Nationwide Geographical and Temporal Distribution of Tick-Borne Diseases in Korean Water Deer (Hydropotes inermis argyropus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Collection
2.3. Nucleic Acids Extraction and Processing
2.4. Detection of Diseases Using Real-Time PCR
2.5. Detection of Diseases Using Conventional PCR
2.6. Genetic Sequencing and Phylogenetic Study
2.7. Statistical Examination
2.8. GenBank Accession Numbers
3. Results
3.1. Prevalence Analysis Using Real-Time PCR
3.2. Prevalence Analysis Using Conventional PCR
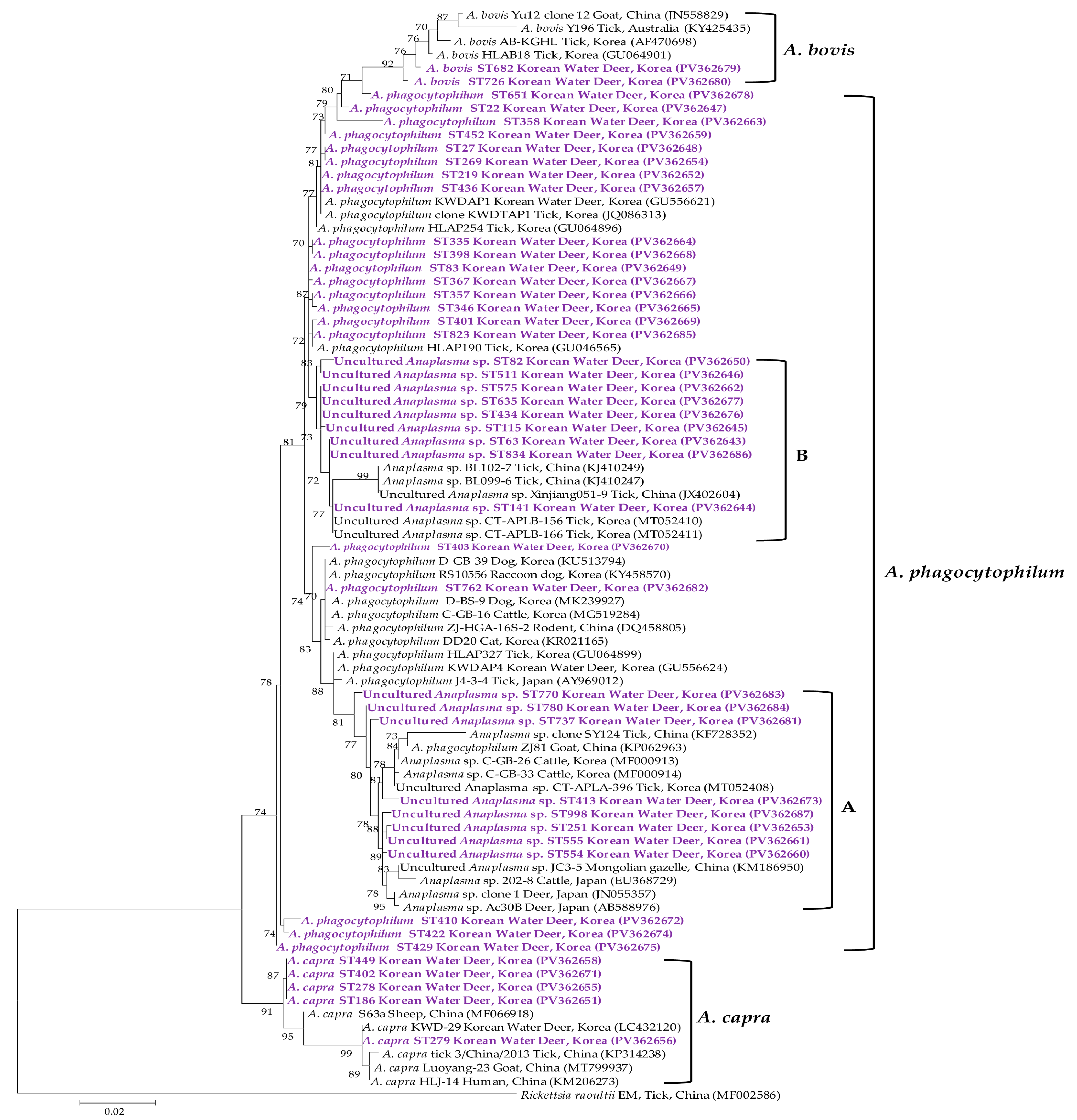
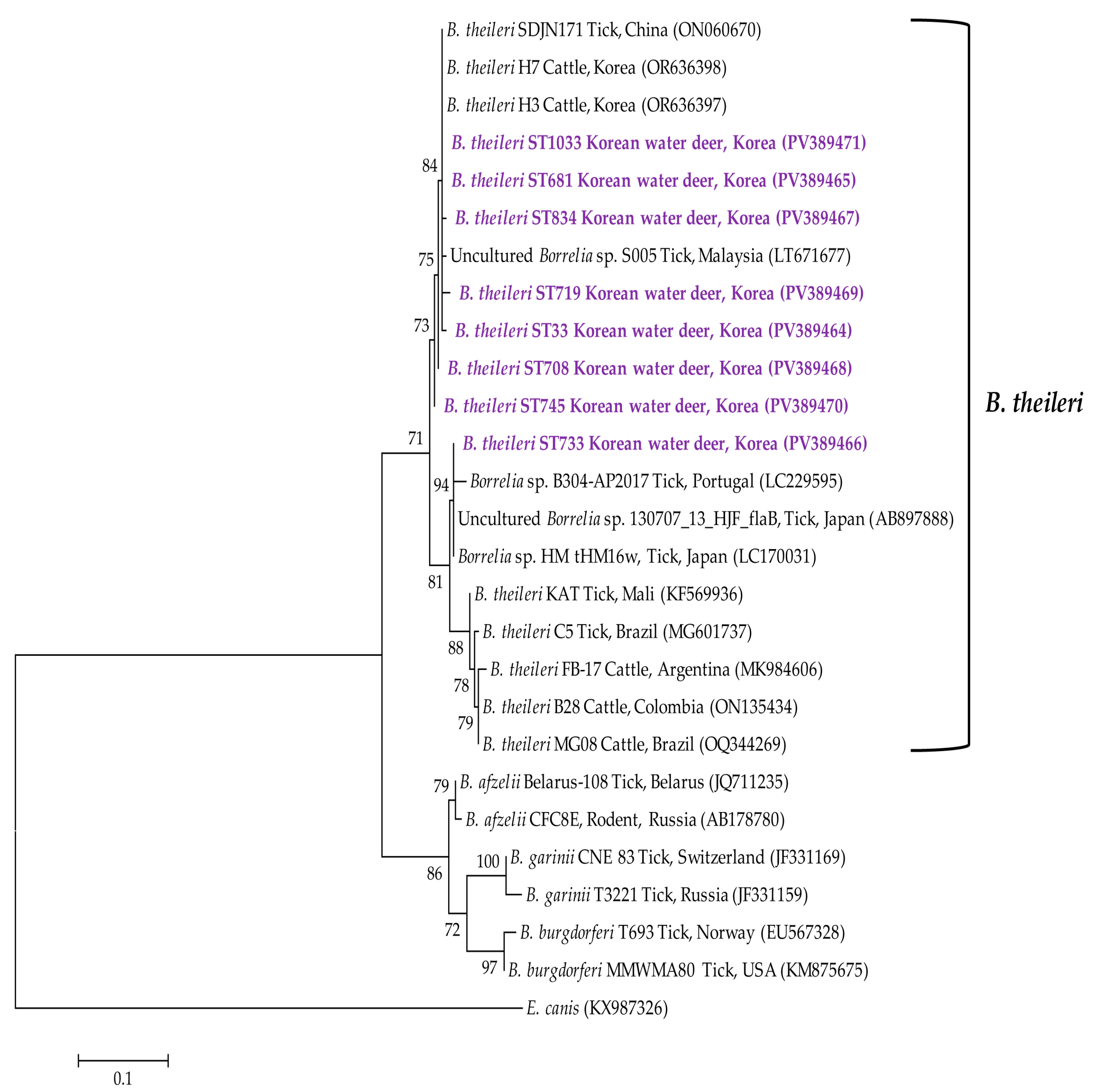
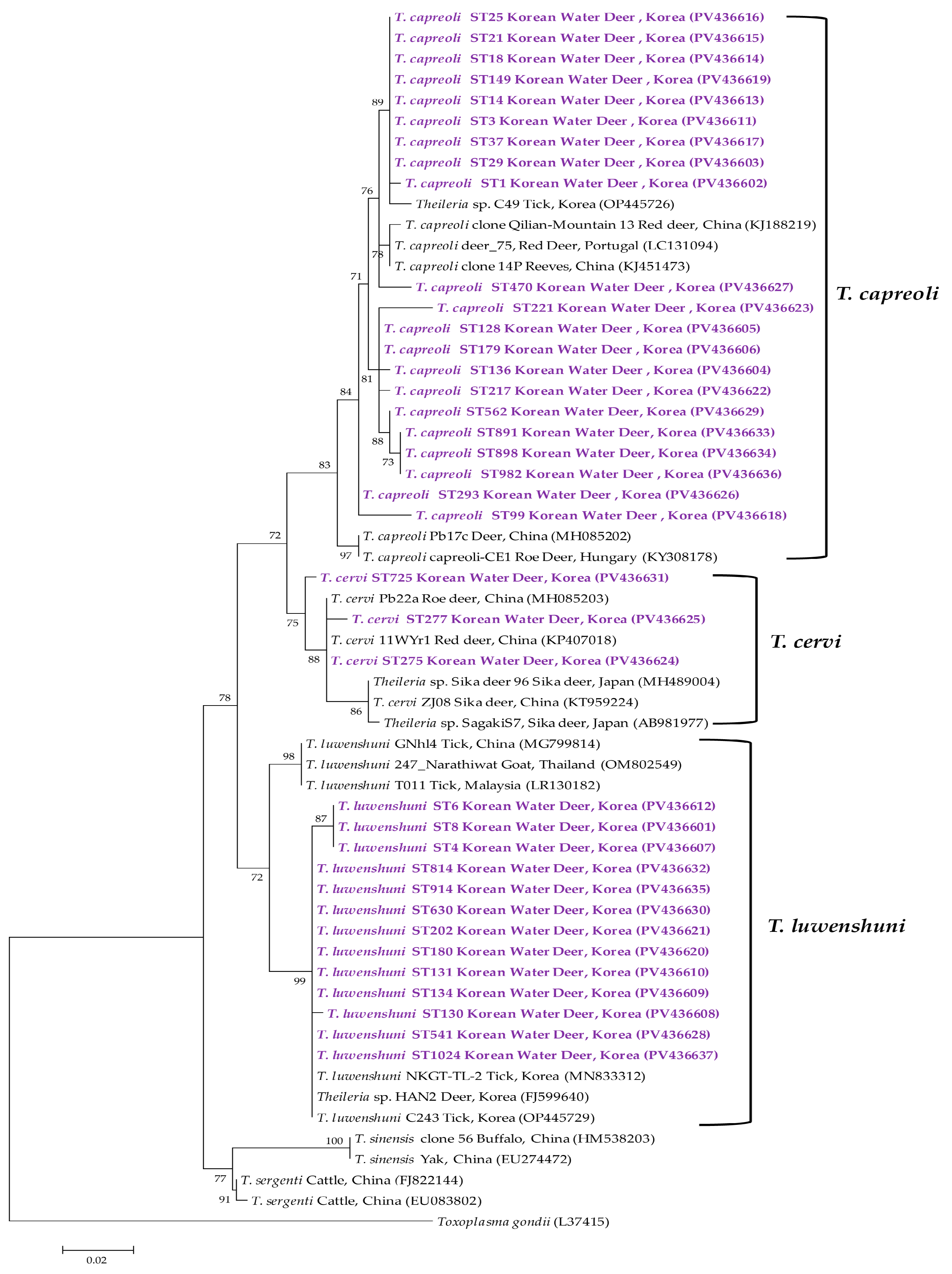
3.3. Molecular and Phylogenetic Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Prepare 1.5 mL microcentrifuge tubes and add β-mercaptoethanol (reducing agent) at a ratio of 10 μL per 700 μL of DLD buffer (i.e., 10 μL + 700 μL per sample).
- Add 45 mL of 100% ethanol to the WA buffer and 66 mL to the wash buffer as instructed by the kit.
- Preheat the elution buffer (EB) to 37 °C prior to use.
- Protocol Steps.
- Add 710 μL of prepared lysis buffer (700 μL DLD + 10 μL β-mercaptoethanol) to ~10 mg of spleen tissue in a homogenization tube.
- Homogenize using a tissue grinder at 3500 rpm for 30 s, pause for 10 s, and repeat twice. If homogenization is incomplete, repeat up to 1–2 additional cycles.
- Vortex thoroughly and incubate at room temperature for 5–10 min. Then, centrifuge at 13,000 rpm for 1 min.
- Label a pre-treatment column and collection tube. Transfer the supernatant (~710 μL) to the column and centrifuge at 13,000 rpm for 1 min.
- Discard the upper spin column. To the remaining flow-through (~710 μL), add 710 μL of 100% ethanol (final volume: 1420 μL).
- Attach a binding column to a new collection tube. Mix the ethanol-added lysate by pipetting 3–4 times.
- Load 710 μL of the solution into the column and centrifuge at 13,000 rpm for 1 min. Discard the flow-through and wipe the tube rim.
- Load the remaining 710 μL of the lysate and repeat the centrifugation step.
- Add 700 μL of WA buffer, centrifuge at 13,000 rpm for 1 min, discard the flow-through, and reattach the column.
- Add 500 μL of wash buffer, centrifuge at 13,000 rpm for 1 min, discard the flow-through, and repeat with another 500 μL followed by centrifugation for 3 min.
- Transfer the column to a clean 1.5 mL tube. Add 200 μL of preheated EB buffer (37 °C) to the column and incubate at room temperature for 5 min.
- Centrifuge at 13,000 rpm for 1 min to collect the final eluate.
- Store nucleic acid extracts at –70 °C until analysis.
References
- Taylor, M.A. Emerging parasitic diseases of sheep. Vet. Parasitol. 2012, 189, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A one health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- Cummins, G.A.; Traub, S.J. Tick-borne diseases. In Auerbach’s Wilderness Medicine; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1–2, pp. 968–993. [Google Scholar] [CrossRef]
- Harris, R.B.; Duckworth, J.W. The IUCN Red List of Threatened Species. [CrossRef]
- Kim, B.J.; Oh, D.H.; Chun, S.H.; Lee, S.D. Distribution, density, and habitat use of the Korean water deer (Hydropotes inermis argyropus) in Korea. Landsc. Ecol. Eng. 2011, 7, 291–297. [Google Scholar] [CrossRef]
- Severo, M.S.; Stephens, K.D.; Kotsyfakis, M.; Pedra, J.H. Anaplasma phagocytophilum: Deceptively simple or simply deceptive? Future Microbiol. 2012, 7, 719–731. [Google Scholar] [CrossRef]
- Qiu, Y.; Squarre, D.; Nakamura, Y.; Lau, A.C.C.; Moonga, L.C.; Kawai, N.; Ohnuma, A.; Hayashida, K.; Nakao, R.; Yamagishi, J.; et al. Evidence of Borrelia theileri in wild and domestic animals in the kafue ecosystem of Zambia. Microorganisms 2021, 9, 2405. [Google Scholar] [CrossRef]
- Mans, B.J.; Pienaar, R.; Latif, A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 2015, 4, 104–118. [Google Scholar] [CrossRef]
- Shin, S.U.; Park, Y.J.; Ryu, J.H.; Jang, D.H.; Hwang, S.; Cho, H.C.; Park, J.; Han, J.I.; Choi, K.S. Identification of zoonotic tick-borne pathogens from Korean water deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis. 2020, 20, 745–754. [Google Scholar] [CrossRef]
- Seong, G.; Han, Y.J.; Oh, S.S.; Chae, J.S.; Yu, D.H.; Park, J.; Park, B.K.; Yoo, J.G.; Choi, K.S. Detection of tick-borne pathogens in the Korean water deer (Hydropotes inermis argyropus) from Jeonbuk province, Korea. Korean J. Parasitol. 2015, 53, 653–659. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, J.; Son, K.; Kim, Y.; Hwang, J.; Jeong, H.; Ahn, T.Y.; Jheong, W.H. Phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in Korean water deer (Hydropotes inermis argyropus) in the Republic of Korea. Ticks Tick-Borne Dis. 2020, 11, 101331. [Google Scholar] [CrossRef]
- Kang, J.G.; Ko, S.; Kim, H.C.; Chong, S.T.; Klein, T.A.; Chae, J.B.; Jo, Y.S.; Choi, K.S.; Yu, D.H.; Park, B.K.; et al. Prevalence of Anaplasma and Bartonella spp. in ticks collected from Korean water deer (Hydropotes inermis argyropus). Korean J. Parasitol. 2016, 54, 87–91. [Google Scholar] [CrossRef] [PubMed]
- VanBik, D.; Lee, S.H.; Seo, M.G.; Jeon, B.R.; Goo, Y.K.; Park, S.J.; Rhee, M.H.; Kwon, O.D.; Kim, T.H.; Geraldino, P.J.L.; et al. Borrelia species detected in ticks feeding on wild Korean water deer (Hydropotes inermis) using molecular and genotypic analyses. J. Med. Entomol. 2017, 54, 1397–1402. [Google Scholar] [CrossRef]
- Seo, M.G.; Kwon, O.D.; Kwak, D. Molecular detection of Rickettsia raoultii, Rickettsia tamurae, and associated pathogens from ticks parasitizing water deer (Hydropotes inermis argyropus) in South Korea. Ticks Tick Borne Dis. 2021, 12, 101712. [Google Scholar] [CrossRef]
- Barlough, J.E.; Madigan, J.E.; DeRock, E.; Bigornia, L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus). Vet. Parasitol. 1996, 63, 319–329. [Google Scholar] [CrossRef]
- Lee, K.; Takano, A.; Taylor, K.; Sashika, M.; Shimozuru, M.; Konnai, S.; Kawabata, H.; Tsubota, T. A relapsing fever group Borrelia sp. similar to Borrelia lonestari found among wild sika deer (Cervus nippon yesoensis) and Haemaphysalis spp. ticks in Hokkaido, Japan. Ticks Tick Borne Dis. 2014, 5, 841–847. [Google Scholar] [CrossRef]
- Casati, S.; Sager, H.; Gern, L.; Piffaretti, J.C. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2006, 13, 65–70. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Im, J.H.; Baek, J.H.; Durey, A.; Kwon, H.Y.; Chung, M.H.; Lee, J.S. Geographic distribution of Tick-borne encephalitis virus complex. J. Vector Borne Dis. 2020, 57, 14–22. [Google Scholar] [CrossRef]
- Tran, N.T.B.; Shimoda, H.; Ishijima, K.; Yonemitsu, K.; Minami, S.; Kuroda, Y.; Tatemoto, K.; Mendoza, M.V.; Kuwata, R.; Takano, A.; et al. Zoonotic infection with Oz virus, a novel thogotovirus. Emerg. Infect. Dis. 2022, 28, 436–439. [Google Scholar] [CrossRef]
- Ogata, Y.; Sato, T.; Kato, K.; Kikuchi, K.; Mitsuhashi, K.; Watari, K.; Tamiya, K.; Goto, A.; Yamaguchi, H.; Hisada, R. A case of tick-borne Yezo virus infection: Concurrent detection in the patient and tick. Int. J. Infect. Dis. 2024, 143, 107038. [Google Scholar] [CrossRef] [PubMed]
- Dagher, H.; Donninger, H.; Hutchinson, P.; Ghildyal, R.; Bardin, P. Rhinovirus detection: Comparison of real-time and conventional PCR. J. Virol. Methods 2004, 117, 113–121. [Google Scholar] [CrossRef]
- Seo, M.G.; Noh, B.E.; Lee, H.S.; Kim, T.K.; Song, B.G.; Lee, H.I. Nationwide temporal and geographical distribution of tick populations and phylogenetic analysis of Severe Fever with Thrombocytopenia Syndrome virus in ticks in Korea, 2020. Microorganisms 2021, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Arraga-Alvarado, C.M.; Qurollo, B.A.; Parra, O.C.; Berrueta, M.A.; Hegarty, B.C.; Breitschwerdt, E.B. Molecular evidence of Anaplasma platys infection in two women from Venezuela. Am. Soc. Trop. Med. Hyg. 2014, 91, 1161–1165. [Google Scholar] [CrossRef]
- García-Pérez, A.L.; Oporto, B.; Espí, A.; del Cerro, A.; Barral, M.; Povedano, I.; Barandika, J.F.; Hurtado, A. Anaplasmataceae in wild ungulates and carnivores in northern Spain. Ticks Tick-Borne Dis. 2016, 7, 264–269. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Im, J.H.; Baek, J.H.; Durey, A.; Kwon, H.Y.; Chung, M.H.; Lee, J.S. Current status of tick-borne diseases in South Korea. Vector-Borne Zoonotic Dis. 2018, 19, 225–233. [Google Scholar] [CrossRef]
- Kim, C.M.; Kim, M.S.; Park, M.S.; Park, J.H.; Chae, J.S. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A. bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vector-Borne Zoonotic Dis. 2003, 3, 17–26. [Google Scholar] [CrossRef]
- Lee, M.; Seo, M.G.; Lee, S.H.; Ouh, I.O.; Kim, Y.H.; Kim, J.K.; Goo, Y.K.; Rhee, M.H.; Kim, T.H.; Kwon, O.D.; et al. Molecular detection and phylogenetic analysis of tick-borne pathogens in wild Korean water deer and farmed elk in Gyeongbuk and Gangwon provinces of Korea. J. Vet. Med. Sci. 2018, 80, 1473–1478. [Google Scholar] [CrossRef]
- Atif, F.A. Alpha proteobacteria of genus Anaplasma (Rickettsiales: Anaplasmataceae): Epidemiology and characteristics of Anaplasma species related to veterinary and public health importance. Parasitology 2016, 143, 659–685. [Google Scholar] [CrossRef]
- Lu, M.; Li, F.; Liao, Y.; Shen, J.J.; Xu, J.M.; Chen, Y.Z.; Li, J.H.; Holmes, E.C.; Zhang, Y.Z. Epidemiology and diversity of Rickettsiales Bacteria in humans and animals in Jiangsu and Jiangxi provinces, China. Sci. Rep. 2019, 9, 13176. [Google Scholar] [CrossRef] [PubMed]
- Karpathy, S.; Kingry, L.; Pritt, B.; Berry, J.; Chilton, N.; Dergousoff, S.; Cortinas, R.; Sheldon, S.; Oatman, S.; Anacker, M.; et al. Anaplasma bovis–like infections in humans, United States, 2015–2017. Emerg. Infect. Dis. J. 2023, 29, 1904. [Google Scholar] [CrossRef]
- Amer, S.; Kim, S.; Yun, Y.M.; Na, K.J. Novel variants of the newly emerged Anaplasma capra from Korean water deer (Hydropotes inermis argyropus) in South Korea. Parasites Vectors 2019, 12, 365. [Google Scholar] [CrossRef]
- Ohashi, N.; Inayoshi, M.; Kitamura, K.; Kawamori, F.; Kawaguchi, D.; Nishimura, Y.; Naitou, H.; Hiroi, M.; Masuzawa, T. Anaplasma phagocytophilum—Infected ticks, Japan. Emerg. Infect. Dis. J. 2005, 11, 1780. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Matsuyama, Y.; Matsuda, H.; Sakamoto, L.; Matsumoto, K.; Yokoyama, N.; Inokuma, H. Detection of Anaplasma bovis and Anaplasma phagocytophilum DNA from Haemaphysalis megaspinosa in Hokkaido, Japan. Vet. Parasitol. 2010, 168, 170–172. [Google Scholar] [CrossRef]
- Jilintai; Seino, N.; Hayakawa, D.; Suzuki, M.; Hata, H.; Kondo, S.; Matsumoto, K.; Yokoyama, N.; Inokuma, H. Molecular survey for Anaplasma bovis and Anaplasma phagocytophilum infection in cattle in a Pastureland where sika deer appear in Hokkaido, Japan. Jpn. J. Infect. Dis. 2009, 62, 73–75. [Google Scholar] [CrossRef]
- Ybañez, A.P.; Matsumoto, K.; Kishimoto, T.; Inokuma, H. Molecular analyses of a potentially novel Anaplasma species closely related to Anaplasma phagocytophilum detected in sika deer (Cervus nippon yesoensis) in Japan. Vet. Microbiol. 2012, 157, 232–236. [Google Scholar] [CrossRef]
- Seo, M.G.; Ouh, I.O.; Kwon, O.D.; Kwak, D. Molecular detection of Anaplasma phagocytophilum-like Anaplasma spp. and pathogenic A. phagocytophilum in cattle from South Korea. Mol. Phylogenetics Evol. 2018, 126, 23–30. [Google Scholar] [CrossRef]
- Seo, M.G.; Lee, H.; Alkathiri, B.; Ahn, K.; Lee, S.H.; Shin, S.; Bae, S.; Kim, K.T.; Jang, M.; Lee, S.K.; et al. Tick populations and molecular analysis of Anaplasma species in ticks from the Republic of Korea. Microorganisms 2023, 11, 820. [Google Scholar] [CrossRef]
- Margos, G.; Vollmer, S.A.; Ogden, N.H.; Fish, D. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect. Genet. Evol. 2011, 11, 1545–1563. [Google Scholar] [CrossRef]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae part 2: Borrelia relapsing fever group and unclassified Borrelia. Biology 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Chae, J.B.; Cho, Y.K.; Jo, Y.S.; Shin, N.S.; Lee, H.; Choi, K.S.; Yu, D.H.; Park, J.; Park, B.K.; et al. Molecular detection of Anaplasma, Bartonella, and Borrelia theileri in raccoon dogs (Nyctereutes procyonoides) in Korea. Am. Soc. Trop. Med. Hyg. 2018, 98, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Hyung, H.J.; Choi, Y.S.; Park, J.; Lee, K.J.; Kang, J.G. Molecular detection of Borrelia theileri in cattle in Korea. Parasites Hosts Dis. 2024, 62, 151–156. [Google Scholar] [CrossRef]
- Kim, M.; Cho, S.; Park, G.; Kim, J.; Rieu, M.; Noh, K.T.; Ha, S.; Park, Q.; Kim, D.H.; Han, S.; et al. Molecular detection of, and Severe fever with thrombocytopenia syndrome virus (SFTSV) in military working dogs and ticks collected from the Republic of Korea army garrisons in Gangwon province in 2021–2022. Entomol. Res. 2024, 54, e12762. [Google Scholar] [CrossRef]
- Monoldorova, S.; Lee, S.; Yun, S.; Park, S.; Jeong, J.U.; Kim, J.; Lee, I.Y.; Jun, H.; Park, C.H.; Byeon, H.S.; et al. Seasonal dynamics of ticks and tick-borne pathogens in Republic of Korea. Pathogens 2024, 13, 1079. [Google Scholar] [CrossRef]
- Schnittger, L.; Yin, H.; Qi, B.; Gubbels, M.J.; Beyer, D.; Niemann, S.; Jongejan, F.; Ahmed, J.S. Simultaneous detection and differentiation of Theileria and Babesia parasites infecting small ruminants by reverse line blotting. Parasitol Res. 2004, 92, 189–196. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Schein, E. Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol Res. 1998, 84, 467–475. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Guan, G.; Liu, A.; Wang, B.; Luo, J.; Yin, H. Molecular detection and identification of piroplasms in sika deer (Cervus nippon) from Jilin province, China. Parasites Vectors 2016, 9, 156. [Google Scholar] [CrossRef]
- Garc, J.; Aurtenetxe, O.; Barral, M.; Marco, I.; Lavin, S.; Garc, A.; Rez, A.P.; Hurtado, A. Molecular detection and characterization of piroplasms infecting cervids and chamois in Northern Spain. Parasitology 2006, 134, 391–398. [Google Scholar] [CrossRef]
- Han, J.I.; Jang, H.J.; Lee, S.J.; Na, K.J. High prevalence of Theileria sp. in wild Chinese Water Deer (Hydropotes inermis argyropus) in South Korea. Vet. Parasitol. 2009, 164, 311–314. [Google Scholar] [CrossRef]
- He, L.; Khan, M.K.; Zhang, W.J.; Zhang, Q.L.; Zhou, Y.Q.; Hu, M.; Zhao, J. Detection and identification of Theileria infection in Sika deer (Cervus nippon) in China. J. Parasitol. 2012, 98, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Fukumoto, S.; Harasawa, R. Prevalence of tick-borne hemolytic microbes in free-living sika deer (Cervus nippon) captured in a deer-overcrowded area. Jpn. J. Zoo Wildl. Med. 2016, 21, 17–27. [Google Scholar] [CrossRef]
- Chae, J.s.; Waghela, S.D.; Craig, T.M.; Kocan, A.A.; Wagner, G.G.; Holman, P.J. Two Theileria cervi SSU rRNA gene sequence types found in isolates from white-tailed deer and elk in North America. J. Wildl. Dis. 1999, 35, 458–465. [Google Scholar] [CrossRef]
- Yin, H.; Schnittger, L.; Luo, J.; Seitzer, U.; Ahmed, J.S. Ovine theileriosis in China: A new look at an old story. Parasitol. Res. 2007, 101 (Suppl. S2), S191–S195. [Google Scholar] [CrossRef]
- Goudarzi, G.; Tavakoli, M.; Ezatpour, B.; Kooshki, H.; Hosseini-Chegeni, A. Molecular detection of Theileria ovis (Apicomplexa: Theileriidae), Anaplasma ovis (Rickettsiales: Anaplasmataceae), and Mycoplasma sp. (Tenericutes: Mycoplasmataceae) from sheep blood in western Iran. Comp. Clin. Pathol. 2019, 28, 1661–1666. [Google Scholar] [CrossRef]
- Moon, K.H.; Lee, S.; Choi, C.Y.; Kim, S.Y.; Kang, C.W.; Lee, K.K.; Yun, Y.M. Investigation of Theileria sp. from ticks and roe deer (Capreolus pygargus) in Jeju Island. J. Vet. Clin. 2014, 31, 6–10. [Google Scholar] [CrossRef]
- Han, Y.J.; Park, J.; Lee, Y.S.; Chae, J.s.; Yu, D.H.; Park, B.K.; Kim, H.C.; Choi, K.S. Molecular identification of selected tick-borne pathogens in wild deer and raccoon dogs from the Republic of Korea. Vet. Parasitol. Reg. Stud. Rep. 2017, 7, 25–31. [Google Scholar] [CrossRef]
| Pathogen | Positive Samples (%) |
|---|---|
| Anaplasma spp. | 937 (90.5; CI 88.7–92.3) |
| Babesia spp. | 325 (31.4; CI 28.6–34.2) |
| Borrelia spp. | 173 (16.7; CI 14.4–19.0) |
| Theileria spp. | 965 (93.2; CI 91.7–94.8) |
| Tick-borne encephalitis virus | 0 |
| OZ virus | 0 |
| Yezo virus | 0 |
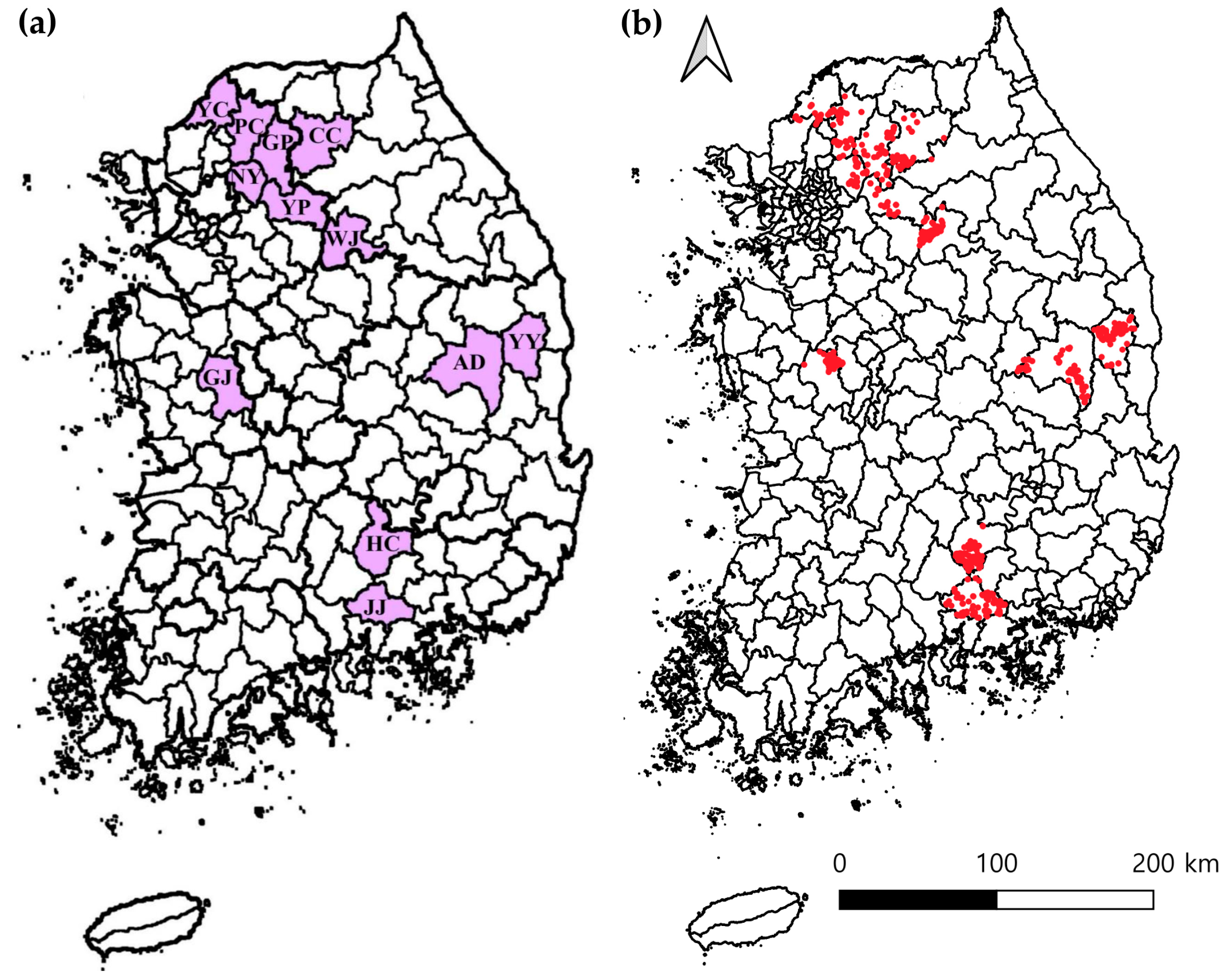
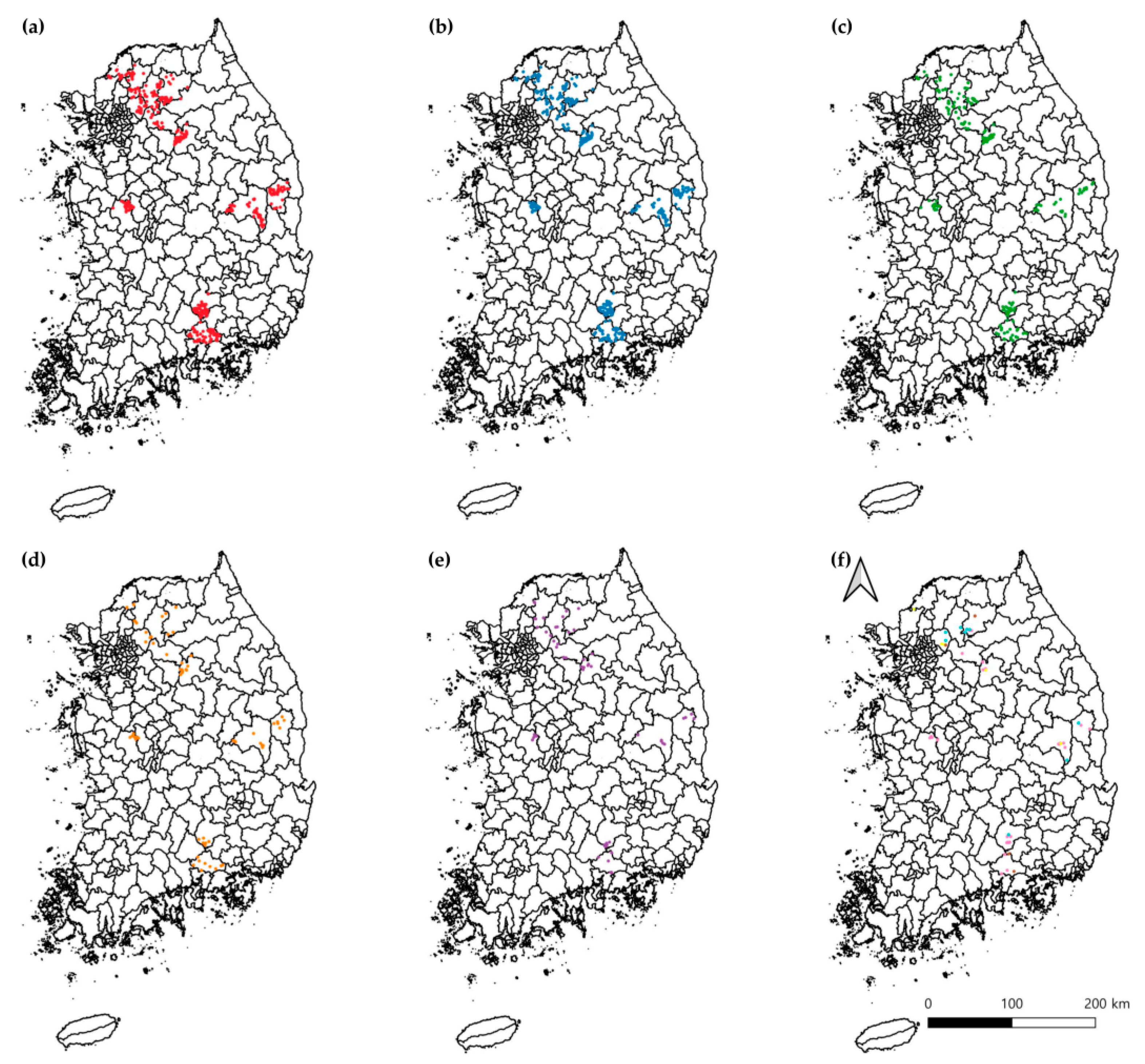
| Area | Region | No. of Tested | No. Positive (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anaplasma phagocytophilum | APLA | APLB | Anaplasma bovis | Anaplasma capra | Borrelia theileri | Theileria capreoli | Theileria cervi | Theileria luwenshuni | Total | ||||
| Northern | Gangwon | Wonju | 134 | 108 (80.6; CI 73.9–87.3) | 0 | 7 (5.2; CI 1.5–9.0) | 3 (2.2; CI 0–4.7) | 0 | 10 (7.5; CI 3.0–11.9) | 40 (29.9; CI 22.1–37.6) | 1 (0.7; CI 0–2.2) | 82 (61.2; CI 52.9–69.4) | 128 (95.5; CI 92.0–99.0) |
| Chuncheon | 72 | 49 (68.1; CI 57.3–78.8) | 3 (4.2; CI 0–8.8) | 4 (5.6; CI 0.3–10.8) | 2 (2.8; CI 0–6.6) | 4 (5.6; CI 0.3–10.8) * | 3 (4.2; CI 0–8.8) | 14 (19.4; CI 10.3–28.6) | 0 | 53 (73.6; CI 63.4–83.8) * | 70 (97.2; CI 93.4–100) | ||
| Gyeonggi | Gapyeong | 60 | 53 (88.3; CI 80.2–96.5) | 1 (1.7; CI 0–4.9) | 4 (6.7; CI 0.4–13.0) | 0 | 0 | 2 (3.3; CI 0–7.9) | 19 (31.7; CI 19.9–43.4) | 0 | 40 (66.7; CI 54.7–78.6) | 59 (98.3; CI 95.1–100) | |
| Namyangju | 56 | 50 (89.3; CI 81.2–97.4) * | 2 (3.6; CI 0–8.4) | 3 (5.4; CI 0–11.3) | 0 | 1 (1.8; CI 0–5.3) | 5 (8.9; CI 1.5–16.4) | 23 (41.1; CI 28.2–54.0) | 3 (5.4; CI 0–11.3) * | 30 (53.6; CI 40.5–66.6) | 56 (100) * | ||
| Yangpyeong | 31 | 23 (74.2; CI 58.8–89.6) | 0 | 5 (16.1; CI 3.2–29.1) | 1 (3.2; CI 0–9.4) | 0 | 2 (6.5; CI 0–15.1) | 17 (54.8; CI 37.3–72.4) * | 0 | 14 (45.2; CI 27.6–62.7) | 31 (100) * | ||
| Yeoncheon | 60 | 40 (66.7; CI 54.7–78.6) | 0 | 2 (3.3; CI 0–7.9) | 0 | 1 (1.7; CI 0–4.9) | 2 (3.3; CI 0–7.9) | 14 (23.3; CI 12.6–34.0) | 1 (1.7; CI 0–4.9) | 30 (50.0; CI 37.3–62.7) | 46 (76.7; CI 66.0–87.4) | ||
| Pocheon | 85 | 72 (84.7; CI 77.1–92.4) | 0 | 7 (8.2; CI 2.4–14.1) | 0 | 0 | 5 (5.9; CI 0.9–10.9) | 37 (43.5; CI 33.0–54.1) | 0 | 45 (52.9; CI 42.3–63.6) | 82 (96.5; CI 92.5–100) | ||
| Central | Gyeongbuk | Andong | 125 | 96 (76.8; CI 69.4–84.2) | 1 (0.8; CI 0–2.4) | 6 (4.8; CI 1.1–8.5) | 5 (4.0; CI 0.6–7.4) | 0 | 9 (7.2; CI 2.7–11.7) | 17 (13.6; CI 7.6–19.6) | 1 (0.8; CI 0–2.4) | 86 (68.8; CI 60.7–76.9) | 117 (93.6; CI 89.3–97.9) |
| Yeongyang | 78 | 48 (61.5; CI 50.7–72.3) | 1 (1.3; CI 0–3.8) | 4 (5.1; CI 0.2–10.0) | 2 (2.6; CI 0–6.1) | 0 | 7 (9.0; CI 2.6–15.3) | 10 (12.8; CI 5.4–20.2) | 0 | 43 (55.1; CI 44.1–66.2) | 59 (75.6; CI 66.1–85.2) | ||
| Chungnam | Gongju | 104 | 75 (72.1; CI 63.5–80.7) | 0 | 6 (5.8; CI 1.3–10.3) | 4 (3.8; CI 0.2–7.5) | 0 | 8 (7.7; CI 2.6–12.8) | 27 (26.0; CI 17.5–34.4) | 0 | 60 (57.7; CI 48.2–67.2) | 95 (91.3; CI 85.9–96.7) | |
| Southern | Gyeongnam | Jinju | 115 | 96 (83.5; CI 76.7–90.3) | 0 | 4 (3.5; CI 0.1–6.8) | 3 (2.6; CI 0–5.5) | 2 (1.7; CI 0–4.1) | 9 (7.8; CI 2.9–12.7) | 33 (28.7; CI 20.4–37.0) | 0 | 76 (66.1; CI 57.4–74.7) | 114 (99.1; CI 97.4–100) |
| Hapcheon | 115 | 94 (81.7; CI 74.7–88.8) | 1 (0.9; CI 0–2.6) | 7 (6.1; CI 1.7–10.5) | 6 (5.2; CI 1.2–9.3) | 0 | 7 (6.1; CI 1.7–10.5) | 33 (28.7; CI 20.4–37.0) | 0 | 69 (60.0; CI 51.0–69.0) | 112 (97.4; CI 94.5–100) | ||
| Total | 1035 | 804 (77.7; CI 75.1–80.2) | 9 (0.9; CI 0.3–1.4) | 59 (5.7; CI 4.3–7.1) | 26 (2.5; CI 1.6–3.5) | 8 (0.8; CI 0.2–1.3) | 69 (6.7; CI 5.1–8.2) | 284 (27.4; CI 24.7–30.2) | 6 (0.6; CI 0.1–1.0) | 628 (60.7; CI 57.7–63.7) | 969 (93.6; CI 92.1–95.1) | ||
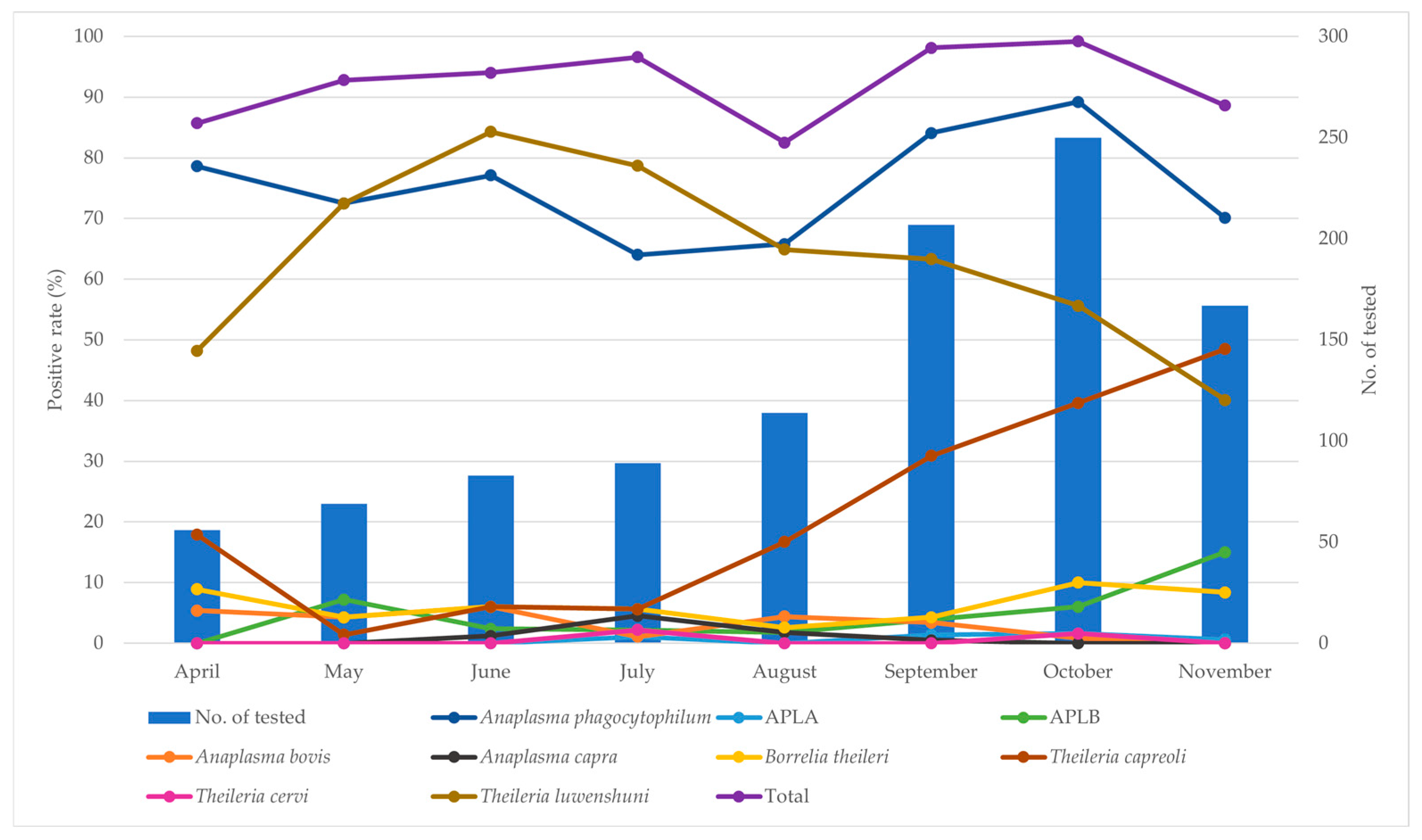
| Collection Month | No. of Tested | No. Positive (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anaplasma phagocytophilum | APLA | APLB | Anaplasma bovis | Anaplasma capra | Borrelia theileri | Theileria capreoli | Theileria cervi | Theileria luwenshuni | Total | ||
| April | 56 | 44 (78.6; CI 67.8–89.3) | 0 | 0 | 3 (5.4; CI 0–11.3) | 0 | 5 (8.9; CI 1.5–16.4) | 10 (17.9; CI 7.8–27.9) | 0 | 27 (48.2; CI 35.1–61.3) | 48 (85.7; CI 76.5–94.9) |
| May | 69 | 50 (72.5; CI 61.9–83.0) | 0 | 5 (7.2; CI 1.1–13.4) | 3 (4.3; CI 0–9.2) | 0 | 3 (4.3; CI 0–9.2) | 1 (1.4; CI 0–4.3) | 0 | 50 (72.5; CI 61.9–83.0) | 64 (92.8; CI 86.6–98.9) |
| June | 83 | 64 (77.1; CI 68.1–86.1) | 0 | 2 (2.4; CI 0–5.7) | 5 (6.0; CI 0.9–11.1) * | 1 (1.2; CI 0–3.6) | 5 (6.0; CI 0.9–11.1) | 5 (6.0; CI 0.9–11.1) | 0 | 70 (84.3; CI 76.5–92.2) * | 78 (94.0; CI 88.9–99.1) |
| July | 89 | 57 (64.0; CI 54.1–74.0) | 1 (1.1; CI 0–3.3) | 2 (2.2; CI 0–5.3) | 1 (1.1; CI 0–3.3) | 4 (4.5; CI 0.2–8.8) * | 5 (5.6; CI 0.8–10.4) | 5 (5.6; CI 0.8–10.4) | 2 (2.2; CI 0–5.3) | 70 (78.7; CI 70.1–87.2) | 86 (96.6; CI 92.9–100) |
| August | 114 | 75 (65.8; CI 57.1–74.5) | 0 | 2 (1.8; CI 0–4.2) | 5 (4.4; CI 0.6–8.1) | 2 (1.8; CI 0–4.2) | 3 (2.6; CI 0–5.6) | 19 (16.7; CI 9.8–23.5) | 0 | 74 (64.9; CI 56.2–73.7) | 94 (82.5; CI 75.5–89.4) |
| September | 207 | 174 (84.1; CI 79.1–89.0) | 3 (1.4; CI 0–3.1) | 8 (3.9; CI 1.2–6.5) | 7 (3.4; CI 0.9–5.8) | 1 (0.5; CI 0–1.4) | 9 (4.3; CI 1.6–7.1) | 64 (30.9; CI 24.6–37.2) | 0 | 131 (63.3; CI 56.7–69.9) | 203 (98.1; CI 96.2–99.9) |
| October | 250 | 223 (89.2; CI 85.4–93.0) * | 4 (1.6; CI 0–3.2) | 15 (6.0; CI 3.1–8.9) | 2 (0.8; CI 0–1.9) | 0 | 25 (10.0; CI 6.3–13.7) | 99 (39.6; CI 33.5–45.7) | 4 (1.6; CI 0–3.2) | 139 (55.6; CI 49.4–61.8) | 248 (99.2; CI 98.1–100) * |
| November | 167 | 117 (70.1; CI 63.1–77.0) | 1 (0.6; CI 0–1.8) | 25 (15.0; CI 9.6–20.4) * | 0 | 0 | 14 (8.4; CI 4.2–12.6) | 81 (48.5; CI 40.9–56.1) * | 0 | 67 (40.1; CI 32.7–47.6) | 148 (88.6; CI 83.8–93.4) |
| Total | 1035 | 804 (77.7; CI 75.1–80.2) | 9 (0.9; CI 0.3–1.4) | 59 (5.7; CI 4.3–7.1) | 26 (2.5; CI 1.6–3.5) | 8 (0.8; CI 0.2–1.3) | 69 (6.7; CI 5.1–8.2) | 284 (27.4; CI 24.7–30.2) | 6 (0.6; CI 0.1–1.0) | 628 (60.7; CI 57.7–63.7) | 969 (93.6; CI 92.1–95.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Chae, S.-J.; Lee, Y.-J.; Shin, H.; Kwak, S.; Jeong, H.; Lee, S.; Kwak, D.; Seo, M.-G. Nationwide Geographical and Temporal Distribution of Tick-Borne Diseases in Korean Water Deer (Hydropotes inermis argyropus). Animals 2025, 15, 1499. https://doi.org/10.3390/ani15101499
Kim B, Chae S-J, Lee Y-J, Shin H, Kwak S, Jeong H, Lee S, Kwak D, Seo M-G. Nationwide Geographical and Temporal Distribution of Tick-Borne Diseases in Korean Water Deer (Hydropotes inermis argyropus). Animals. 2025; 15(10):1499. https://doi.org/10.3390/ani15101499
Chicago/Turabian StyleKim, Beoul, Su-Jin Chae, You-Jeong Lee, Haksub Shin, Sunmin Kwak, Hyesung Jeong, Suwoong Lee, Dongmi Kwak, and Min-Goo Seo. 2025. "Nationwide Geographical and Temporal Distribution of Tick-Borne Diseases in Korean Water Deer (Hydropotes inermis argyropus)" Animals 15, no. 10: 1499. https://doi.org/10.3390/ani15101499
APA StyleKim, B., Chae, S.-J., Lee, Y.-J., Shin, H., Kwak, S., Jeong, H., Lee, S., Kwak, D., & Seo, M.-G. (2025). Nationwide Geographical and Temporal Distribution of Tick-Borne Diseases in Korean Water Deer (Hydropotes inermis argyropus). Animals, 15(10), 1499. https://doi.org/10.3390/ani15101499





