Effects of Substituting Sweet Sorghum for Corn Silage in the Diet on the Growth Performance, Meat Quality, and Rumen Microorganisms of Boer Goats in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Harvesting and Ensiling of Sweet Sorghum
2.2. Microbial Community Analysis of Silage
2.3. Microbial Community Analysis of the Rumen
2.4. Illumina Miseq Sequencing and Data Analysis
2.5. Experimental Design and Diets
2.6. Laboratory Analysis
2.7. Statistical Analysis
3. Results
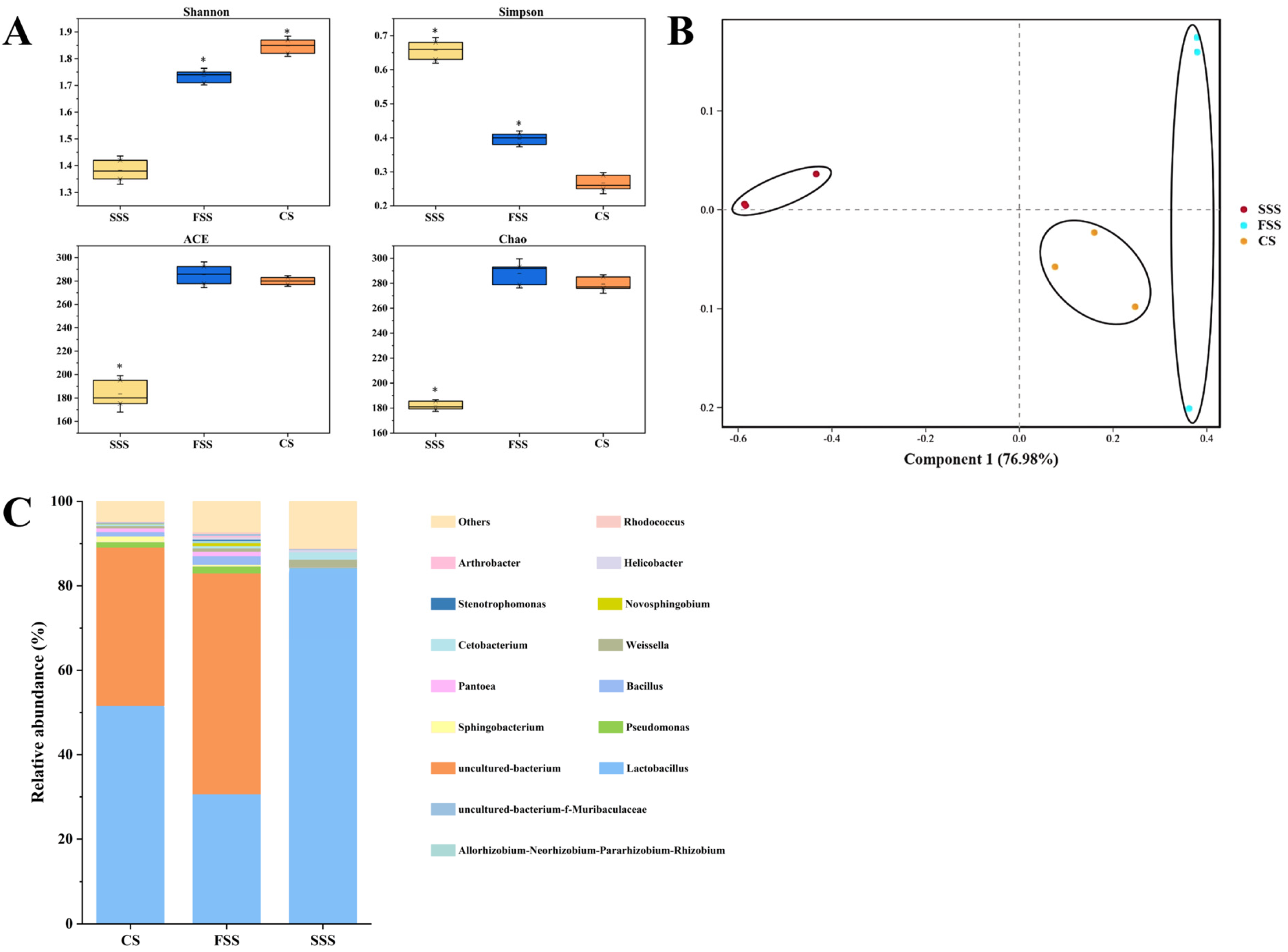
3.1. Analysis of Microbial Community in Silage
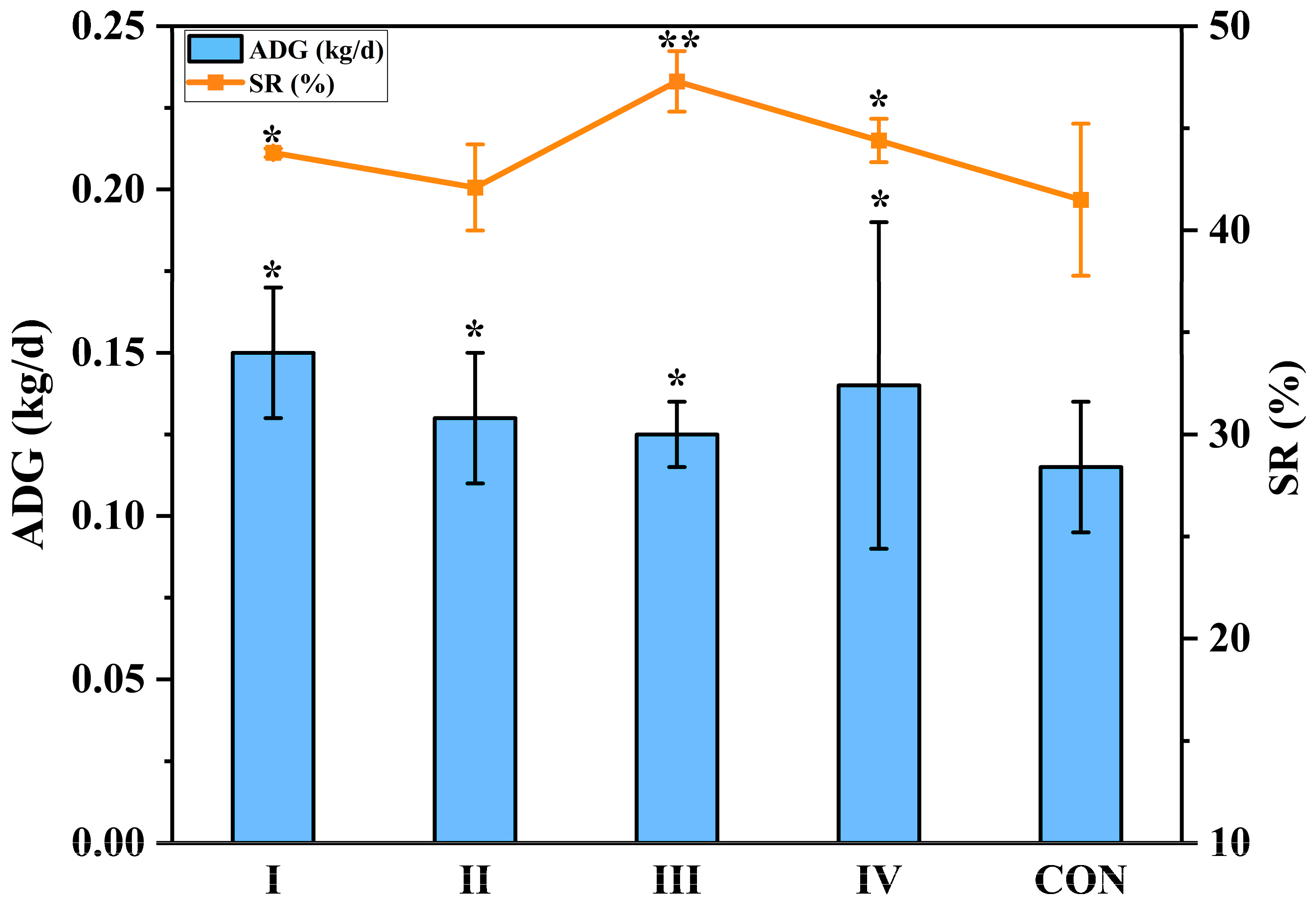
3.2. Goat Growth Performance
3.3. Goat Meat Quality
3.4. The Fatty Acid Composition in Meat
3.5. Comparison of Amino Acids in Meat
3.6. Analysis of Microbial Community in the Rumen of Boer Goats
4. Discussion
4.1. Chemical Composition and Microbial Community of Silage
4.2. Growth Performance and Rumen Microbiological Analysis in Goats
4.3. Goat Meat Quality
4.4. Fatty Acids in Goat Meat
4.5. Amino Acids in Goat Meat
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marsalis, M.A.; Angadi, S.V.; Contreras-Govea, F.E. Dry matter yield and nutritive value of corn, forage sorghum, and BMR forage sorghum at different plant populations and nitrogen rates. Field Crops Res. 2010, 116, 52–57. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Chaudhry, A.S.; Ramdani, D.; Osman, A.; Guo, X.-F.; Edwards, G.R.; Cheng, L. Chemical composition and in vitro fermentation characteristics of high sugar forage sorghum as an alternative to forage maize for silage making in Tarim Basin, China. J. Integr. Agric. 2016, 15, 175–182. [Google Scholar] [CrossRef]
- Penner, G.B.; Oba, M. Increasing dietary sugar concentration may improve dry matter intake, ruminal fermentation, and productivity of dairy cows in the postpartum phase of the transition period. J. Dairy Sci. 2009, 92, 3341–3353. [Google Scholar] [CrossRef]
- Borghi, E.; Crusciol, C.A.C.; Nascente, A.S.; Sousa, V.V.; Martins, P.O.; Mateus, G.P.; Costa, C. Costa Sorghum grain yield, forage biomass production and revenue as affected by intercropping time. Eur. J. Agron. 2013, 51, 130–139. [Google Scholar] [CrossRef]
- Li, B.-Z.; Balan, V.; Yuan, Y.J.; Dale, B.E. Process optimization to convert forage and sweet sorghum bagasse to ethanol based on ammonia fiber expansion (AFEX) pretreatment. Bioresour. Technol. 2010, 101, 1285–1292. [Google Scholar] [CrossRef]
- dos Passos Bernardes, A.; Tremblay, G.F.; Bélanger, G.; Seguin, P.; Brégard, A.; Vanasse, A. Bagasse Silage from Sweet Pearl Millet and Sweet Sorghum as Influenced by Harvest Dates and Delays between Biomass Chopping and Pressing. BioEnergy Res. 2016, 9, 88–97. [Google Scholar] [CrossRef]
- Ward, J.D.; Redfearn, D.D.; McCormick, M.E.; Cuomo, G.J. Chemical Composition, Ensiling Characteristics, and Apparent Digestibility of Summer Annual Forages in a Subtropical Double-Cropping System with Annual Ryegrass1. J. Dairy Sci. 2001, 84, 177–182. [Google Scholar] [CrossRef]
- Schittenhelm, S.; Schroetter, S. Comparison of Drought Tolerance of Maize, Sweet Sorghum and Sorghum-Sudangrass Hybrids. J. Agron. Crop Sci. 2014, 200, 46–53. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Lu, S.; Chaudhry, A.S.; Tarla, D.; Khanaki, H.; Raja, I.H.; Shan, A. Effects of Sweet and Forge Sorghum Silages Compared to Maize Silage without Additional Grain Supplement on Lactation Performance and Digestibility of Lactating Dairy Cows. Animals 2024, 14, 1702. [Google Scholar] [CrossRef]
- Gao, X.; Hou, M.J.; Fu, X.Y.; Wang, H.C.; Shang, Z.H.; Zhu, H.D. Effect of replacing corn straw by sweet sorghum silage or whole plant corn silage in sheep diets on rumen fermentation and bacterial flora. Animal 2023, 17, 100906. [Google Scholar] [CrossRef]
- Casey, N.H.; Van Niekerk, W.A. The boer goat. I. Origin, adaptability, performance testing, reproduction and milk production. Small Rumin. Res. 1988, 1, 291–302. [Google Scholar] [CrossRef]
- Erasmus, J.A. Adaptation to various environments and resistance to disease of the Improved Boer goat. Small Rumin. Res. 2000, 36, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Dong, M.; Li, Q.; Xu, F.; Wang, S.; Chen, J.; Li, W. Effects of microbial inoculants on the fermentation characteristics and microbial communities of sweet sorghum bagasse silage. Sci. Rep. 2020, 10, 837. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Wu, P.; Fu, X.; Wang, H.; Hou, M.; Shang, Z. Effect of Silage Diet (Sweet Sorghum vs. Whole-Crop Corn) and Breed on Growth Performance, Carcass Traits, and Meat Quality of Lambs. Animals 2021, 11, 3120. [Google Scholar] [CrossRef]
- Lv, X.; Chen, L.; Zhou, C.; Zhang, G.; Xie, J.; Kang, J.; Tan, Z.; Tang, S.; Kong, Z.; Liu, Z.; et al. Application of different proportions of sweet sorghum silage as a substitute for corn silage in dairy cows. Food Sci. Nutr. 2023, 11, 3575–3587. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, X.F.; Liu, J.B.; Gao, L.J.; Ishii, M.; Igarashi, Y.; Cui, Z.J. Effects of water-soluble carbohydrate content on silage fermentation of wheat straw. J. Biosci. Bioeng. 2006, 101, 232–237. [Google Scholar] [CrossRef]
- Dehghani, M.R.; Weisbjerg, M.R.; Hvelplund, T.; Kristensen, N.B. Effect of enzyme addition to forage at ensiling on silage chemical composition and NDF degradation characteristics. Livest. Sci. 2012, 150, 51–58. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, X.J.; Li, J.F.; Wang, S.R.; Dong, Z.H.; Shao, T. Effects of developmental stage and store time on the microbial community and fermentation quality of sweet sorghum silage. Ital. J. Anim. Sci. 2022, 21, 1543–1557. [Google Scholar] [CrossRef]
- Manninen, M.; Virkajärvi, P.; Jauhiainen, L. Effect of whole-crop barley and oat silages on the performance of mature suckler cows and their progeny in outdoor winter feeding. Anim. Feed. Sci. Technol. 2005, 121, 227–242. [Google Scholar] [CrossRef]
- Rezaei, J.; Rouzbehan, Y.; Fazaeli, H.; Zahedifar, M. Effects of substituting amaranth silage for corn silage on intake, growth performance, diet digestibility, microbial protein, nitrogen retention and ruminal fermentation in fattening lambs. Anim. Feed. Sci. Technol. 2014, 192, 29–38. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Song, X.; Zhong, L.; Lyu, N.; Liu, F.; Li, B.; Hao, Y.; Xue, Y.; Li, J.; Feng, Y.; Ma, Y.; et al. Inulin Can Alleviate Metabolism Disorders in ob/ob Mice by Partially Restoring Leptin-related Pathways Mediated by Gut Microbiota. Genom. Proteom. Bioinform. 2019, 17, 64–75. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chiu, C.C.; Hung, S.W.; Huang, W.C.; Lee, Y.P.; Liu, J.Y.; Huang, Y.T.; Chen, T.H.; Chuang, H.L. Gnotobiotic mice inoculated with Firmicutes, but not Bacteroidetes, deteriorate nonalcoholic fatty liver disease severity by modulating hepatic lipid metabolism. Nutr. Res. 2019, 69, 20–29. [Google Scholar] [CrossRef]
- Abhijith, A.; Warner, R.D.; Ha, M.; Dunshea, F.R.; Leury, B.J.; Zhang, M.; Joy, A.; Osei-Amponsah, R.; Chauhan, S.S. Effect of slaughter age and post-mortem days on meat quality of longissimus and semimembranosus muscles of Boer goats. Meat Sci. 2021, 175, 108466. [Google Scholar] [CrossRef]
- Chauhan, S.S.; England, E.M. Postmortem glycolysis and glycogenolysis: Insights from species comparisons. Meat Sci. 2018, 144, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Hopkins, D.L.; Bruce, H.; Li, D.; Baldi, G.; Bekhit, A.E. Causes and Contributing Factors to “Dark Cutting” Meat: Current Trends and Future Directions: A Review. Compr. Rev. Food. Sci. Food Saf. 2017, 16, 400–430. [Google Scholar] [CrossRef]
- Husin, N.; Zulkhairi, M.; Rahim, M.Z.A.; Azizan, M.; Noor, M.A.M.; Ismail-Fitry, M.R.; Rashedi, M.; Hassan, N. Real-time monitoring of food freshness using delphinidin-based visual indicator. Malays. J. Anal. Sci. 2020, 24, 558–569. [Google Scholar]
- Devi, R.; Rasane, P.; Kaur, S.; Singh, J.; Mca, S. Meat and Meat losses: Influence on meat quality. SSRN Electron. J. 2019, 6, 762–786. [Google Scholar]
- Cadavez, V.A.P.; Popova, T.; Bermúdez, R.; Osoro, K.; Purriños, L.; Bodas, R.; Lorenzo, J.M.; Gonzales-Barron, U. Compositional attributes and fatty acid profile of lamb meat from Iberian local breeds. Small Rumin. Res. 2020, 193, 106244. [Google Scholar] [CrossRef]
- Morán, L.; Giráldez, F.J.; Panseri, S.; Aldai, N.; Jordán, M.J.; Chiesa, L.M.; Andrés, S. Effect of dietary carnosic acid on the fatty acid profile and flavour stability of meat from fattening lambs. Food Chem. 2013, 138, 2407–2414. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Jiang, F.; Song, J.; Yang, X.; Shu, N.; Yuan, L.; Tan, C.P.; Liu, Y. Understanding of the Role of Pretreatment Methods on Rapeseed Oil from the Perspective of Phenolic Compounds. J. Agric. Food Chem. 2020, 68, 8847–8854. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.E.; Cadwallader, K.R. Identification and Quantitation of Potent Odorants in Spearmint Oils. J. Agric. Food Chem. 2018, 66, 2414–2421. [Google Scholar] [CrossRef]
- Banskalieva, V.; Sahlu, T.; Goetsch, A.L. Fatty acid composition of goat muscles and fat depots: A review. Small Rumin. Res. 2000, 37, 255–268. [Google Scholar] [CrossRef]
- Meinert, L.; Andersen, L.T.; Bredie, W.L.; Bjergegaard, C.; Aaslyng, M.D. Chemical and sensory characterisation of pan-fried pork flavour: Interactions between raw meat quality, ageing and frying temperature. Meat Sci. 2007, 75, 229–242. [Google Scholar] [CrossRef]
- Li, H.; Xu, M.; Lee, J.; He, C.; Xie, Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1234–E1244. [Google Scholar] [CrossRef]
- Su, W.; Xu, W.; Zhang, H.; Ying, Z.; Zhou, L.; Zhang, L.; Wang, T. Effects of dietary leucine supplementation on the hepatic mitochondrial biogenesis and energy metabolism in normal birth weight and intrauterine growth-retarded weanling piglets. Nutr. Res. Pract. 2017, 11, 121–129. [Google Scholar] [CrossRef]

| Item | Silage | ||
|---|---|---|---|
| Forage Sweet Sorghum Silage (FSS) | Sugar Sweet Sorghum Silage (SSS) | Whole-Corp Corn Silage (CS) | |
| DM, % | 32.98 | 51.71 | 28.31 |
| pH | 4.59 | 5.08 | 4.78 |
| WSCs, % DM | 22.35 | 17.16 | 10.56 |
| CP, % DM | 5.76 | 5.87 | 5.18 |
| NDF, % DM | 75.98 | 83.93 | 63.58 |
| ADF, % DM | 46.62 | 55.86 | 44.71 |
| Crude fiber, % DM | 40.14 | 47.32 | 30.5 |
| Ash, % DM | 8.45 | 7.34 | 8.08 |
| Lactic acid, g/kg DM | 10.03 | 8.32 | 8.5 |
| Acetic acid, g/kg DM | 2.30 | 2.18 | 2.34 |
| Propionic acid, g/kg DM | 0.87 | 1.01 | 1.18 |
| Item | Silage | ||||
|---|---|---|---|---|---|
| Group I | Group II | Group III | Group IV | CON | |
| Concentrate diet | |||||
| Ingredient, % of diet DM | |||||
| Maize, % | 64.0 | 64.0 | 64.0 | 64.0 | 64.0 |
| Soybean meal, % | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Wheat bran, % | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Salt, % | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Premix, % | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Roughage diet | |||||
| Ingredient, % of diet FM | |||||
| Forage sweet sorghum silage, % | 50.0 | 70.0 | - | - | - |
| Sugar sweet sorghum silage, % | - | - | 50.0 | 70.0 | - |
| Corn silage, % | - | - | - | - | 50.0 |
| Wheat stalk, % | 30.0 | 10.0 | 30.0 | 10.0 | 30.0 |
| Alfalfa, % | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Item | Treatment | ||||
|---|---|---|---|---|---|
| Group I | Group II | Group III | Group IV | CON | |
| pH | 6.08 ± 0.14 | 6.00 ± 0.27 | 5.80 ± 0.21 | 5.90 ± 0.16 | 5.86 ± 0.09 |
| Moisture, % | 77.40 ± 1.56 | 78.75 ± 2.33 | 77.5 ± 2.97 | 77.4 ± 0.57 | 76.45 ± 1.34 |
| CP, % | 23.44 ± 2.93 | 23.20 ± 2.76 | 24.66 ± 3.03 * | 24.21 ± 2.31* | 22.86 ± 3.39 |
| Crude fat, % | 4.54 ± 0.23 | 4.62 ± 0.12 | 5.02 ± 0.11 * | 4.96 ± 0.21 | 4.52 ± 0.18 |
| Ash, % | 1.08 ± 0.0020 | 1.00 ± 0.0010 | 1.04 ± 0.0004 | 0.96 ± 0.0001 | 1.01 ± 0.0006 |
| Item | Treatment | ||||
|---|---|---|---|---|---|
| Group I | Group II | Group III | Group IV | CON | |
| C6:0 | 0.22 ± 0.07 | 0.21 ± 0.01 | 0.23 ± 0.02 | 0.22 ± 0.03 | 0.24 ± 0.11 |
| C10:0 | 0.17 ± 0.04 | 0. 15 ± 0.02 | 0.14 ± 0.03 | 0.16 ± 0.01 | 0.18 ± 0.02 |
| C12:0 | 0.23 ± 0.67 | 0.01 ± 0.00 | 0.03 ± 0.02 | 0.27 ± 0.02 | 0.29 ± 0.12 |
| C13:0 | 0.09 ± 0.02 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.05 | 0.10 ± 0.03 |
| C14:0 | 0.45 ± 0.68 | 0.43 ± 0.42 | 0.47 ± 0.23 | 0.42 ± 0.23 | 0.47 ± 0.23 |
| C15:0 | 0.21 ± 0.08 | 0.19 ± 0.03 | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.03 |
| C16:0 | 25.40 ± 1.51 | 26.42 ± 1.67 | 27.14 ± 1.76 | 27.56 ± 1.68 | 30.36 ± 1.81 |
| C17:0 | 0.16 ± 0.06 | 0.16 ± 0.04 | 0.12 ± 0.01 | 0.14 ± 0.03 | 0.13 ± 0.02 |
| C18:0 | 21.91 ± 1.41 | 22.23 ± 1.68 | 23.01 ± 1.59 | 23.31 ± 1.57 | 22.21 ± 1.41 |
| C20:0 | 0.77 ± 0.48 | 0.79 ± 0.77 | 0.67 ± 0.23 | 0.80 ± 0.23 | 0.79 ± 0.23 |
| C21:0 | 0.12 ± 0.04 | 0.11 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.03 | 0.10 ± 0.03 |
| C22:0 | 0.23 ± 0.65 | 0.25 ± 0.46 | 0.29 ± 0.19 | 0.28 ± 0.03 | 0.28 ± 0.21 |
| C23:0 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| C16:1 | 6.83 ± 0.60 | 7.75 ± 1.02 | 6.87 ± 0.57 | 6.70 ± 1.20 | 6.26 ± 1.23 |
| C18:1 n-9 trans | 1.23 ± 0.51 | 1.22 ± 0.84 | 1.24 ± 0.81 | 1.23 ± 0.81 | 1.22 ± 0.79 |
| C18:1 n-9 cis | 28.69 ± 1.34 * | 29.41 ± 1.96 * | 29.31 ± 1.52 * | 26.70 ± 1.41 | 24.84 ± 1.07 |
| C20:1 | 0.11 ± 0.04 | 0.12 ± 0.02 | 0.13 ± 0.03 | 0.11 ± 0.02 | 0.13 ± 0.04 |
| C22:1 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| C18:2 n-6 | 2.53 ± 0.11 | 2.85 ± 0.09 | 2.79 ± 0.10 | 2.60 ± 0.10 | 2.54 ± 0.10 |
| C18:3 n-6 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| C20:3 n-6 | 0.12 ± 0.02 | 0.11 ± 0.01 | 0.12 ± 0.03 | 0.12 ± 0.02 | 0.11 ± 0.02 |
| C20:4 n-6 | 4.37 ± 0.67 | 4.78 ± 0.23 * | 4.47 ± 0.23 | 3.98 ± 0.48 | 3.82 ± 1.01 |
| C22:4 n-6 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.01 |
| C18:3 n-3 | 1.95 ± 0.23 | 0.78 ± 0.48 | 0.55 ± 0.42 | 1.68 ± 0.41 | 1.60 ± 0.39 |
| C20:3 n-3 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.00 |
| C20:5 n-3 | 0.21 ± 0.01 | 0.22 ± 0.04 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.21 ± 0.03 |
| C22:5 n-3 | 0.31 ± 0.07 | 0. 30 ± 0.07 | 0.29 ± 0.06 | 0.29 ± 0.08 | 0.31 ± 0.07 |
| C22:6 n-3 | 0.17 ± 0.03 | 0.16 ± 0.02 | 0.17 ± 0.03 | 0.18 ± 0.03 | 0.18 ± 0.02 |
| SFA | 49.97 ± 1.63 * | 51.07 ± 1.10 | 52.51 ± 1.04 | 53.57 ± 2.02 | 55.37 ± 2.11 |
| MUFA | 36.88 ± 1.95 | 38.52 ± 2.01 * | 37.59 ± 1.84 | 34.76 ± 1.68 | 32.47 ± 1.51 |
| PUFA | 9.82 ± 0.21 * | 9.38 ± 1.18 | 8.78 ± 1.86 | 9.25 ± 1.23 | 8.97 ± 1.08 |
| UFA | 46.70 ± 1.65 * | 47.90 ± 1.14 * | 46.36 ± 1.43 * | 44.01 ± 1.52 | 41.44 ± 1.37 |
| UFA/SFA | 0.935 ± 0.02 ** | 0.938 ± 0.01 ** | 0.883 ± 0.01* | 0.822 ± 0.02 | 0.748 ± 0.01 |
| Item | Treatment | ||||
|---|---|---|---|---|---|
| Group I | Group II | Group III | Group IV | CON | |
| Thr | 3.60 ± 0.03 | 3.71 ± 0.02 | 2.83 ± 0.01 | 2.76 ± 0.01 | 3.50 ± 0.02 |
| Val | 3.90 ± 0.01 * | 3.89 ± 0.2 * | 2.98 ± 0.01 | 3.04 ± 0.01 | 3.64 ± 0.03 |
| Met | 0.23 ± 0.00 | 0.93 ± 0.01 ** | 0.11 ± 0.01 | 0.94 ± 0.01 ** | 0.09 ± 0.00 |
| Ile | 3.62 ± 0.02 * | 3.61 ± 0.01 * | 2.76 ± 0.01 | 2.67 ± 0.01 | 3.34 ± 0.03 |
| Leu | 6.24 ± 0.17 * | 6.30 ± 0.13 * | 4.86 ± 0.09 | 4.85 ± 0.08 | 5.93 ± 0.11 |
| Phe | 3.00 ± 0.05 | 3.05 ± 0.04 | 2.68 ± 0.02 | 2.40 ± 0.01 | 2.88 ± 0.03 |
| Lys | 6.35 ± 0.13 | 6.43 ± 0.13 * | 4.86 ± 0.03 | 4.84 ± 0.02 | 6.02 ± 0.13 |
| Trp | 1.11 ± 0.04 * | 0.98 ± 0.02 | 0.68 ± 0.01 * | 0.81 ± 0.01 | 0.98 ± 0.02 |
| Asp | 7.13 ± 0.17 | 7.21 ± 0.15 | 5.60 ± 0.11 | 5.66 ± 0.09 | 6.99 ± 0.12 |
| Ser | 2.96 ± 0.01 | 3.04 ± 0.03 | 2.40 ± 0.02 * | 2.39 ± 0.01 * | 2.86 ± 0.03 |
| Glu | 13.02 ± 0.21 | 13.48 ± 0.23 | 10.62 ± 0.21 | 10.68 ± 0.22 | 12.90 ± 0.27 |
| Pro | 5.74 ± 0.12 * | 5.84 ± 0.11 * | 5.19 ± 0.16 | 5.86 ± 0. 17 * | 5.34 ± 0.16 |
| Gly | 3.18 ± 0.02 | 3.30 ± 0.04 | 2.82 ± 0.01 | 3.81 ± 0.05 * | 2.97 ± 0.02 |
| Ala | 4.41 ± 0.15 | 4.44 ± 0.13 | 3.55 ± 0.11 | 3.93 ± 0.09 | 4.20 ± 0.02 |
| Tyr | 2.43 ± 0.02 | 2.39 ± 0.03 | 1.76 ± 0.05 | 1.84 ± 0.04 | 2.06 ± 0.04 |
| His | 2.34 ± 0.01 | 2.22 ± 0.02 | 1.59 ± 0.02 | 1.63 ± 0.01 | 1.95 ± 0.03 |
| Arg | 4.69 ± 0.12 | 4.81 ± 0.13 * | 3.74 ± 0.11 | 4.00 ± 0.12 * | 4.55 ± 0.15 |
| Cys | 2.00 ± 0.01 | 2.18 ± 0.02 | 1.52 ± 0.00 | 1.93 ± 0.01 | 2.32 ± 0.02 |
| EAA | 28.05 ± 0.67 * | 28.90 ± 0.47 * | 21.76 ± 0.65 | 22.31 ± 0.47 | 26.38 ± 0.42 |
| NEAA | 47.90 ± 1.85 | 48.91 ± 1.42 * | 38.79 ± 1.21 | 41.73 ± 1.54 | 46.14 ± 1.35 |
| Total | 74.84 ± 2.21 * | 76.83 ± 2.31 * | 59.87 ± 2.01 | 63.23 ± 2.43 | 71.54 ± 2.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Guo, F.; Wang, Y.; Dong, M.; Wang, J.; Xiao, G. Effects of Substituting Sweet Sorghum for Corn Silage in the Diet on the Growth Performance, Meat Quality, and Rumen Microorganisms of Boer Goats in China. Animals 2025, 15, 1492. https://doi.org/10.3390/ani15101492
Wang S, Guo F, Wang Y, Dong M, Wang J, Xiao G. Effects of Substituting Sweet Sorghum for Corn Silage in the Diet on the Growth Performance, Meat Quality, and Rumen Microorganisms of Boer Goats in China. Animals. 2025; 15(10):1492. https://doi.org/10.3390/ani15101492
Chicago/Turabian StyleWang, Shuyang, Fangzhu Guo, Yuchen Wang, Miaoyin Dong, Junkai Wang, and Guoqing Xiao. 2025. "Effects of Substituting Sweet Sorghum for Corn Silage in the Diet on the Growth Performance, Meat Quality, and Rumen Microorganisms of Boer Goats in China" Animals 15, no. 10: 1492. https://doi.org/10.3390/ani15101492
APA StyleWang, S., Guo, F., Wang, Y., Dong, M., Wang, J., & Xiao, G. (2025). Effects of Substituting Sweet Sorghum for Corn Silage in the Diet on the Growth Performance, Meat Quality, and Rumen Microorganisms of Boer Goats in China. Animals, 15(10), 1492. https://doi.org/10.3390/ani15101492








