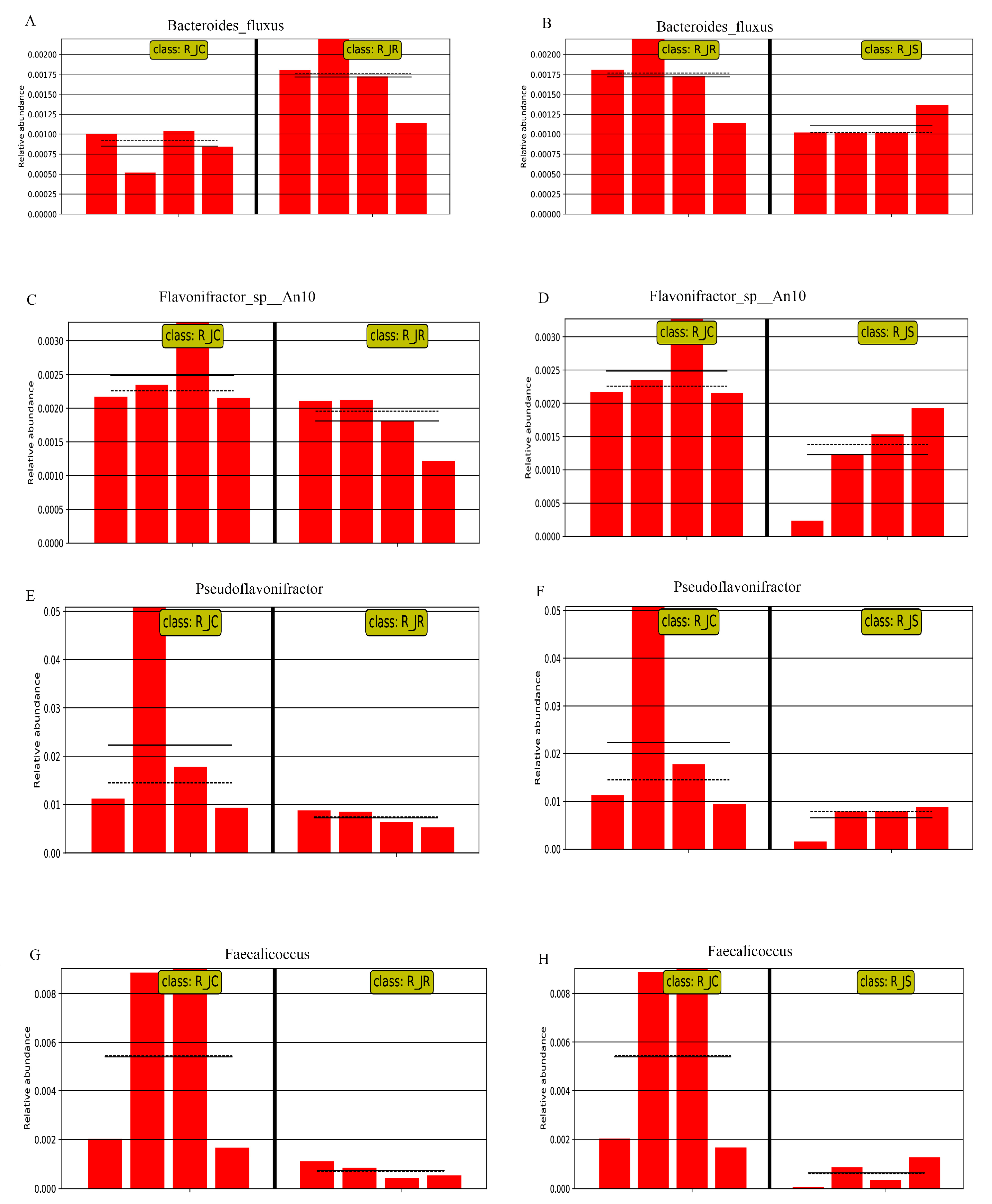
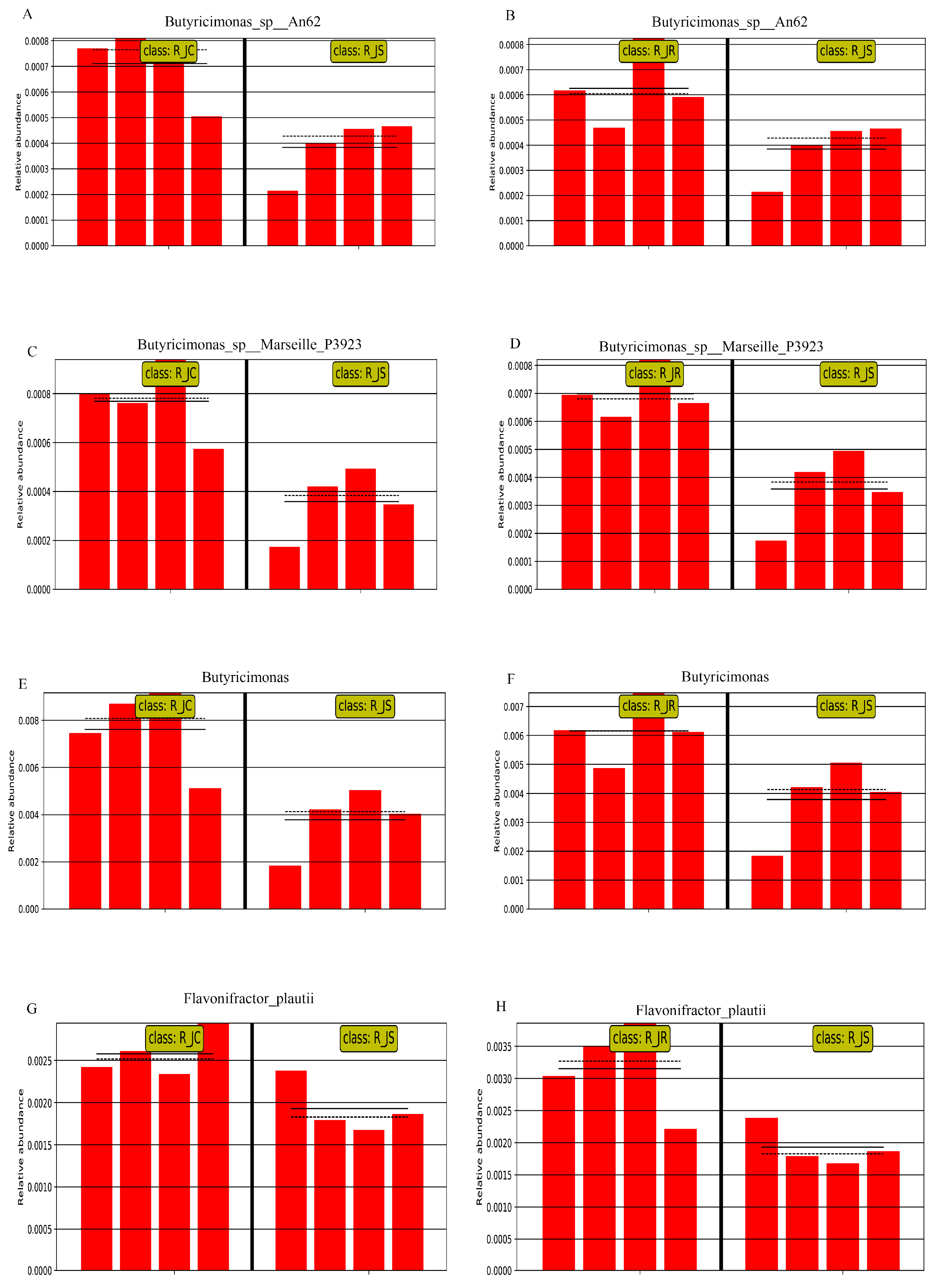
3.6. Differential Abundance Analysis of Cecal Microbiota Between Different Resistance Groups During Recovery
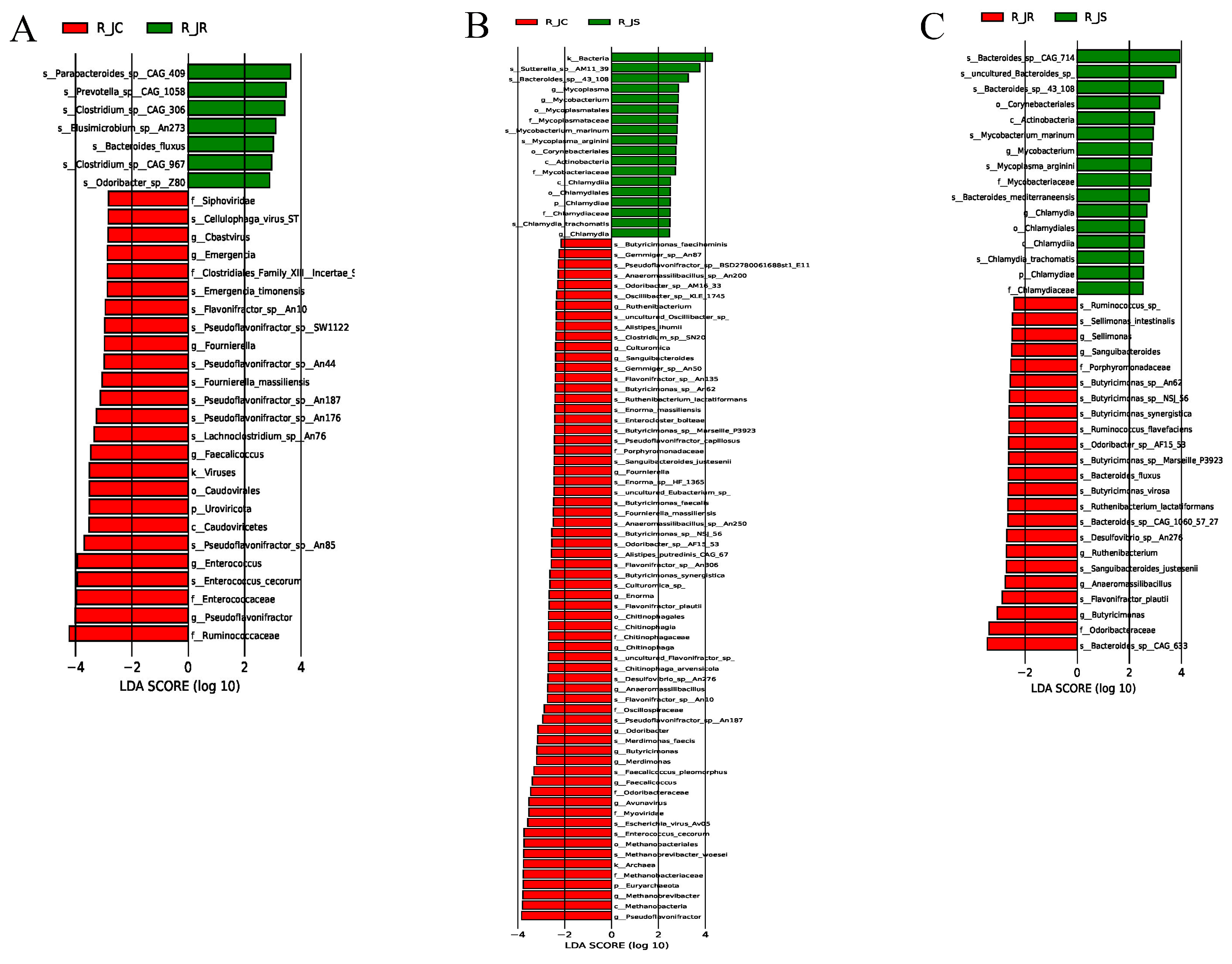
3.7. LEfSe Analysis of Differential Cecal Microbiota Between Different Resistance Groups During Recovery
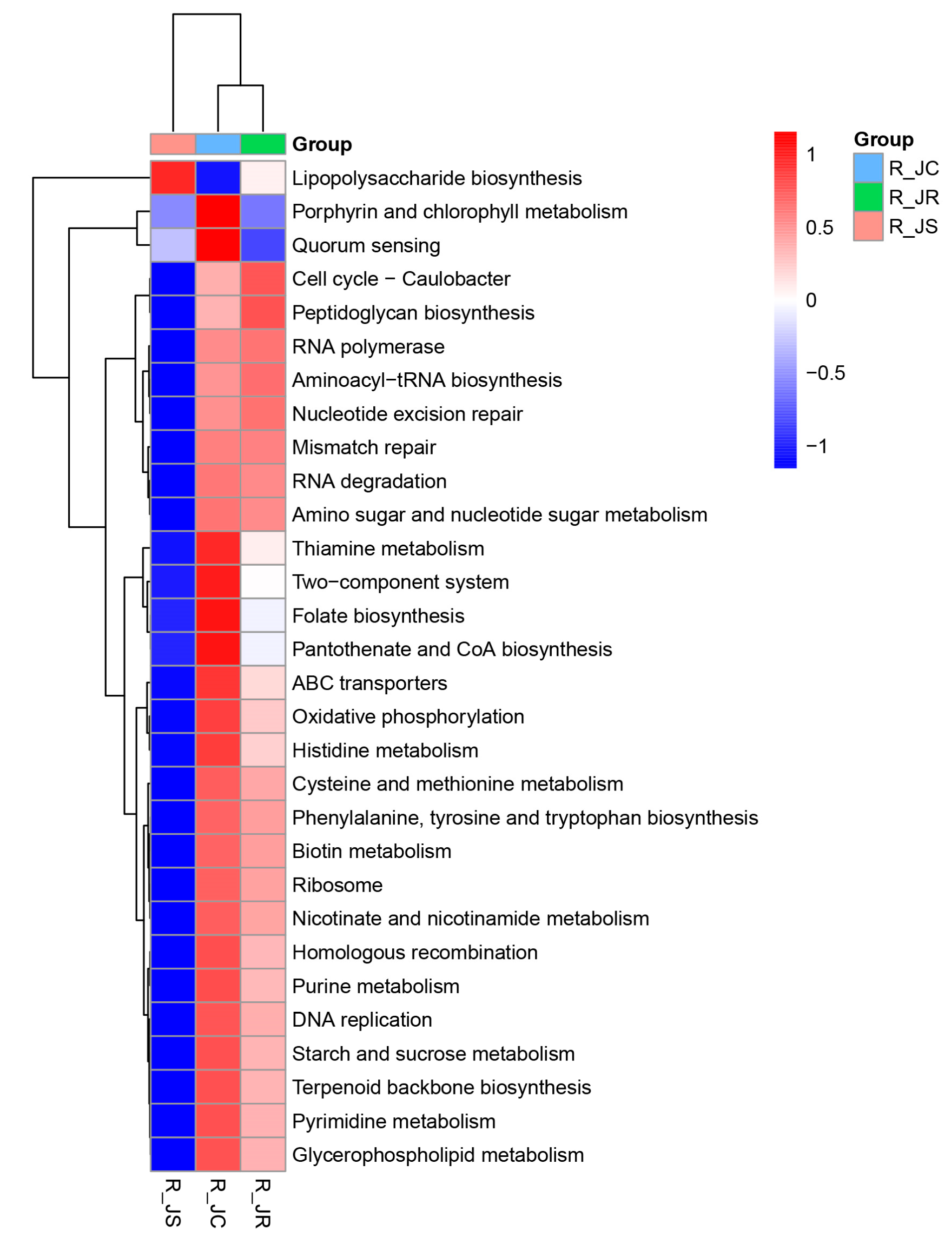
3.8. Functional Enrichment Analysis of Cecal Microbiota Between Different Resistance Groups During Recovery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Zou, W.; Wang, X.; Dai, G.; Zhang, T.; Zhang, G.; Xie, K.; Wang, J.; Shi, H. Research Note: Correlation analysis of interleukin-6, interleukin-8, and C-C motif chemokine ligand 2 gene expression in chicken spleen and cecal tissues after Eimeria tenella infection in vivo. Poult. Sci. 2020, 99, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Wickramasuriya, S.S.; Park, I.; Lee, Y.; Richer, L.M.; Przybyszewski, C.; Gay, C.G.; van Oosterwijk, J.G.; Lillehoj, H.S. Orally delivered Bacillus subtilis expressing chicken NK-2 peptide stabilizes gut microbiota and enhances intestinal health and local immunity in coccidiosis-infected broiler chickens. Poult. Sci. 2023, 102, 102590. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Vrba, V.; Xia, D.; Jatau, I.D.; Spiro, S.; Nolan, M.J.; Underwood, G.; Tomley, F.M. Genetic and biological characterisation of three cryptic Eimeria operational taxonomic units that infect chickens (Gallus gallus domesticus). Int. J. Parasitol. 2021, 51, 621–634. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhao, X.Y.; Zhao, G.X.; Huang, H.B.; Li, H.R.; Shi, C.W.; Yang, W.T.; Jiang, Y.L.; Wang, J.Z.; Ye, L.P.; et al. Dissection of the cecal microbial community in chickens after Eimeria tenella infection. Parasites Vectors 2020, 13, 56. [Google Scholar] [CrossRef]
- Jebessa, E.; Guo, L.; Chen, X.; Bello, S.F.; Cai, B.; Girma, M.; Hanotte, O.; Nie, Q. Influence of Eimeria maxima coccidia infection on gut microbiome diversity and composition of the jejunum and cecum of indigenous chicken. Front. Immunol. 2022, 13, 994224. [Google Scholar] [CrossRef]
- Song, X.; Gao, Y.; Xu, L.; Yan, R.; Li, X. Partial protection against four species of chicken coccidia induced by multivalent subunit vaccine. Vet. Parasitol. 2015, 212, 80–85. [Google Scholar] [CrossRef]
- Wang, B.; Du, P.; Huang, S.; He, D.; Chen, J.; Wen, X.; Yang, J.; Xian, S.; Cheng, Z. Comparison of the caecal microbial community structure and physiological indicators of healthy and infection Eimeria tenella chickens during peak of oocyst shedding. Avian Pathol. 2023, 52, 51–61. [Google Scholar] [CrossRef]
- Yu, H.; Mi, C.; Wang, Q.; Dai, G.; Zhang, T.; Zhang, G.; Xie, K.; Zhao, Z. Long noncoding RNA profiling reveals that LncRNA BTN3A2 inhibits the host inflammatory response to Eimeria tenella infection in chickens. Front. Immunol. 2022, 13, 891001. [Google Scholar] [CrossRef]
- Burrell, A.; Tomley, F.M.; Vaughan, S.; Marugan-Hernandez, V. Life cycle stages, specific organelles and invasion mechanisms of Eimeria species. Parasitology 2020, 147, 263–278. [Google Scholar] [CrossRef]
- Leung, H.; Yitbarek, A.; Snyder, R.; Patterson, R.; Barta, J.R.; Karrow, N.; Kiarie, E. Responses of broiler chickens to Eimeria challenge when fed a nucleotide-rich yeast extract. Poult. Sci. 2019, 98, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.R.A.; Shah, M.A.; Riaz, A.; Malik, A.M.; ul Hasan, M.; Li, X.R.; Babar, W.; Shahid, S.K.A. Identification and Molecular Characterization of Eimeria tenella based on EtMic5 Gene in Pakistan. Pak. Vet. J. 2020, 40, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- Rubio, L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2019, 98, 695–706. [Google Scholar] [CrossRef]
- Baldwin, S.; Hughes, R.J.; Hao Van, T.T.; Moore, R.J.; Stanley, D. At-hatch administration of probiotic to chickens can introduce beneficial changes in gut microbiota. PLoS ONE 2018, 13, e0194825. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, S.; Zhou, C.; Tang, X.; Li, C.; Wang, C.; Tang, X.; Suo, J.; Jia, Y.; El-Ashram, S.; et al. Influence of Eimeria falciformis Infection on Gut Microbiota and Metabolic Pathways in Mice. Infect. Immun. 2018, 86, e00073-18. [Google Scholar] [CrossRef]
- Madlala, T.; Okpeku, M.; Adeleke, M.A. Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: A review. Parasite 2021, 28, 48. [Google Scholar] [CrossRef]
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A review on prebiotics and probiotics for the control of dysbiosis: Present status and future perspectives. Animal 2015, 9, 43–48. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Nolan, M.J.; Harman, K.; Boulton, K.; Hume, D.A.; Tomley, F.M.; Stabler, R.A.; Blake, D.P. Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. PLoS ONE 2017, 12, e0184890. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.R.; Fukata, T.; Baba, E.; Arakawa, A. Effect of Eimeria tenella infection on Salmonella enteritidis infection in chickens. Poult. Sci. 1995, 74, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.T.; Hofacre, C.L.; Payne, A.M.; Anderson, D.B.; Kaiser, P.; Mackie, R.I.; Gaskins, H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef]
- Macdonald, S.E.; van Diemen, P.M.; Martineau, H.; Stevens, M.P.; Tomley, F.M.; Stabler, R.A.; Blake, D.P. Impact of Eimeria tenella Coinfection on Campylobacter jejuni Colonization of the Chicken. Infect. Immun. 2019, 87, e00772-18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.H.; Jia, L.S.; Wei, S.S.; Ding, H.Y.; Yang, J.Y.; Wang, H.W. Effects of Eimeria tenella infection on the barrier damage and microbiota diversity of chicken cecum. Poult. Sci. 2020, 99, 1297–1305. [Google Scholar] [CrossRef]
- Bishop, S.C.; Woolliams, J.A. Genomics and disease resistance studies in livestock. Livest. Sci. 2014, 166, 190–198. [Google Scholar] [CrossRef]
- Jeffers, T.K.; Challey, J.R.; McGibbon, W.H. Response of several lines of fowl and their single-cross progeny to experimental infection with Eimeria tenella. Avian Dis. 1970, 14, 203–210. [Google Scholar] [CrossRef]
- Palafox, A.L.; Alicata, J.E.; Kartman, L. Breeding chickens for resistance to cecal coccidiosis. World’s Poult. Sci. J. 1949, 5, 84–87. [Google Scholar] [CrossRef]
- Choi, J.; Ko, H.; Tompkins, Y.H.; Teng, P.Y.; Lourenco, J.M.; Callaway, T.R.; Kim, W.K. Effects of Eimeria tenella Infection on Key Parameters for Feed Efficiency in Broiler Chickens. Animals 2021, 11, 3428. [Google Scholar] [CrossRef]
- Du, S.; Song, Z.; Cen, Y.; Fan, J.; Li, P.; Si, H.; Hu, D. Susceptibility and cecal microbiota alteration to Eimeria-infection in Yellow-feathered broilers, Arbor Acres broilers and Lohmann pink layers. Poult. Sci. 2024, 103, 103824. [Google Scholar] [CrossRef]
- Tang, J.; Wang, Q.; Yu, H.; Dong, L.; Tang, M.; Arif, A.; Zhang, G.; Zhang, T.; Xie, K.; Su, S.; et al. A Comparison of the Cecal Microbiota between the Infection and Recovery Periods in Chickens with Different Susceptibilities to Eimeria tenella. Animals 2024, 14, 2709. [Google Scholar] [CrossRef] [PubMed]
- Bandara, R.M.A.S.; Torrey, S.; Turner, P.V.; Zur Linden, A.; Schwean-Lardner, K.; Widowski, T.M. Efficacy of three different cervical dislocation methods for on-farm killing of layer chicks. Poult. Sci. 2023, 102, 102538. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Deng, J.; Yang, Z.; Zeng, L.; Yang, X. Metagenomic analysis reveals linkages between cecal microbiota and feed efficiency in Xiayan chickens. Poult. Sci. 2020, 99, 7066–7075. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Xiang, J.; Li, J.; Yang, M.; Zhang, Z.; Zhang, L.; Zhang, G.; Yang, Y.; Liu, G.; Lu, Y.; et al. Natural Magnolol ameliorates coccidiosis infected with Eimeria tenella by affecting antioxidant, anti-inflammatory, and gut microbiota of chicks. Poult. Sci. 2023, 102, 102975. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, W.; Wang, D.; Xu, W. Effect of myoglobin, hemin, and ferric iron on quality of chicken breast meat. Anim. Biosci. 2021, 34, 1382–1391. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2014, 42, D199–D205. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.R.; Apajalahti, J.H. The influence of nutrition on intestinal disease with emphasis on coccidiosis. Avian Pathol. 2022, 51, 504–520. [Google Scholar] [CrossRef]
- van Eerden, E.; Santos, R.R.; Molist, F.; Dardi, M.; Pantoja-Millasm, L.A.; Molist-Badiola, J.; Baratelli, M.; Pages, M. Efficacy of an attenuated vaccine against avian coccidiosis in combination with feed additives based on organic acids and essential oils on production performance and intestinal lesions in broilers experimentally challenged with necrotic enteritis. Poult. Sci. 2022, 101, 101848. [Google Scholar] [CrossRef]
- Attree, E.; Sanchez-Arsuaga, G.; Jones, M.; Xia, D.; Marugan-Hernandez, V.; Blake, D.; Tomley, F. Controlling the causative agents of coccidiosis in domestic chickens; an eye on the past and considerations for the future. CABI Agric. Biosci. 2021, 2, 37. [Google Scholar] [CrossRef]
- Boulton, K.; Nolan, M.J.; Wu, Z.; Psifidi, A.; Riggio, V.; Harman, K.; Bishop, S.C.; Kaiser, P.; Abrahamsen, M.S.; Hawken, R.; et al. Phenotypic and genetic variation in the response of chickens to Eimeria tenella induced coccidiosis. Genet. Sel. Evol. 2018, 50, 63. [Google Scholar] [CrossRef]
- Jordan, A.; Caldwell, D.J.; Klein, J.; Coppedge, J.; Pohl, S.; Fitz-Coy, S.; Lee, J.T. Eimeria tenella oocyst shedding and output in cecal or fecal contents following experimental challenge in broilers. Poult. Sci. 2011, 90, 990–995. [Google Scholar] [CrossRef]
- Rubin, D.C.; Levin, M.S. Mechanisms of intestinal adaptation. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 237–248. [Google Scholar] [CrossRef]
- Wang, H.W.; Liu, J.; Zhao, W.P.; Zhang, Z.H.; Li, S.Q.; Li, S.H.; Zhu, S.Q.; Zhou, B.H. Effect of Fluoride on Small Intestine Morphology and Serum Cytokine Contents in Rats. Biol. Trace Elem. Res. 2019, 189, 511–518. [Google Scholar] [CrossRef]
- Kong, C.; Yang, M.; Yue, N.; Zhang, Y.; Tian, C.; Wei, D.; Shi, R.; Yao, J.; Wang, L.; Li, D. Restore Intestinal Barrier Integrity: An Approach for Inflammatory Bowel Disease Therapy. J. Inflamm. Res. 2024, 17, 5389–5413. [Google Scholar] [CrossRef]
- Cereijido, M.; Shoshani, L.; Contreras, R.G. Molecular physiology and pathophysiology of tight junctions I. Biogenesis of tight junctions and epithelial polarity. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G477–G482. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- McCauley, H.A.; Guasch, G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015, 21, 492–503. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Hansson, G.C.; Johansson, M.E. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes 2010, 1, 51–54. [Google Scholar] [CrossRef]
- Round, A.N.; Rigby, N.M.; Garcia de la Torre, A.; Macierzanka, A.; Mills, E.N.; Mackie, A.R. Lamellar structures of MUC2-rich mucin: A potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules 2012, 13, 3253–3261. [Google Scholar] [CrossRef]
- Zhou, B.H.; Wang, H.W.; Wang, X.Y.; Zhang, L.F.; Zhang, K.Y.; Xue, F.Q. Eimeria tenella: Effects of diclazuril treatment on microneme genes expression in second-generation merozoites and pathological changes of caeca in parasitized chickens. Exp. Parasitol. 2010, 125, 264–270. [Google Scholar] [CrossRef]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef]
- Lamendella, R.; Domingo, J.W.; Ghosh, S.; Martinson, J.; Oerther, D.B. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 2011, 11, 103. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Medinger, R.; Nolte, V.; Pandey, R.V.; Jost, S.; Ottenwälder, B.; Schlötterer, C.; Boenigk, J. Diversity in a hidden world: Potential and limitation of next-generation sequencing for surveys of molecular diversity of eukaryotic microorganisms. Mol. Ecol. 2010, 19 (Suppl. 1), 32–40. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Yang, X.; Pan, X.; Jia, Z.; Bai, B.; Zhi, W.; Chen, H.; Ma, C.; Ma, D. Oral administration of Lactobacillus brevis 23017 combined with ellagic acid attenuates intestinal inflammatory injury caused by Eimeria infection by activating the Nrf2/HO-1 antioxidant pathway. Vet. Res. 2022, 53, 21. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Biggs, P.; Parsons, C.M. The effects of several organic acids on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult. Sci. 2008, 87, 2581–2589. [Google Scholar] [CrossRef]
- Dwars, R.M.; Davelaar, F.G.; Smit, H.F. Infection of broiler parent hens with avian intestinal spirochaetes: Effects on egg production and chick quality. Avian Pathol. 1993, 22, 693–701. [Google Scholar] [CrossRef]
- Kollarcikova, M.; Kubasova, T.; Karasova, D.; Crhanova, M.; Cejkova, D.; Sisak, F.; Rychlik, I. Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poult. Sci. 2019, 98, 2347–2353. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important Metabolic Pathways and Biological Processes Expressed by Chicken Cecal Microbiota. Appl. Environ. Microbiol. 2015, 82, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Medvecky, M.; Cejkova, D.; Polansky, O.; Karasova, D.; Kubasova, T.; Cizek, A.; Rychlik, I. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genom. 2018, 19, 561. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.; Glastras, S.J.; Wong, M.Y.W.; Pollock, C.A.; Saad, S. The Role of the Gut Microbiome in Diabetes and Obesity-Related Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9641. [Google Scholar] [CrossRef] [PubMed]
- Cobo, F.; Pérez-Carrasco, V.; Gómez-Vicente, E.; Martín-Hita, L.; García-Salcedo, J.A.; Navarro-Marí, J.M. First case of abdominal infection caused by Bacteroides fluxus. Anaerobe 2021, 69, 102363. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Lane, N.E.; Wu, J.; Yang, T.; Li, J.; He, H.; Wei, J.; Zeng, C.; Lei, G. Population-based metagenomics analysis reveals altered gut microbiome in sarcopenia: Data from the Xiangya Sarcopenia Study. J. Cachexia Sarcopenia Muscle 2022, 13, 2340–2351. [Google Scholar] [CrossRef]
- Gavande, P.V.; Nath, P.; Kumar, K.; Ahmed, N.; Fontes, C.M.G.A.; Goyal, A. Highly efficient, processive and multifunctional recombinant endoglucanase RfGH5_4 from Ruminococcus flavefaciens FD-1 v3 for recycling lignocellulosic plant biomasses. Int. J. Biol. Macromol. 2022, 209 Pt A, 801–813. [Google Scholar] [CrossRef]
- Helaszek, C.T.; White, B.A. Cellobiose uptake and metabolism by Ruminococcus flavefaciens. Appl. Environ. Microbiol. 1991, 57, 64–68. [Google Scholar] [CrossRef]
- Kim, J.A.; Jung, M.Y.; Kim, D.H.; Kim, Y. Genome analysis of Bacteroides sp. CACC 737 isolated from feline for its potential application. J. Anim. Sci. Technol. 2020, 62, 952–955. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Kaplan, H.M.; Ok, F.; Demir, E. Alpha-linolenic Acid Attenuates Lipopolysaccharide Induced Cystitis. Int. J. Pharmacol. 2017, 13, 649–653. [Google Scholar]
- Duan, Y.T.; Wang, W.D.; Qin, S.Y.; Xu, X.; Li, B.X.; Chen, M.J.; Zheng, C.L. Study on the performance of Anerinibacillus sp. in degrading cyanide wastewater and its metabolic mechanism. Chemosphere 2023, 345, 140354. [Google Scholar] [CrossRef] [PubMed]
- Derqaoui, S.; Oukessou, M.; Attrassi, K.; Elftouhy, F.Z.; Nassik, S. Detection of Sutterella spp. in Broiler Liver and Breast. Front. Vet. Sci. 2022, 9, 859902. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Van Immerseel, F.; Croubels, S.; De Baere, S.; Haesebrouck, F.; Ducatelle, R.; Louis, P.; Vandamme, P. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microb. Biotechnol. 2011, 4, 503–512. [Google Scholar] [CrossRef]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral administration of Flavonifractor plautii attenuates inflammatory responses in obese adipose tissue. Mol. Biol. Rep. 2020, 47, 6717–6725. [Google Scholar] [CrossRef]
- Sun, H.; Li, J.; Wang, L.; Yin, K.; Xu, C.; Liu, G.; Xiao, T.; Huang, B.; Wei, Q.; Gong, M.; et al. Comparative Proteomics Analysis for Elucidating the Interaction Between Host Cells and Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2021, 11, 643001. [Google Scholar] [CrossRef]
- Grabacka, M.; Pierzchalska, M.; Płonka, P.M.; Pierzchalski, P. The Role of PPAR Alpha in the Modulation of Innate Immunity. Int. J. Mol. Sci. 2021, 22, 10545. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef]




| Items | JC | JR | JS |
|---|---|---|---|
| 0 to 5 days PI (Infection period) | |||
| Body weight * (BW; g) | 717.75 ± 47.29 A | 646.33 ± 28.14 B | 618.30 ± 36.37 B |
| Average daily gain (ADG; g/d) | 25.90 ± 2.47 A | 15.37 ± 3.22 B | 4.36 ± 1.37 C |
| Average daily feed intake (ADFI; g/d) | 54.51 ± 6.16 A | 48.13 ± 1.06 A | 32.49 ± 4.67 B |
| Feed conversion ratio (FCR; g/g) | 2.10 ± 0.17 C | 3.13 ± 0.74 B | 7.45 ± 1.85 A |
| Items | R_JC | R_JR | R_JS |
| 6 to 27 days PI (Recovery period) | |||
| Body weight ** (BW; g) | 1112.00 ± 131.57 A | 940.00 ± 67.11 B | 804.75 ± 131.43 C |
| Average daily gain (ADG; g/d) | 13.54 ± 2.28 A | 10.40 ± 3.49 B | 8.04 ± 1.91 B |
| Average daily feed intake (ADFI; g/d) | 60.24 ± 2.26 B | 64.05 ± 2.40 B | 74.81 ± 1.41 A |
| Feed conversion ratio (FCR; g/g) | 4.45 ± 0.69 C | 6.16 ± 0.53 B | 9.30 ± 0.91 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Dong, L.; Tang, M.; Arif, A.; Zhang, H.; Zhang, G.; Zhang, T.; Xie, K.; Su, S.; Zhao, Z.; et al. Metagenomic Analysis Reveals the Characteristics of Cecal Microbiota in Chickens with Different Levels of Resistance During Recovery from Eimeria tenella Infection. Animals 2025, 15, 1500. https://doi.org/10.3390/ani15101500
Tang J, Dong L, Tang M, Arif A, Zhang H, Zhang G, Zhang T, Xie K, Su S, Zhao Z, et al. Metagenomic Analysis Reveals the Characteristics of Cecal Microbiota in Chickens with Different Levels of Resistance During Recovery from Eimeria tenella Infection. Animals. 2025; 15(10):1500. https://doi.org/10.3390/ani15101500
Chicago/Turabian StyleTang, Jianqiang, Liyue Dong, Meihui Tang, Areej Arif, Honghong Zhang, Genxi Zhang, Tao Zhang, Kaizhou Xie, Shijie Su, Zhenhua Zhao, and et al. 2025. "Metagenomic Analysis Reveals the Characteristics of Cecal Microbiota in Chickens with Different Levels of Resistance During Recovery from Eimeria tenella Infection" Animals 15, no. 10: 1500. https://doi.org/10.3390/ani15101500
APA StyleTang, J., Dong, L., Tang, M., Arif, A., Zhang, H., Zhang, G., Zhang, T., Xie, K., Su, S., Zhao, Z., & Dai, G. (2025). Metagenomic Analysis Reveals the Characteristics of Cecal Microbiota in Chickens with Different Levels of Resistance During Recovery from Eimeria tenella Infection. Animals, 15(10), 1500. https://doi.org/10.3390/ani15101500






