Highly Pathogenic Avian Influenza H5N1 in Cats (Felis catus): A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Sources of Information and Strategy of Search
2.2. Selection of Studies and Extraction of Data
2.3. Assessment of Bias Risk
2.4. Publication Bias Assessment
2.5. Data Analysis
3. Findings
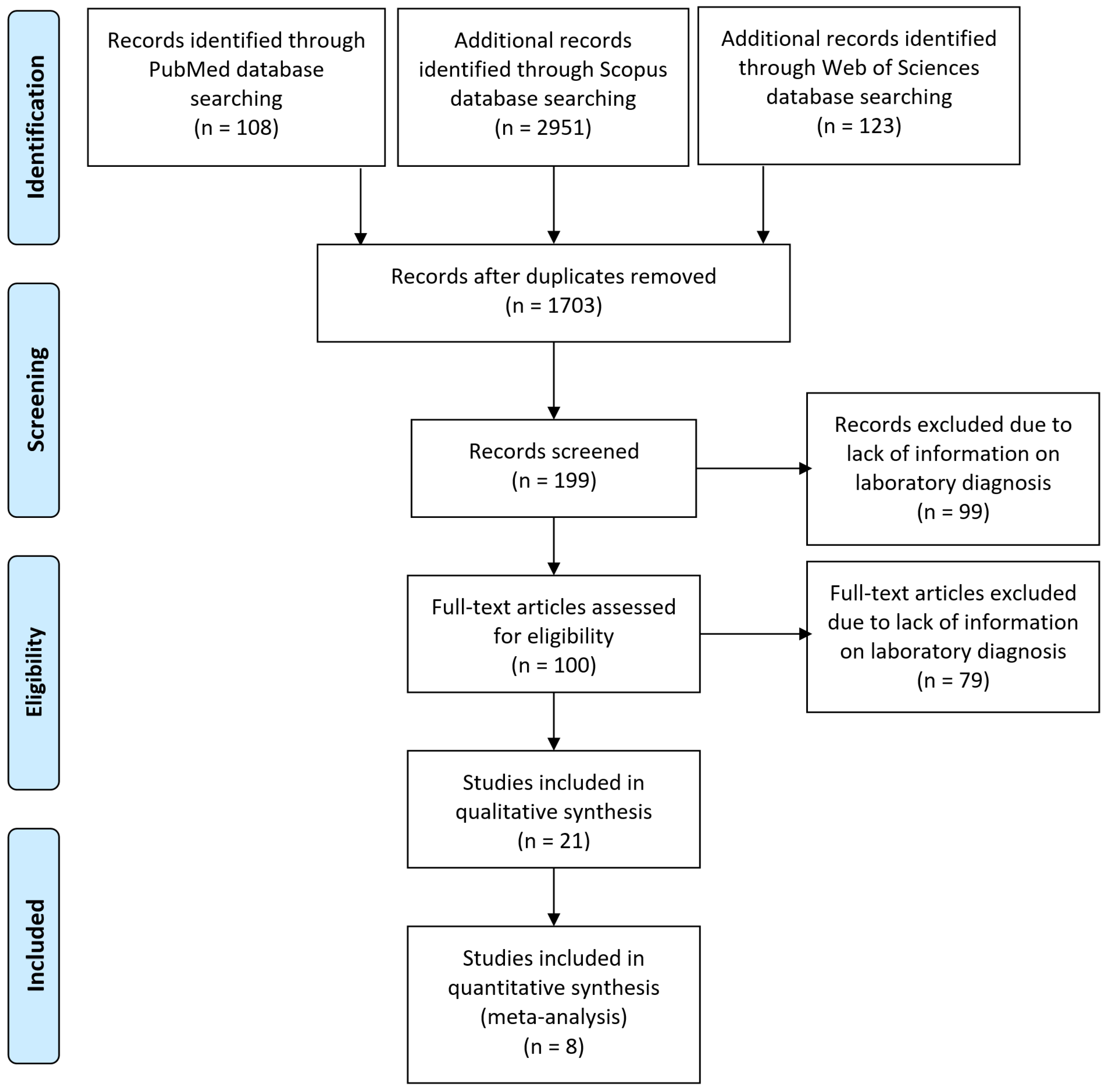
3.1. Selection of Studies
3.2. Characteristics of Included Studies
3.3. Risk of Bias Assessment
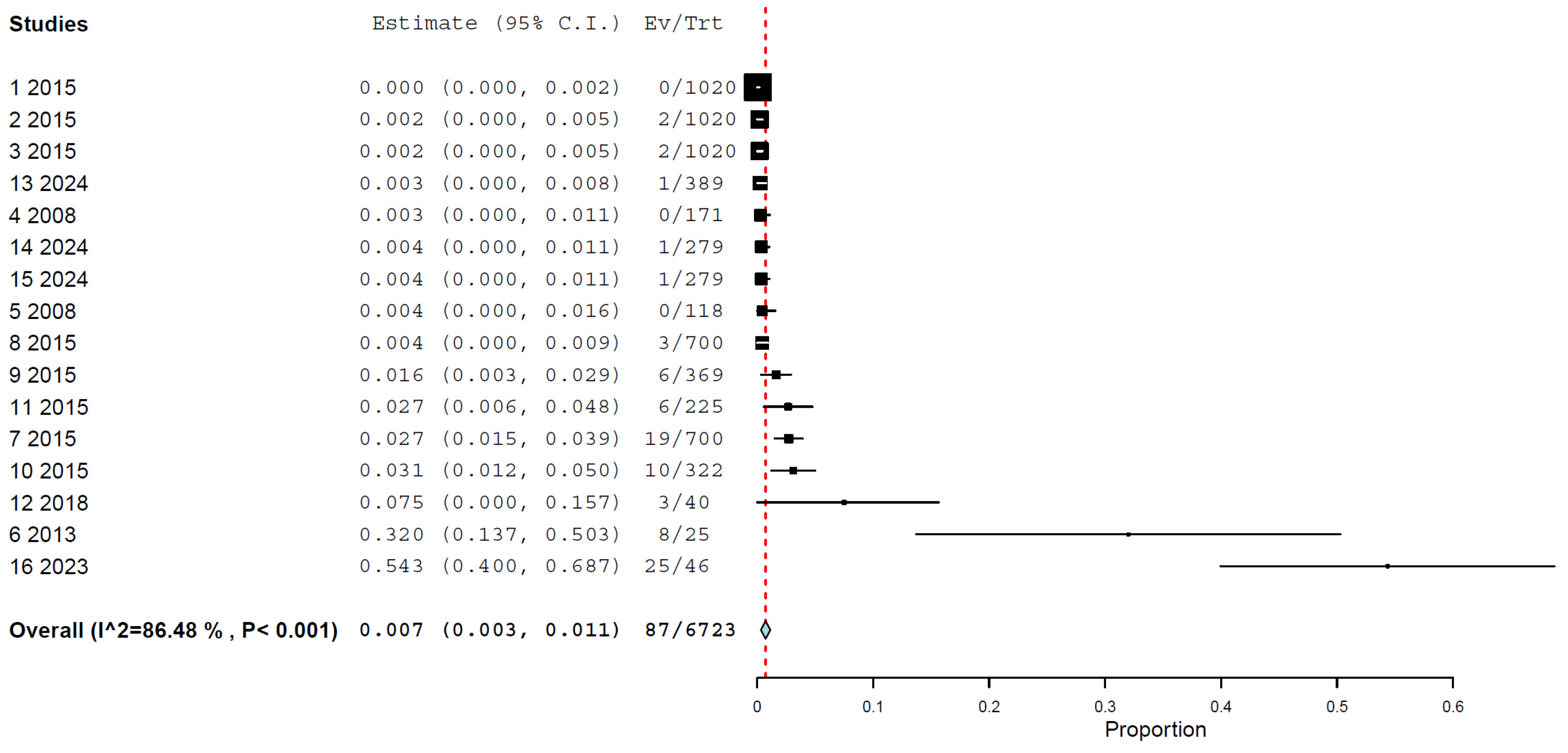
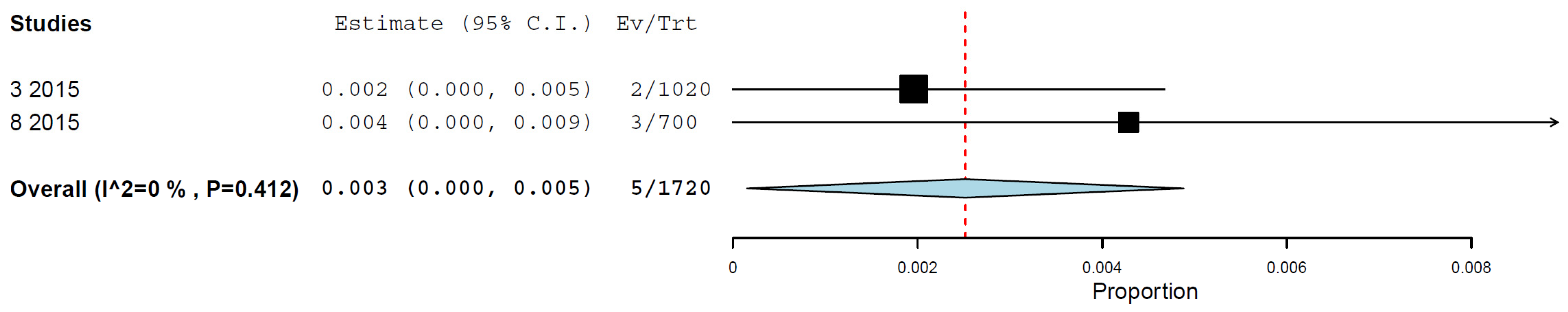
3.4. Prevalence/Seroprevalence of H5N1 in Cats by Any Method
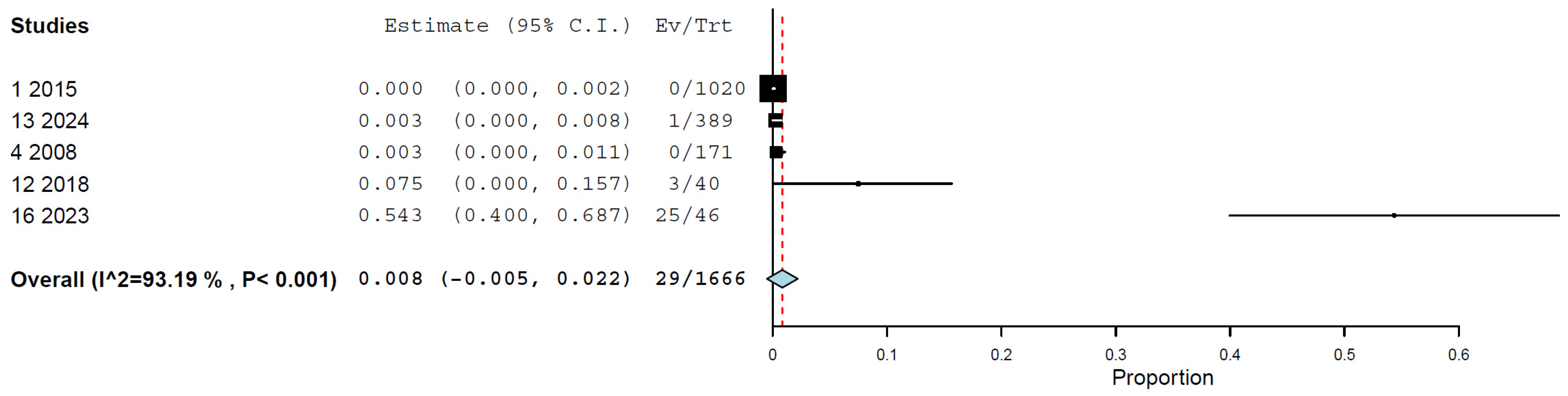
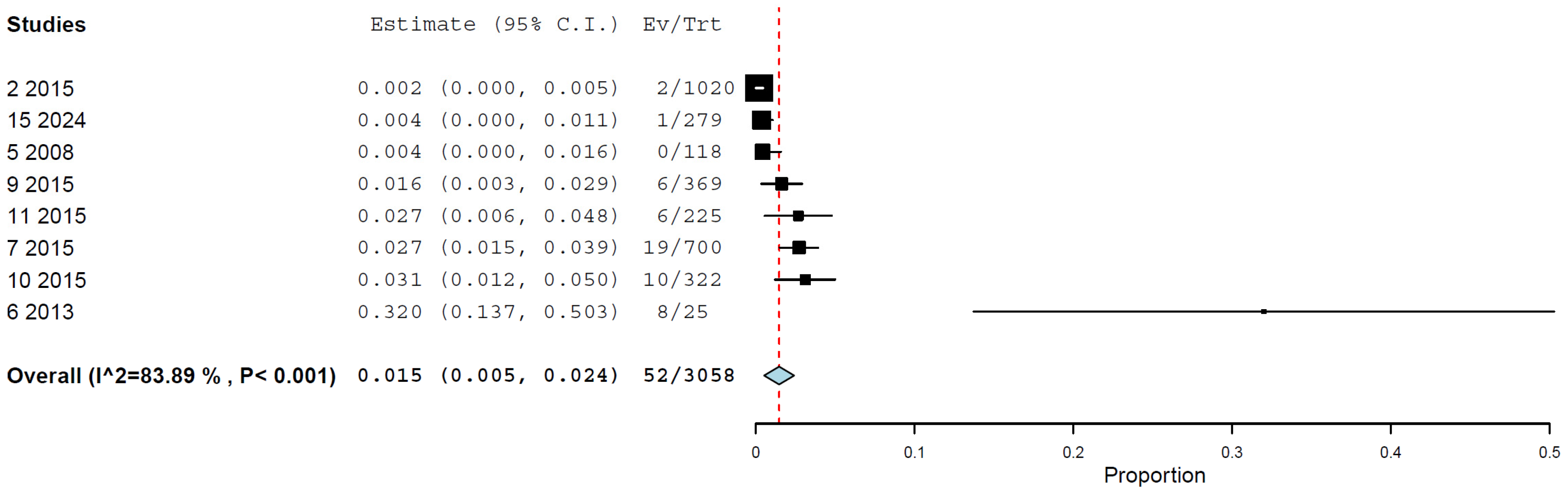
3.5. Prevalence/Seroprevalence of H5N1 in Cats by Molecular and Immunological Methods
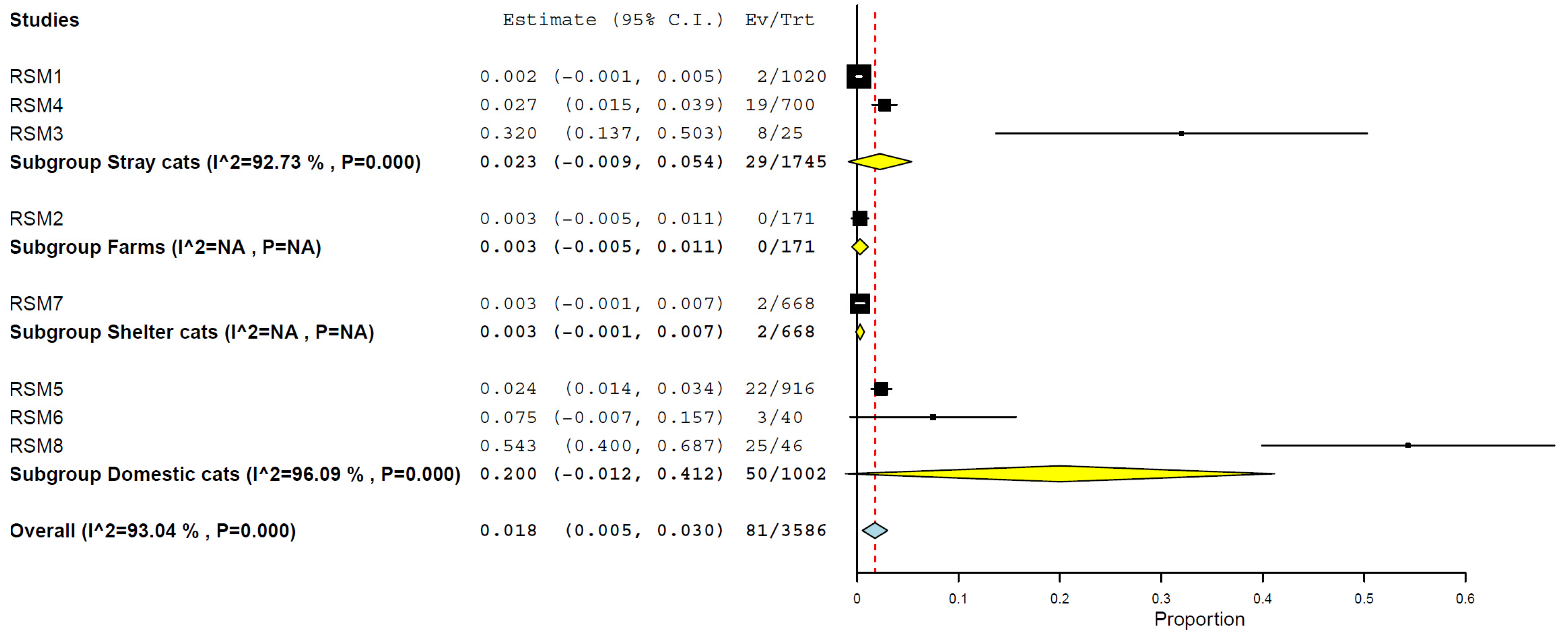
3.6. Prevalence/Seroprevalence of H5N1 in Cats by Type of Cats
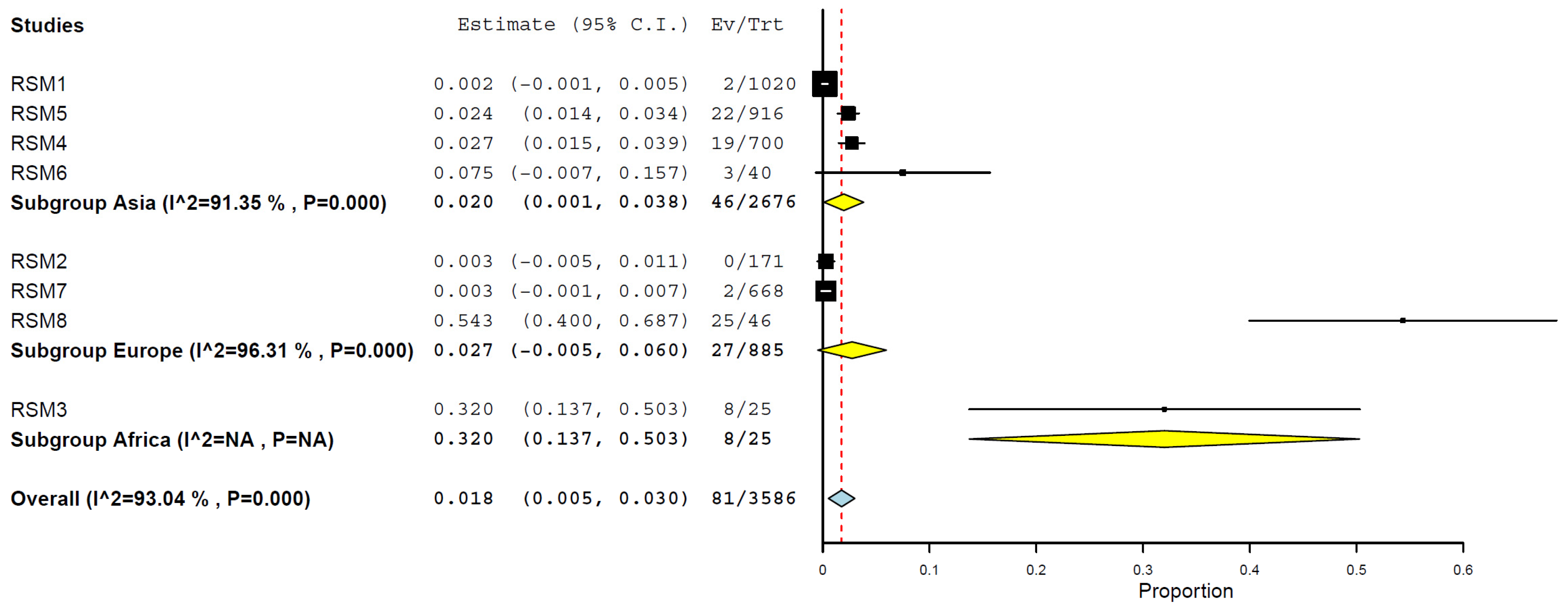
3.7. Prevalence/Seroprevalence of H5N1 in Cats by Geographical Regions
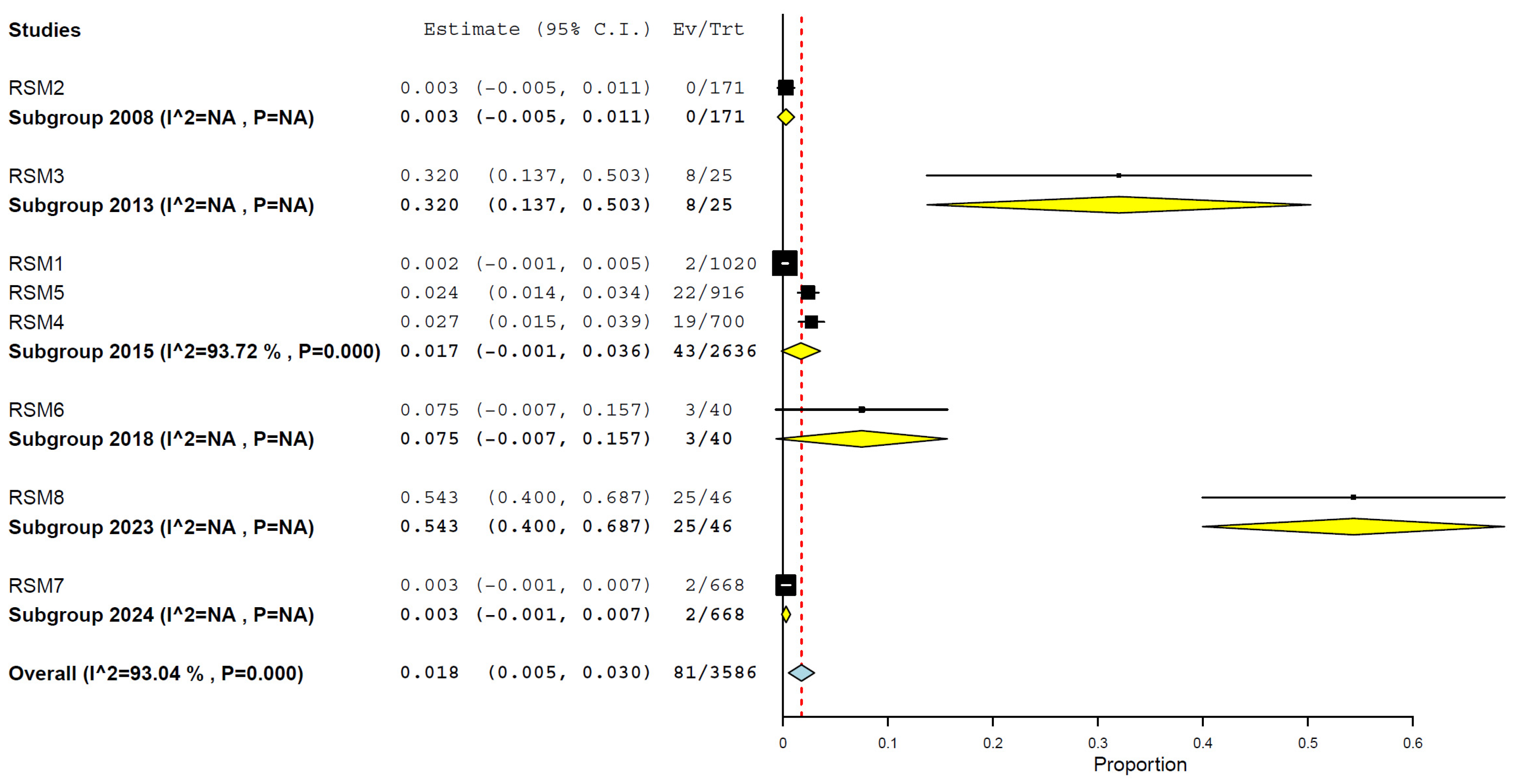
3.8. Prevalence/Seroprevalence of H5N1 in Cats by Years and Periods
3.9. Molecular and Serological Findings from Case Reports
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonilla-Aldana, D.K.; Calle-Hernández, D.M.; Ulloque-Badaracco, J.R.; Alarcón-Braga, E.A.; Hernández-Bustamante, E.A.; Cabrera-Guzmán, J.C.; Quispe-Vasquez, S.M.; Huayta-Cortez, M.A.; Benites-Zapata, V.A.; Rodriguez-Morales, A.J. Highly pathogenic avian influenza A(H5N1) in animals: A systematic review and meta-analysis. New Microbes New Infect. 2024, 60–61, 101439. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Aldana, D.K.; Aguirre-Florez, M.; Villamizar-Peña, R.; Gutiérrez-Ocampo, E.; Henao-Martínez, J.F.; Cvetkovic-Vega, A.; Dhama, K.; Rabaan, A.; Sah, R.; Rodriguez-Morales, A.J.; et al. After SARS-CoV-2, will H5N6 and other influenza viruses follow the pandemic path? Infez. Med. 2020, 28, 475–485. [Google Scholar]
- Rodriguez-Morales, A.J.; Bonilla-Aldana, D.K.; Paniz-Mondolfi, A.E. Concerns about influenza H5N8 outbreaks in humans and birds: Facing the next airborne pandemic? Travel. Med. Infect. Dis. 2021, 41, 102054. [Google Scholar] [CrossRef] [PubMed]
- Priyanka; Khandia, R.; Chopra, H.; Choudhary, O.P.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. The re-emergence of H3N2 influenza: An update on the risk and containment. New Microbes New Infect. 2023, 53, 101147. [Google Scholar] [CrossRef]
- Srivastava, S.; Jayaswal, N.; Kumar, S.; Rao, G.; Budha, R.R.; Mohanty, A.; Mehta, R.; Apostolopoulos, V.; Sah, S.; Bonilla-Aldana, D.K.; et al. Targeting H3N2 influenza: Advancements in treatment and vaccine strategies. Expert. Rev. Anti Infect. Ther. 2025, 23, 5–18. [Google Scholar] [CrossRef]
- Chaudhary, R.K.; Patil, P.; Mateti, U.V.; Sah, S.; Mohanty, A.; Rath, R.S.; Padhi, B.K.; Malik, S.; Jassim, K.H.; Al-Shammari, M.A.; et al. System Biology Approach to Identify the Hub Genes and Pathways Associated with Human H5N1 Infection. Vaccines 2023, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Choy, K.T.; Cheng, K.M.; Brackman, C.J.; Cheng, S.M.; Sit, T.H.; Tse, A.C.; Sims, L.D.; Gu, H.; Tang, A.W.; et al. Detection and characterisation of high pathogenicity avian influenza virus (H5N1/H5N8) clade 2.3.4.4b, Hong Kong SAR, China, 2021 to 2024. Euro Surveill. 2025, 30. [Google Scholar] [CrossRef]
- CDC. Isolation of avian influenza A(H5N1) viruses from humans—Hong Kong, May–December 1997. MMWR Morb. Mortal. Wkly. Rep. 1997, 46, 1204–1207. [Google Scholar]
- CDC. Update: Isolation of avian influenza A(H5N1) viruses from humans—Hong Kong, 1997–1998. MMWR Morb. Mortal. Wkly. Rep. 1998, 46, 1245–1247. [Google Scholar]
- Apostolopoulos, V.; Chavda, V.P.; Mehta, R.; Rodriguez-Morales, A.J.; Henao-MartÍnez, A.F.; Sah, R. Alert and surveillance on H5N1 influenza virus: Risks to agriculture and public health. Ther. Adv. Infect. Dis. 2024, 11, 20499361241266521. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Hui, D.S.; Ippolito, G.; Traore, T.; Satta, G.; Everett, D.B.; Zumla, A. Avian Influenza - The next travel-associated pandemic? Proactive One Health surveillance is required to reduce the risk of the spread. Travel. Med. Infect. Dis. 2025, 65, 102829. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Reinhart, K.; Couture, A.; Kniss, K.; Davis, C.T.; Kirby, M.K.; Murray, E.L.; Zhu, S.; Kraushaar, V.; Wadford, D.A.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infections in Humans. N. Engl. J. Med. 2025, 392, 843–854. [Google Scholar] [CrossRef]
- Vasudevan, G.; Vanamayya, P.R.; Nagarajan, S.; Rajukumar, K.; Suba, S.; Venketash, G.; Tosh, C.; Sood, R.; Nissly, R.H.; Kuchipudi, S.V. Infectious dose-dependent accumulation of live highly pathogenic avian influenza H5N1 virus in chicken skeletal muscle-implications for public health. Zoonoses Public Health 2018, 65, e243–e247. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Haque, S.; Tawil, S.; Husni, R.; Bonilla-Aldana, D.K.; Montenegro-Idrogo, J.J.; Rodriguez-Morales, A.J. Avian influenza spillover to humans: Are we prepared to deal with another potential pandemic? Travel. Med. Infect. Dis. 2023, 55, 102634. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Naguib, M.M.; Nogales, A.; Barre, R.S.; Stewart, J.P.; García-Sastre, A.; Martinez-Sobrido, L. Avian influenza A (H5N1) virus in dairy cattle: Origin, evolution, and cross-species transmission. mBio 2024, 15, e0254224. [Google Scholar] [CrossRef]
- Sreenivasan, C.C.; Li, F.; Wang, D. Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts. Viruses 2024, 16, 1703. [Google Scholar] [CrossRef]
- Renaud, C.; Osborn, A.; Parmley, E.J.; Hatchette, T.F.; LeBlanc, J.; Weese, J.S.; Misra, V.; Yamamura, D.; Forgie, S.; Renwick, S.; et al. Highly pathogenic avian influenza: Unprecedented outbreaks in Canadian wildlife and domestic poultry. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2023, 8, 187–191. [Google Scholar] [CrossRef]
- Tomás, G.; Marandino, A.; Panzera, Y.; Rodríguez, S.; Wallau, G.L.; Dezordi, F.Z.; Pérez, R.; Bassetti, L.; Negro, R.; Williman, J.; et al. Highly pathogenic avian influenza H5N1 virus infections in pinnipeds and seabirds in Uruguay: Implications for bird-mammal transmission in South America. Virus Evol. 2024, 10, veae031. [Google Scholar] [CrossRef]
- Van Leeuw, V.; Depoorter, P.; Mauroy, A.; Beck, O.; Claeys, H.; De Regge, N.; De Waele, V.; De Winter, P.; Heymans, J.F.; Hooyberghs, J.; et al. Susceptibility of Mammals to Highly Pathogenic Avian Influenza: A Qualitative Risk Assessment From the Belgian Perspective. Zoonoses Public. Health 2025, 72, 150–165. [Google Scholar] [CrossRef]
- Sah, R.; Srivastava, S.; Kumar, S.; Mehta, R.; Donovan, S.; Sierra-Carrero, L.; Luna, C.; Woc-Colburn, L.; Cardona-Ospina, J.A.; Hinestroza-Jordan, M.; et al. Concerns on H5N1 avian influenza given the outbreak in U.S. dairy cattle. Lancet Reg. Health Am. 2024, 35, 100785. [Google Scholar] [CrossRef]
- Kalthoff, D.; Hoffmann, B.; Harder, T.; Durban, M.; Beer, M. Experimental infection of cattle with highly pathogenic avian influenza virus (H5N1). Emerg. Infect. Dis. 2008, 14, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Octaviani, C.P.; Huang, P.; Bi-Hung, P.; Gray, G.C.; Tseng, C.K. Superior replication, pathogenicity, and immune evasion of a Texas dairy cattle H5N1 virus compared to a historical avian isolate. Sci. Rep. 2025, 15, 8797. [Google Scholar] [CrossRef]
- Halwe, N.J.; Cool, K.; Breithaupt, A.; Schön, J.; Trujillo, J.D.; Nooruzzaman, M.; Kwon, T.; Ahrens, A.K.; Britzke, T.; McDowell, C.D.; et al. H5N1 clade 2.3.4.4b dynamics in experimentally infected calves and cows. Nature 2025, 637, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Burrough, E.R.; Magstadt, D.R.; Petersen, B.; Timmermans, S.J.; Gauger, P.C.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.C.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg. Infect. Dis. 2024, 30, 1335–1343. [Google Scholar] [CrossRef]
- Giménez-Lirola, L.G.; Cauwels, B.; Mora-Díaz, J.C.; Magtoto, R.; Hernández, J.; Cordero-Ortiz, M.; Nelli, R.K.; Gorden, P.J.; Magstadt, D.R.; Baum, D.H. Detection and Monitoring of Highly Pathogenic Influenza A Virus 2.3.4.4b Outbreak in Dairy Cattle in the United States. Viruses 2024, 16, 1376. [Google Scholar] [CrossRef]
- Rzymski, P. Avian influenza outbreaks in domestic cats: Another reason to consider slaughter-free cell-cultured poultry? Front. Microbiol. 2023, 14, 1283361. [Google Scholar] [CrossRef] [PubMed]
- Szaluś-Jordanow, O.; Golke, A.; Dzieciątkowski, T.; Chrobak-Chmiel, D.; Rzewuska, M.; Czopowicz, M.; Sapierzyński, R.; Kardas, M.; Biernacka, K.; Mickiewicz, M.; et al. A Fatal A/H5N1 Avian Influenza Virus Infection in a Cat in Poland. Microorganisms 2023, 11, 2263. [Google Scholar] [CrossRef]
- Briand, F.X.; Souchaud, F.; Pierre, I.; Beven, V.; Hirchaud, E.; Hérault, F.; Planel, R.; Rigaudeau, A.; Bernard-Stoecklin, S.; Van der Werf, S.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Domestic Cat, France, 2022. Emerg. Infect. Dis. 2023, 29, 1696–1698. [Google Scholar] [CrossRef]
- Naraharisetti, R.; Weinberg, M.; Stoddard, B.; Stobierski, M.G.; Dodd, K.A.; Wineland, N.; Beal, M.; Morse, J.; Hatter, S.; Sledge, D.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection of Indoor Domestic Cats Within Dairy Industry Worker Households - Michigan, May 2024. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 61–65. [Google Scholar] [CrossRef]
- Perez-Acle, T.; Ravello, C.; Rosemblatt, M. Are we cultivating the perfect storm for a human avian influenza pandemic? Biol. Res. 2024, 57, 96. [Google Scholar] [CrossRef]
- Koopmans, M.P.G.; Barton Behravesh, C.; Cunningham, A.A.; Adisasmito, W.B.; Almuhairi, S.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Cediel Becerra, N.; Charron, D.F.; et al. The panzootic spread of highly pathogenic avian influenza H5N1 sublineage 2.3.4.4b: A critical appraisal of One Health preparedness and prevention. Lancet Infect. Dis. 2024, 24, e774–e781. [Google Scholar] [CrossRef]
- Brüssow, H. The Arrival of Highly Pathogenic Avian Influenza Viruses in North America, Ensuing Epizootics in Poultry and Dairy Farms and Difficulties in Scientific Naming. Microb. Biotechnol. 2024, 17, e70062. [Google Scholar] [CrossRef] [PubMed]
- Eisfeld, A.J.; Biswas, A.; Guan, L.; Gu, C.; Maemura, T.; Trifkovic, S.; Wang, T.; Babujee, L.; Dahn, R.; Halfmann, P.J.; et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature 2024, 633, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Chothe, S.K.; Srinivas, S.; Misra, S.; Nallipogu, N.C.; Gilbride, E.; LaBella, L.; Mukherjee, S.; Gauthier, C.H.; Pecoraro, H.L.; Webb, B.T.; et al. Marked neurotropism and potential adaptation of H5N1 clade 2.3.4.4.b virus in naturally infected domestic cats. Emerg. Microbes Infect. 2025, 14, 2440498. [Google Scholar] [CrossRef]
- Misra, S.; Gilbride, E.; Ramasamy, S.; Pond, S.L.K.; Kuchipudi, S.V. Enhanced Diversifying Selection on Polymerase Genes in H5N1 Clade 2.3.4.4b: A Key Driver of Altered Species Tropism and Host Range Expansion. bioRxiv 2024. [Google Scholar] [CrossRef]
- Amonsin, A.; Songserm, T.; Chutinimitkul, S.; Jam-On, R.; Sae-Heng, N.; Pariyothorn, N.; Payungporn, S.; Theamboonlers, A.; Poovorawan, Y. Genetic analysis of influenza A virus (H5N1) derived from domestic cat and dog in Thailand. Arch. Virol. 2007, 152, 1925–1933. [Google Scholar] [CrossRef]
- Songserm, T.; Amonsin, A.; Jam-on, R.; Sae-Heng, N.; Meemak, N.; Pariyothorn, N.; Payungporn, S.; Theamboonlers, A.; Poovorawan, Y. Avian influenza H5N1 in naturally infected domestic cat. Emerg. Infect. Dis. 2006, 12, 681–683. [Google Scholar] [CrossRef]
- Kang, Y.M.; Heo, G.B.; An, S.H.; Lee, H.; Park, E.; Cha, R.M.; Jang, Y.Y.; Sagong, M.; Kim, A.Y.; Kim, J.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in Cats, South Korea, 2023. Emerg. Infect. Dis. 2024, 30, 2510–2520. [Google Scholar] [CrossRef]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef]
- Rosenke, K.; Griffin, A.; Kaiser, F.; Altynova, E.; Mukesh, R.; Bushmaker, T.; Flagg, M.; Tipih, T.; Goldin, K.; Wickenhagen, A.; et al. Pathogenesis of bovine H5N1 clade 2.3.4.4b infection in macaques. Nature 2025. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Castano-Betancourt, K.J.; Ortega-Martinez, J.M.; Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Benites-Zapata, V.A.; Rodriguez-Morales, A.J. Prevalence of zoonotic and non-zoonotic Rickettsia in horses: A systematic review and meta-analysis. New Microbes New Infect. 2023, 51, 101068. [Google Scholar] [CrossRef]
- Barker, T.H.; Migliavaca, C.B.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting proportional meta-analysis in different types of systematic reviews: A guide for synthesisers of evidence. BMC Med. Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef]
- Hunter, J.P.; Saratzis, A.; Sutton, A.J.; Boucher, R.H.; Sayers, R.D.; Bown, M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014, 67, 897–903. [Google Scholar] [CrossRef]
- Lee, K.; Yeom, M.; Vu, T.T.H.; Do, H.Q.; Na, W.; Lee, M.; Jeong, D.G.; Cheon, D.S.; Song, D. Characterization of highly pathogenic avian influenza A (H5N1) viruses isolated from cats in South Korea, 2023. Emerg. Microbes Infect. 2024, 13, 2290835. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Wolf, P.U.; Uhl, W.; Gerst, S.; Harder, T.; Starick, E.; Vahlenkamp, T.W.; Mettenleiter, T.C.; Teifke, J.P. Distribution of lesions and antigen of highly pathogenic avian influenza virus A/Swan/Germany/R65/06 (H5N1) in domestic cats after presumptive infection by wild birds. Vet. Pathol. 2007, 44, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Mainenti, M.; Siepker, C.; Magstadt, D.R.; Gauger, P.; Baum, D.; Petersen, B.; Aubrey, T.; Sett, K.; Burrough, E.R. Distribution of lesions and detection of influenza A(H5N1) virus, clade 2.3.4.4b, in ante- and postmortem samples from naturally infected domestic cats on U.S. dairy farms. J. Vet. Diagn. Investig. 2025, 37, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rabalski, L.; Milewska, A.; Pohlmann, A.; Gackowska, K.; Lepionka, T.; Szczepaniak, K.; Swiatalska, A.; Sieminska, I.; Arent, Z.; Beer, M.; et al. Emergence and potential transmission route of avian influenza A (H5N1) virus in domestic cats in Poland, June 2023. Euro Surveill. 2023, 28. [Google Scholar] [CrossRef]
- Sillman, S.J.; Drozd, M.; Loy, D.; Harris, S.P. Naturally occurring highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b infection in three domestic cats in North America during 2023. J. Comp. Pathol. 2023, 205, 17–23. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, P.; He, S.; Luo, Y.; Jia, K.; Fu, C.; Sun, Y.; He, H.; Tu, L.; Ning, Z.; et al. Sparse serological evidence of H5N1 avian influenza virus infections in domestic cats, northeastern China. Microb. Pathog. 2015, 82, 27–30. [Google Scholar] [CrossRef]
- Duijvestijn, M.; Schuurman, N.; Vernooij, J.C.M.; van Leeuwen, M.; van den Brand, J.M.A.; Wagenaar, J.A.; van Kuppeveld, F.J.M.; Egberink, H.F.; de Haan, C.A.M.; Verhagen, J.H. Highly pathogenic avian influenza (HPAI) H5 virus exposure in domestic cats and rural stray cats, the Netherlands, October 2020 to June 2023. Euro Surveill. 2024, 29. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Prince, A.; Fawzy, A.; Nadra, E.; Abdou, M.I.; Omar, L.; Fayed, A.; Salem, M. Sero-prevalence of avian influenza in animals and human in Egypt. Pak. J. Biol. Sci. 2013, 16, 524–529. [Google Scholar] [CrossRef]
- Zhao, F.R.; Zhou, D.H.; Zhang, Y.G.; Shao, J.J.; Lin, T.; Li, Y.F.; Wei, P.; Chang, H.Y. Detection prevalence of H5N1 avian influenza virus among stray cats in eastern China. J. Med. Virol. 2015, 87, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Domańska-Blicharz, K.; Świętoń, E.; Świątalska, A.; Monne, I.; Fusaro, A.; Tarasiuk, K.; Wyrostek, K.; Styś-Fijoł, N.; Giza, A.; Pietruk, M.; et al. Outbreak of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in cats, Poland, June to July 2023. Euro Surveill. 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Marschall, J.; Schulz, B.; Harder Priv-Doz, T.C.; Vahlenkamp Priv-Doz, T.W.; Huebner, J.; Huisinga, E.; Hartmann, K. Prevalence of influenza A H5N1 virus in cats from areas with occurrence of highly pathogenic avian influenza in birds. J. Feline Med. Surg. 2008, 10, 355–358. [Google Scholar] [CrossRef]
- Zhou, H.; He, S.Y.; Sun, L.; He, H.; Ji, F.; Sun, Y.; Jia, K.; Ning, Z.; Wang, H.; Yuan, L.; et al. Serological evidence of avian influenza virus and canine influenza virus infections among stray cats in live poultry markets, China. Vet. Microbiol. 2015, 175, 369–373. [Google Scholar] [CrossRef]
- Mahardika, G.N.; Adi, A.A.A.M.; Besung, N.K.; Dharmawan, N.S.; Kencana, G.A.Y.; Rompis, A.L.T.; Sampurna, P.; Setiasih, L.E.; Suardana, W.; Suardana, I.B.K.; et al. Surveillance of avian influenza virus of H5N1 subtype in backyard animals and its introduction in Bali, Indonesia. Pak. Vet. J. 2018, 38, 7–12. [Google Scholar] [CrossRef]
- Annand, E.J.; High, H.; Wong, F.Y.K.; Phommachanh, P.; Chanthavisouk, C.; Happold, J.; Dhingra, M.S.; Eagles, D.; Britton, P.N.; Alders, R.G. Detection of highly pathogenic avian influenza in Sekong Province Lao PDR 2018-Potential for improved surveillance and management in endemic regions. Transbound. Emerg. Dis. 2021, 68, 168–182. [Google Scholar] [CrossRef]
- Houston, D.D.; Azeem, S.; Lundy, C.W.; Sato, Y.; Guo, B.; Blanchong, J.A.; Gauger, P.C.; Marks, D.R.; Yoon, K.J.; Adelman, J.S. Evaluating the role of wild songbirds or rodents in spreading avian influenza virus across an agricultural landscape. PeerJ 2017, 5, e4060. [Google Scholar] [CrossRef]
- Wasik, B.R.; Voorhees, I.E.H.; Parrish, C.R. Canine and Feline Influenza. Cold Spring Harb. Perspect. Med. 2021, 11, a038562. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Alm, E.; Enkirch, T.; Lamb, F.; Melidou, A.; Willgert, K.; Marangon, S.; Monne, I.; Stegeman, J.A.; Delacourt, R.; et al. Drivers for a pandemic due to avian influenza and options for One Health mitigation measures. EFSA J. 2024, 22, e8735. [Google Scholar] [CrossRef]
- Sun, X.; Belser, J.A.; Maines, T.R. Adaptation of H9N2 Influenza Viruses to Mammalian Hosts: A Review of Molecular Markers. Viruses 2020, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, G.; Wang, C.; Jiang, M.; Gao, W.; Wang, M.; Sun, H.; Sun, Y.; Chang, K.C.; Liu, J.; et al. Enhanced pathogenicity and neurotropism of mouse-adapted H10N7 influenza virus are mediated by novel PB2 and NA mutations. J. Gen. Virol. 2017, 98, 1185–1195. [Google Scholar] [CrossRef]
- Cavicchio, L.; Campalto, M.; Carrino, M.; Lucchese, L.; Ceglie, L.; Fincato, A.; Boscolo Cegion, L.; Mazzotta, E.; Beato, M.S.; Natale, A. Influenza in feral cat populations: Insights from a study in North-East Italy. Front. Vet. Sci. 2024, 11, 1439354. [Google Scholar] [CrossRef]
- Moreno, A.; Bonfante, F.; Bortolami, A.; Cassaniti, I.; Caruana, A.; Cottini, V.; Cereda, D.; Farioli, M.; Fusaro, A.; Lavazza, A.; et al. Asymptomatic infection with clade 2.3.4.4b highly pathogenic avian influenza A(H5N1) in carnivore pets, Italy, April 2023. Euro Surveill. 2023, 28. [Google Scholar] [CrossRef]
- Anis, A.; AboElkhair, M.; Ibrahim, M. Characterization of highly pathogenic avian influenza H5N8 virus from Egyptian domestic waterfowl in 2017. Avian Pathol. 2018, 47, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Chestakova, I.V.; van der Linden, A.; Bellido Martin, B.; Caliendo, V.; Vuong, O.; Thewessen, S.; Hartung, T.; Bestebroer, T.; Dekker, J.; Jonge Poerink, B.; et al. High number of HPAI H5 virus infections and antibodies in wild carnivores in the Netherlands, 2020–2022. Emerg. Microbes Infect. 2023, 12, 2270068. [Google Scholar] [CrossRef]
- Kovalenko, G.; Galat, M.; Ishchenko, L.; Halka, I. Serological Evidence for Influenza A Viruses Among Domestic Dogs and Cats in Kyiv, Ukraine. Vector Borne Zoonotic Dis. 2021, 21, 483–489. [Google Scholar] [CrossRef]
| Study | Publication Year | Study Years | Country | Study Type | N | Population | Test Employed | Reference |
|---|---|---|---|---|---|---|---|---|
| Prevalence of influenza A H5N1 virus in cats from areas with the occurrence of highly pathogenic avian influenza in birds | 2008 | 2006–2007 | Germany | Prevalence | 171 | Cats living near H5N1-positive poultry farms | RT-PCR, HI assay | [56] |
| Sero-prevalence of Avian Influenza in Animals and Humans in Egypt | 2013 | Egypt | Prevalence | 25 | Stray cats | HI assay | [53] | |
| Detection Prevalence of H5N1 Avian Influenza Virus Among Stray Cats in Eastern China * | 2015 | 2010–2011 | China | Prevalence | 1020 | Stray cats | RT-PCR, HI assay, and NT assay | [54] |
| Serological evidence of avian influenza virus and canine influenza virus infections among stray cats in live poultry markets, China | 2015 | China | Seroprevalence | 700 | Stray cats | HI assay and NT assay | [57] | |
| Sparse serological evidence of H5N1 avian influenza virus infections in domestic cats, northeastern China | 2015 | 2013 | China | Seroprevalence | 916 | Domestic cats | HI assay | [51] |
| Surveillance of Avian Influenza Virus of H5N1 Subtype in Backyard Animals and Its Introduction in Bali, Indonesia | 2018 | 2005–2006 | Indonesia | Prevalence | 40 | Domestic cats | RT-PCR | [58] |
| Outbreak of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in cats, Poland, June to July 2023 ** | 2023 | 2023 | Poland | Prevalence | 46 | Domestic cats | RT-PCR, Genome sequencing, and phylogeny | [55] |
| Influenza in feral cat populations: insights from a study in North-East Italy | 2024 | 2021–2022 | Italy | Prevalence | 668 | Shelter cats | RT-PCR, HI assay | [65] |
| Meta-Analysis | Number of Studies | N | Pool Prevalence (%) | 95% Confidence Interval | I2 ‡ | p |
|---|---|---|---|---|---|---|
| All studies | 8 | 3586 | 0.7 | 0.3–1.1% | 86.48% | <0.001 |
| By RT-PCR | 5 | 1666 | 0.8 | 0.0–2.2% | 93.19% | <0.001 |
| By hemagglutination inhibition assay | 6 | 3058 | 1.5 | 0.5–2.4% | 83.89% | <0.001 |
| By neutralisation assay | 2 | 1720 | 0.3 | 0.0–0.5% | 0.0% | 0.412 |
| Domestic cats | 3 | 1002 | 20.0 | 0.0–41.2% | 96.09% | <0.001 |
| Stray cats | 3 | 1745 | 2.3 | 0.0–5.4% | 92.73% | <0.001 |
| Shelter cats | 1 | 668 | 0.3 | 0.0–0.7% | n/a | n/a |
| Farm cats | 1 | 171 | 0.3 | 0.0–1.1% | n/a | n/a |
| Africa | 1 | 25 | 32.0 | 13.7–50.3% | n/a | n/a |
| Europe | 3 | 885 | 2.7 | 0.0–6.0% | 96.31% | <0.001 |
| Asia | 4 | 2676 | 2.0 | 0.1–3.8% | 91.35% | <0.001 |
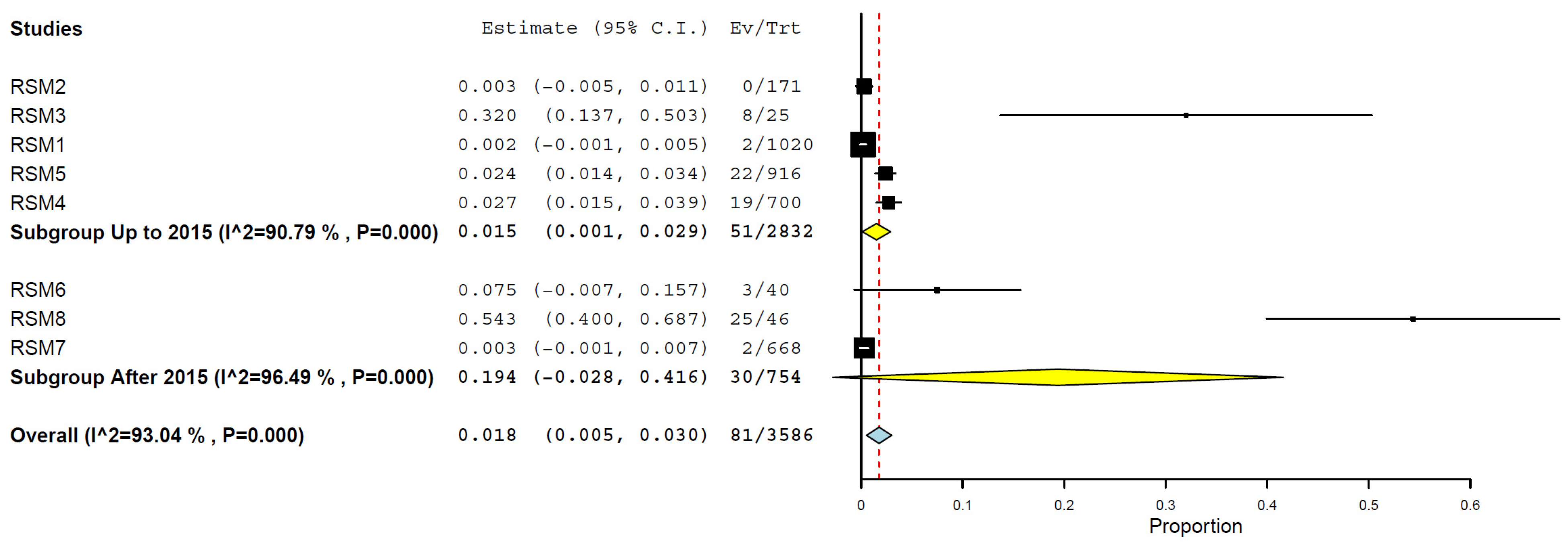
| 2016–2024 | 3 | 754 | 19.4 | 0.0–41.6% | 96.49% | <0.001 |
| 2008–2015 | 5 | 2832 | 1.5 | 0.1–2.9% | 90.79% | <0.001 |
| Cat No. | Year | Country | Type of Cat | Age, Years | Sex | Outcome | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 2006 | Thailand | Domestic | 2 | Male | Dead | The cat had eaten a pigeon 5 days before its death. | [37] |
| 2 | 2007 | Germany | Shelter | N/R | Male | Dead | They were found dead on the island of Rügen, approximately 1.6 km from sites where wild birds infected with highly pathogenic avian influenza virus (HPAIV H5N1), mainly swans and whooper swans as well as several species of geese and ducks, had been continuously detected during the previous three weeks. Note: They had access to the outdoors. Cat 2: found approximately 4 miles away from Cat 1. Cat 3: found 0.5 miles from the site where Cat 1 had been detected. | [47] |
| 3 | N/R | Male | Dead | |||||
| 4 | N/R | Male | Dead | |||||
| 5 | 2023 | Poland | Domestic | 6 | Male | Dead | Access to the outside, the diet included raw chicken meat, and the animal was vaccinated. | [27] |
| 6 | 2023 | Italy | Domestic | N/R | N/R | Unknown | Outbreak in birds within the same farm. | [66] |
| 7 | 2023 | France | Shelter | N/R | N/R | Unknown | A highly pathogenic H5N1 virus infected a domestic cat near a French duck farm, showing mammalian adaptation mutations and highlighting public health concerns. Diagnosed with RT-PCR, sequencing clade 2.3.4.4b. | [28] |
| 8 | 2023 | USA | Shelter | N/R | Male | Dead | He was brought to a clinic in northeast Nebraska because he was unable to walk and was anorexic. He had been kept outdoors and was missing for several days before being found in an abnormal condition. Three other free-roaming outdoor cats were housed at the facility. Diagnosed with RT-PCR, sequencing clade 2.3.4.4b. | [50] |
| 9 | 0.5 | Female | Dead | A cat from the same residence presented clinical signs 24 h after case 1. It was diagnosed with RT-PCR, sequencing clade 2.3.4.4b. | ||||
| 10 | 0.7 | Female | Dead | Difficulty walking, lethargy, and anorexia. This animal lived outdoors and was one of nine cats on the premises. Diagnosed with RT-PCR, sequencing clade 2.3.4.4b. | ||||
| 11 | 2023 | Poland | Domestic | N/R | N/R | Survived | A fatal H5N1 outbreak in Polish cats revealed viral mammalian adaptations, likely transmitted via contaminated poultry meat. Most infected cats died, raising public health and zoonotic transmission concerns. | [49] |
| 12 | 2023 | Poland | Domestic | N/R | N/R | Dead | ||
| 13 | 2023 | Poland | Domestic | N/R | N/R | Survived | ||
| 14 | 2023 | Poland | Domestic | N/R | N/R | Dead | ||
| 15 | 2024 | Republic of Korea | Shelter | N/R | N/R | Dead | Respiratory and neurological symptoms. | [46] |
| 16 | 2024 | Republic of Korea | Shelter | N/R | N/R | Dead | In July 2023, H5N1 outbreaks occurred in two cat shelters in Seoul, resulting in high mortality rates linked to contaminated raw duck meat. At least 39 cats died; others were infected or seropositive. The virus exhibited mammalian adaptations, underscoring concerns about zoonotic transmission and food safety. This case series analysed 7 of those confirmed H5N1 infection in cats. | [38] |
| 17 | 2024 | Republic of Korea | Shelter | N/R | N/R | Dead | ||
| 18 | 2024 | Republic of Korea | Shelter | N/R | N/R | Dead | ||
| 19 | 2024 | Republic of Korea | Shelter | N/R | N/R | Dead | ||
| 20 | 2024 | Republic of Korea | Shelter | N/R | N/R | Unknown | ||
| 21 | 2024 | Republic of Korea | Shelter | N/R | N/R | Unknown | ||
| 22 | 2024 | Republic of Korea | Shelter | N/R | N/R | Unknown | ||
| 23 | 2024 | Republic of Korea | Shelter | N/R | N/R | Dead | ||
| 24 | 2024 | USA | Shelter | Adults | Female | Dead | The cats were found dead with no apparent signs of injury and belonged to a resident population of approximately 24 domestic cats that had been fed milk from diseased cows. RT-PCR diagnosed both, sequencing clade 2.3.4.4b. | [24] |
| 25 | 2024 | USA | Shelter | Adults | Male | Dead | ||
| 26 | 2025 | USA | Domestic | 5 | Female | Dead | Household 1: Lives exclusively inside the house. The owner worked with cattle. Diagnosed by RT-PCR, sequencing clade 2.3.4.4b, genotype B3.13. | [29] |
| 27 | 2025 | USA | Domestic | 0.5 | Male | Unknown | Household 2: Six days after the cat case, the cat was raised indoors. Diagnosed by RT-PCR, sequencing clade 2.3.4.4b, genotype B3.13. | |
| 28 | 2025 | USA | Stray | 1.5 | N/R | Dead | H5N1 clade 2.3.4.4b caused fatal neurotropic infections in cats, with high brain viral loads, unique mutations, and potential adaptation suggesting zoonotic and reassortment risks. RT-PCR diagnosed both, sequencing clade 2.3.4.4b. | [34] |
| 29 | 2025 | USA | Stray | 0.5 | N/R | Dead | ||
| 30 | 2025 | USA | Domestic | 0.5–2 | N/R | Dead | They were fed mice, rats, and birds and offered raw and pasteurised colostrum and milk from diseased cows, which was diverted from bulk commercial milk. RT-PCR diagnosed them, sequencing for H5 and H5 2.3.4.4b in the three cats. | [48] |
| 31 | 2025 | USA | Domestic | 0.5–2 | N/R | Dead | ||
| 32 | 2025 | USA | Domestic | 0.5–2 | Female | Dead | ||
| 33 | 2025 | USA | Domestic | 0.5–2 | N/R | Dead | ||
| 34 | 2025 | USA | Domestic | 5 | N/R | Unknown | Indoor cats reportedly had no contact with people outside the dairy farm where the outbreak occurred. RT-PCR diagnosed them, sequencing for H5 and H5 2.3.4.4b in the three cats. | |
| 35 | 2025 | USA | Domestic | 13 | N/R | Dead |
| Systems | Manifestations | n | % |
|---|---|---|---|
| Asymptomatic | 1 | 3 | |
| Symptomatic | 34 | 97 | |
| Neurological | Lethargy | 10 | 29 |
| Ataxia | 8 | 23 | |
| Progressive neurological deterioration | 8 | 23 | |
| Depressed | 6 | 17 | |
| Inability to get up | 4 | 11 | |
| Reduction in the threat reflex | 4 | 11 | |
| Blindness | 4 | 11 | |
| Cranial nerve abnormality | 2 | 6 | |
| Anisocoria | 2 | 6 | |
| Hypersensitivity | 2 | 6 | |
| Hiding behaviour | 1 | 3 | |
| Sleepiness | 1 | 3 | |
| Seizures | 1 | 3 | |
| Disorientation | 1 | 3 | |
| Nystagmus | 1 | 3 | |
| Tremors | 1 | 3 | |
| Systemic | Fever | 7 | 20 |
| Weakness | 4 | 11 | |
| Dehydration | 3 | 9 | |
| Panting | 1 | 3 | |
| Tachycardia | 1 | 3 | |
| Dyspnoea | 1 | 3 | |
| Hypothermia | 1 | 3 | |
| Shivering | 1 | 3 | |
| Sialorrhea | 1 | 3 | |
| Ocular | Eye and nose discharge | 6 | 17 |
| Inflammation of the eyes and nose | 1 | 3 | |
| Runny nose | 1 | 3 | |
| Purulent-watery ocular discharge | 1 | 3 | |
| Conjunctival injection | 1 | 3 | |
| Enteric | Loss of appetite | 5 | 14 |
| Anorexia | 3 | 9 | |
| Reduced appetite | 2 | 6 | |
| Respiratory | Tachypnea | 5 | 14 |
| Miscellaneous | Jaw inflammation | 1 | 3 |
| Organ | Finding | n | % |
|---|---|---|---|
| Lung | Interstitial pneumonia | 11 | 61 |
| Pulmonary oedema | 5 | 28 | |
| Lung congestion | 2 | 11 | |
| Bronchitis | 2 | 11 | |
| Bronchiolitis | 2 | 11 | |
| Lung necrosis | 2 | 11 | |
| Pulmonary hyperemia | 2 | 11 | |
| Lung perivasculitis | 2 | 11 | |
| Lung interstitial haemorrhage | 1 | 6 | |
| Liver | Liver necrosis foci | 5 | 28 |
| Liver inflammatory infiltrates | 3 | 17 | |
| Neurological | Multifocal encephalitis | 5 | 28 |
| Neuronal necrosis | 3 | 17 | |
| Necrotising meningoencephalitis | 2 | 11 | |
| Meningitis | 2 | 11 | |
| Brain perivascular infiltrate | 1 | 6 | |
| Brain congestion | 1 | 6 | |
| Heart | Heart vasculitis | 2 | 11 |
| Necrotising myocarditis | 2 | 11 | |
| Multisystemic | Necrosis foci | 1 | 6 |
| Multifocal necrosis | 1 | 6 | |
| Spleen | Splenomegaly | 1 | 6 |
| Spleen Congestion | 1 | 6 | |
| Kidney | Kidney congestion | 1 | 6 |
| Necrotic lesions and inflammation of the mesenteric plexus | 1 | 6 | |
| Intestine/Mesenteric | Intestinal serous haemorrhage | 1 | 6 |
| Chorioretinitis | 1 | 6 | |
| Eye | Conjunctivitis | 1 | 6 |
| Cat No. | Detail Description |
|---|---|
| 1 | Nonsuppurative encephalitis, gliosis, mononuclear infiltration in the Virchow–Robin space, vasculitis, and cerebrum congestion. A microscopic lesion in the lung was caused by severe pulmonary oedema, interstitial pneumonia, and congestion. Multifocal necrosis was found in the liver, and tubulonephritis and lymphoid depletion were present in the spleen. Influenza H5N1 diagnosed by RT-PCR. |
| 2 | At necropsy, the cat exhibited pulmonary hyperemia with multiple consolidated areas, predominantly peribronchiolar, characterised by a dark red discolouration and irregular shape. The spleen was markedly enlarged and congested. The liver was dark tan, firm, and regular in appearance. In the small intestine, numerous adult roundworms and some cestodes were found in an otherwise empty gastrointestinal tract. Influenza H5N1 diagnosed by RT-PCR. |
| 3 | The cat’s lungs were diffusely dark red with multiple coalescing yellow consolidated nodules. On cross-section, they primarily revealed dark red, irregularly shaped, peribronchiolar areas of consolidation. The bronchi and bronchioles contained abundant, tenacious yellow mucus. The pulmonary lymph nodes were moderately enlarged and moist on the cross-section. The liver contained several well-circumscribed, randomly arranged, grey to white areas measuring millimetres or up to 3 mm in diameter (necrosis). Numerous adult roundworms and some cestodes were present in the small intestine. The heart showed moderate dilation of the right ventricle. Influenza H5N1 diagnosed by RT-PCR. |
| 4 | The cat exhibited extensive postmortem loss of skin, subcutaneous tissue, and portions of skeletal muscles in the cervical and shoulder regions, likely due to scavenging. A few well-circumscribed, light brown to yellow areas, up to 2 mm in diameter, were observed in the liver, indicative of necrosis. The retropharyngeal and pulmonary lymph nodes were moderately swollen, with some ecchymosis. The lungs were severely oedematous with numerous, well-circumscribed yellow nodules. A hemohydrothorax of approximately 20 mL was observed. The mucosa of the nasal cavity, pharynx, and trachea was diffusely hyperemic. Diffuse haemorrhages were found retroperitoneally and intramuscularly in the diaphragm, within the perinephric tissue, and in the pancreas. Influenza H5N1 diagnosed by RT-PCR. |
| 5, 6, and 7 | Liver areas of necrosis with an inflammatory infiltrate composed of lymphocytes and histiocytes. Lungs, atelectasis, hyperemia, interstitial and alveolar haemorrhages. Macrophages within the alveolar lumen. Brain, perivascular infiltrates of lymphocytes and histiocytes in the white and grey matter. Perivascular infiltrate of lymphocytes and some histiocytes at the base of the choroid plexus. Bowel wall, vacuolisation and necrosis of cells in the myenteric plexus, accompanied by an inflammatory infiltrate of lymphocytes and histiocytes. Influenza H5N1 was diagnosed in cats 5 and 7 by RT-PCR. |
| 8 | Lung Interstitial pneumonia is characterised by acute vascular congestion, alveolar oedema, and fibrin exudation mixed with sparse neutrophils and macrophages into the alveolar septa and lumen. Thrombosis at the liver, multifocal random foci of acute necrosis of variable sizes (arrowheads). The necrotic areas contain fibrin, karyrhectic debris, erythrocytes, and sparse neutrophils and/or macrophages. Adrenal cortex, large foci of random acute necrosis (arrowheads). Pancreas: Severe, multifocal to coalescent necrosis manifested by extensive loss of pancreatic exocrine cells and collapse of lobules (arrowheads). An admixture of inflammatory leukocytes and karyrhectic debris is seen in the remaining stroma. The region of normal pancreatic exocrine cells is indicated for comparison with a pancreas with endothelial necrosis, fibrinoid change, and thrombosis. Influenza H5N1 diagnosed by RT-PCR. |
| 9 | The lung presented with interstitial pneumonia similar to case 1, characterised by adding more macrophages populating the alveolar septa, less evident vascular thrombosis, and some foci of mild type II pneumocyte hyperplasia. The myocardial interstitium contained some random groups of lymphocytes. The cerebral cortex presented areas of wedge-shaped lamellar malacia with variable rarefaction, cavitation, and haemorrhage. Influenza H5N1 diagnosed by RT-PCR. |
| 10 | Histologically, the brain lesions were similar to those in cases 1 and 2, with wedge-shaped areas of inflammation most frequently located in the cerebral cortex. However, the areas of glial and mononuclear cell inflammation, with mixed lymphocytes, plasma cells, and macrophages, were more extensive and numerous than those seen in previous cases, often presenting with an increased abundance of cellular debris, degenerated neurons, glial cells, and neuronal satellitosis. Influenza H5N1 diagnosed by RT-PCR. |
| 24 and 25 | Minor haemorrhages in the subcutaneous tissue over the dorsal region of the skull and multifocal meningeal haemorrhages in the brain of both cats. Microscopically, severe subacute multifocal necrotising and lymphocytic meningoencephalitis with vasculitis and neuronal necrosis, moderate subacute multifocal necrotising and lymphocytic interstitial pneumonia, moderate to severe subacute multifocal necrotising and lymphohistiocytic myocarditis, and moderate subacute multifocal lymphoplasmacytic chorioretinitis with ganglion cell necrosis and attenuation of the inner plexiform and nuclear layers. Influenza H5N1 diagnosed by RT-PCR. |
| 28 and 29 | The lung showed interstitial pneumonia, bronchiolitis, and bronchitis. The brain showed meningitis and encephalitis, while the hippocampus showed no lesions. Immunohistochemical (IHC) analysis (IL) revealed the presence of avian influenza virus (IAV) nucleoprotein in each of these organs. The brain tissue exhibited a higher level of nucleoprotein staining than the lung tissue, with a notable presence of nucleoprotein in the cerebellum and hippocampus. Influenza H5N1 diagnosed by RT-PCR. |
| 30 | Lesions in the brainstem, cerebrum, lung, and heart (subcutaneous and mild meningeal haemorrhage, mononuclear and necrotising encephalitis, and interstitial pneumonia). Influenza H5N1 diagnosed by RT-PCR. |
| 31 | Lesions in the brainstem, cerebrum, lung, heart, and eye (retina, choroid), mononuclear and necrotising encephalitis, interstitial pneumonia, and chorioretinitis. Influenza H5N1 diagnosed by RT-PCR. |
| 32 | Lesions in the brainstem, cerebellum, cerebrum, spinal cord, eye (retina, choroid), lung, tonsil, submandibular salivary gland, minor salivary glands (tongue), heart, liver, adrenal gland, and thyroid gland (mononuclear and necrotising encephalitis, interstitial pneumonia, chorioretinitis). Influenza H5N1 diagnosed by RT-PCR. |
| 33 | Lesions in the brain, eyelid conjunctiva, nasal turbinates, cribriform plate, lung, submandibular salivary gland, minor salivary glands in the pharynx, and parotid gland (mononuclear and necrotizing encephalitis, interstitial pneumonia). Influenza H5N1 diagnosed by RT-PCR. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla-Aldana, D.K.; Bonilla-Aldana, J.L.; Acosta-España, J.D.; Rodriguez-Morales, A.J. Highly Pathogenic Avian Influenza H5N1 in Cats (Felis catus): A Systematic Review and Meta-Analysis. Animals 2025, 15, 1441. https://doi.org/10.3390/ani15101441
Bonilla-Aldana DK, Bonilla-Aldana JL, Acosta-España JD, Rodriguez-Morales AJ. Highly Pathogenic Avian Influenza H5N1 in Cats (Felis catus): A Systematic Review and Meta-Analysis. Animals. 2025; 15(10):1441. https://doi.org/10.3390/ani15101441
Chicago/Turabian StyleBonilla-Aldana, D. Katterine, Jorge Luis Bonilla-Aldana, Jaime David Acosta-España, and Alfonso J. Rodriguez-Morales. 2025. "Highly Pathogenic Avian Influenza H5N1 in Cats (Felis catus): A Systematic Review and Meta-Analysis" Animals 15, no. 10: 1441. https://doi.org/10.3390/ani15101441
APA StyleBonilla-Aldana, D. K., Bonilla-Aldana, J. L., Acosta-España, J. D., & Rodriguez-Morales, A. J. (2025). Highly Pathogenic Avian Influenza H5N1 in Cats (Felis catus): A Systematic Review and Meta-Analysis. Animals, 15(10), 1441. https://doi.org/10.3390/ani15101441







