Development of a Monoclonal Antibody Against Duck IFN-γ Protein and the Application for Intracellular Cytokine Staining
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Plasmids and Cells
2.3. Expression and Purification of the Recombinant IFN-γ Proteins
2.4. Preparation and Purification of the Monoclonal Antibody
2.5. Western Blot Analysis
2.6. Antibody Subclass Detection
2.7. Intracellular Cytokine Staining
2.8. Statistical Analysis
3. Results
3.1. Expression and Purification of Recombinant Duck IFN-γ Protein
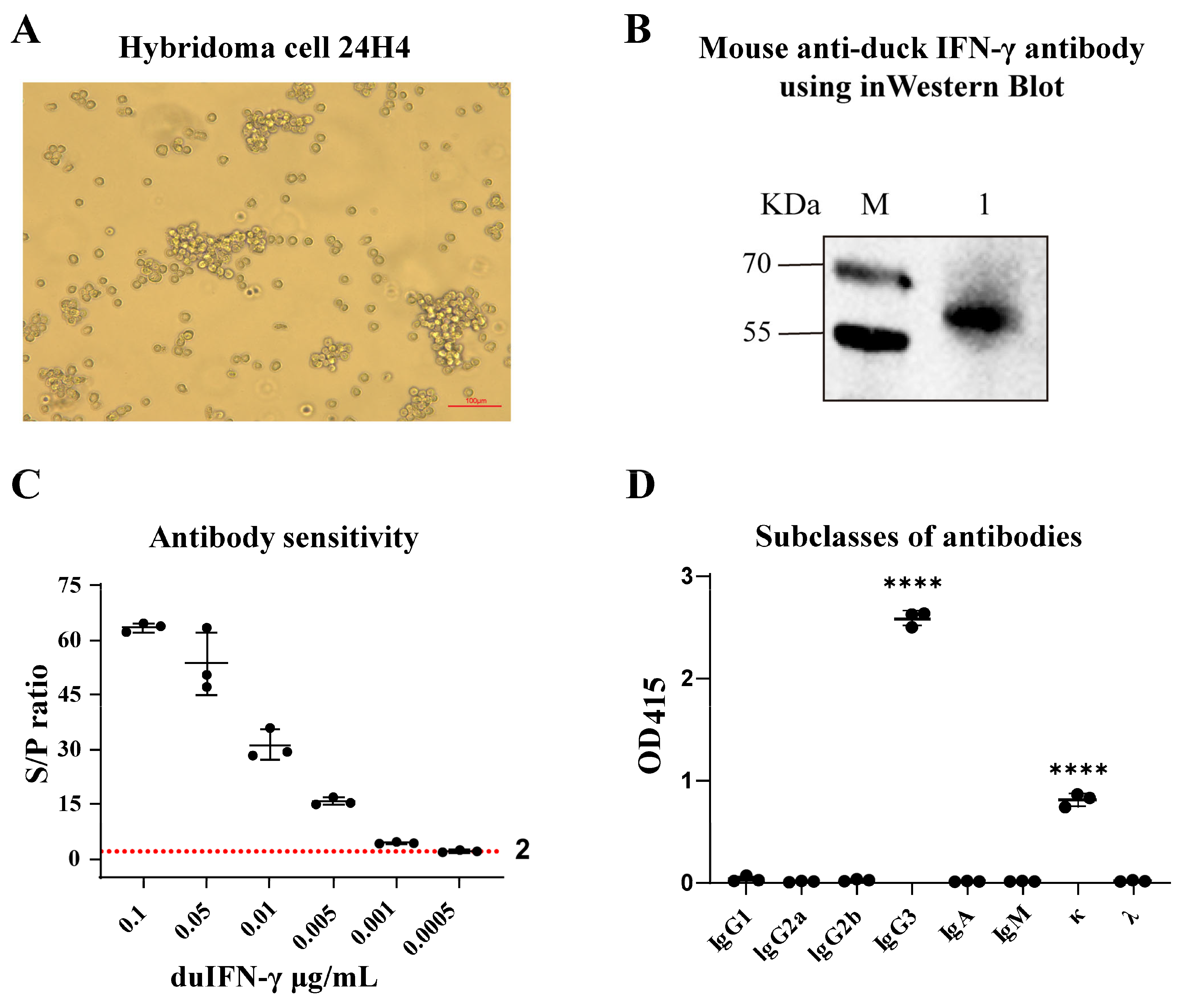
3.2. Preparation of the mAb Against Duck IFN-γ
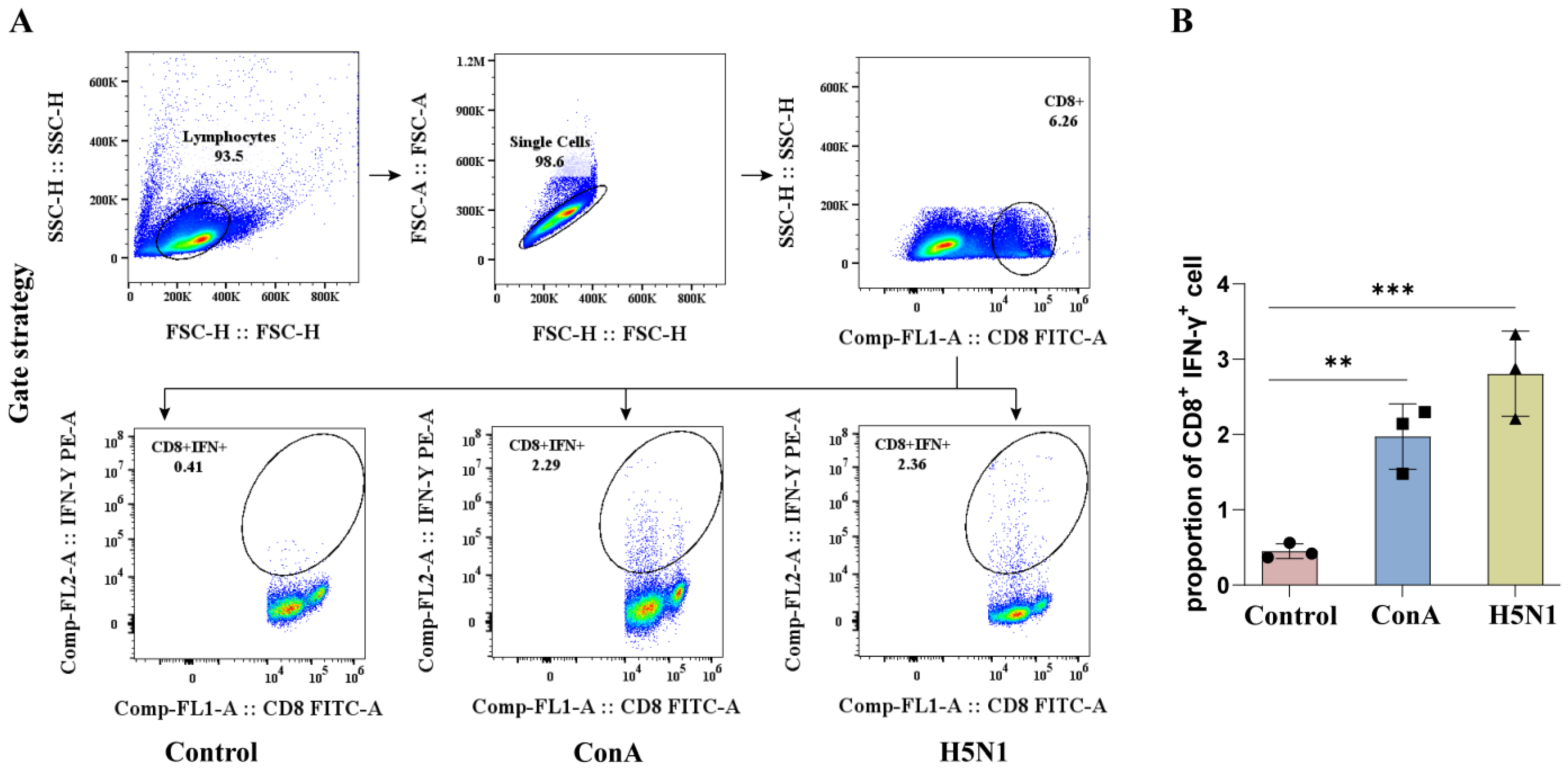
3.3. Establishment an ICS Assay Protocol to Detect IFN-γ Expression in CD8+ T Cells from Duck PBMCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masuda, Y.; Matsuda, A.; Usui, T.; Sugai, T.; Asano, A.; Yamano, Y. Biological effects of chicken type III interferon on expression of interferon-stimulated genes in chickens: Comparison with type I and type II interferons. J. Vet. Med. Sci. 2012, 74, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, G.; Yu, Z.; Sun, H.; Wang, L. Role of interferon-gamma (IFN-gamma) and IFN-gamma receptor 1/2 (IFNgammaR1/2) in regulation of immunity, infection, and cancer development: IFN-gamma-dependent or independent path-way. Biomed. Pharmacother. 2022, 155, 113683. [Google Scholar] [CrossRef]
- Zhu, B.; Zhu, J.; Liu, A.; Yao, B.; Liao, F.; Yang, S. Transcriptomic and metabolomic analysis based on different aggres-sive pecking phenotypes in ducks. Sci. Rep. 2024, 14, 22321. [Google Scholar]
- Dhama, K.; Kumar, N.; Saminathan, M.; Tiwari, R.; Karthik, K.; Kumar, M.A.; Palanivelu, M.; Shabbir, M.Z.; Malik, Y.S.; Singh, R.K. Duck virus enteritis (duck plague)—A comprehensive update. Vet. Q. 2017, 37, 57–80. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, S.; Yang, Z.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Capsular polysaccharide determines the serotyping of Riemerella anatipestifer. Microbiol. Spectr. 2023, 11, e180423. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Liu, W.; Hu, Z. Current status and future direction of duck hepatitis A virus vaccines. Avian Pathol. 2023, 52, 89–99. [Google Scholar] [CrossRef]
- El-Shall, N.A.; El Naby, W.S.A.; Hussein, E.; Yonis, A.E.; Sedeik, M.E. The pathogenicity of H5N8 avian influenza virus in chickens and in duck breeds and the role of MX1 and IFN-alpha in infection outcome and transmission to contact birds. Comp. Immunol. Microbiol. Infect. Dis. 2023, 100, 102039. [Google Scholar] [CrossRef]
- James, J.; Billington, E.; Warren, C.J.; De Sliva, D.; Di Genova, C.; Airey, M.; Meyer, S.M.; Lewis, T.; Peers-Dent, J.; Thomas, S.S.; et al. Clade 2.3.4.4b H5N1 high pathogenicity avian influenza virus (HPAIV) from the 2021/22 epizootic is highly duck adapted and poorly adapted to chickens. J. Gen. Virol. 2023, 104, 001852. [Google Scholar] [CrossRef]
- Dai, M.; Sun, H.; Zhao, L.; Wu, Q.; You, B.; Xu, F.; Liao, J.; Zhu, S.; Li, Z.; Yao, Y.; et al. Duck CD8(+) T Cell Response to H5N1 Highly Pathogenic Avian Influenza Virus Infection In Vivo and In Vitro. J. Immunol. 2022, 209, 979–990. [Google Scholar] [CrossRef]
- Tan, Y.; Raheem, M.A.; Rahim, M.A.; Xin, H.; Zhou, Y.; Hu, X.; Dai, Y.; Ataya, F.S.; Chen, F. Isolation, characterization, evaluation of pathogenicity, and immunomodulation through interferon production of duck adenovirus type-3 (DAdV-3). Poult. Sci. 2024, 103, 103411. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Tang, Z.; Han, L.; Wei, X.; Xie, X.; Ren, S.; Meng, K.; Liu, Y.; Xu, M.; et al. Efficient Identification of Tembusu Virus CTL Epitopes in Inbred HBW/B4 Ducks Using a Novel MHC Class I-Restricted Epitope Screening Scheme. J. Immunol. 2022, 209, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Hu, Y.; Liu, Z.; Zhong, W.; Cao, H.; Chen, H.; Jin, M. Efficient strategy for constructing duck enteritis virus-based live attenuated vaccine against homologous and heterologous H5N1 avian influenza virus and duck enteritis virus infection. Vet. Res. 2015, 46, 42. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, P.; Maecker, H.T. Multiparameter intracellular cytokine staining. Methods Mol. Biol. 2011, 699, 165–178. [Google Scholar] [PubMed]
- Reap, E.; Sônego, F.; O’Koren, E.; Gaëlle, M.; Avrutskaya, A.; Hauser, J.; Ball, R.; Bui, T.; Belle, I.; Hall, C.; et al. Abstract 5635: Enhanced anti-tumor lymphocyte function and frequencies measured by multichromatic flow cytometry in human CTLA-4 knock-in mice in a colorectal carcinoma model after treatment with ipilimumab. Cancer Res. 2020, 80 (Suppl. S16), 5635. [Google Scholar] [CrossRef]
- Gong, Z.; Li, Q.; Shi, J.; Ren, G. An Artifact in Intracellular Cytokine Staining for Studying T Cell Responses and Its Alleviation. Front. Immunol. 2022, 13, 759188. [Google Scholar] [CrossRef]
- Yu, E.D.; Wang, E.; Garrigan, E.; Sutherland, A.; Khalil, N.; Kearns, K.; Pham, J.; Schulten, V.; Peters, B.; Frazier, A.; et al. Ex vivo assays show human gamma-delta T cells specific for common allergens are Th1-polarized in allergic donors. Cell Rep. Methods 2022, 2, 100350. [Google Scholar] [CrossRef]
- Qin, Y.; Tu, K.; Teng, Q.; Feng, D.; Zhao, Y.; Zhang, G. Identification of Novel T-Cell Epitopes on Infectious Bronchitis Virus N Protein and Development of a Multi-epitope Vaccine. J. Virol. 2021, 95, e66721. [Google Scholar] [CrossRef]
- Dai, M.; Li, S.; Shi, K.; Liao, J.; Sun, H.; Liao, M. Systematic Identification of Host Immune Key Factors Influencing Viral Infection in PBL of ALV-J Infected SPF Chicken. Viruses 2020, 12, 114. [Google Scholar] [CrossRef]
- De Antonio, E.M.; Husmann, R.J.; Hansen, R.; Lunney, J.K.; Strom, D.; Martin, S.; Zuckermann, F.A. Quantitative detec-tion of porcine interferon-gamma in response to mitogen, superantigen and recall viral antigen. Vet. Immunol. Immunopathol. 1998, 61, 265–277. [Google Scholar] [CrossRef]
- Gutmann, S.; Zawatzky, R.; Müller, M. Characterisation and quantification of equine interferon gamma. Vet. Immunol. Immunopathol. 2005, 104, 105–115. [Google Scholar] [CrossRef]
- Sipka, A.; Babasyan, S.; Asbie, S.; Freer, H.; Wagner, B. Optimization of a bovine cytokine multiplex assay using a new bovine and cross-reactive equine monoclonal antibodies. Vet. Immunol. Immunopathol. 2024, 273, 110789. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.I.; Haq, K.; Brisbin, J.T.; Mian, M.F.; Kulkarni, R.R.; Sharif, S. Assessment of bioactivity of a recombinant chicken interferon-gamma expressed using a baculovirus expression system. J. Interf. Cytokine Res. 2011, 31, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Scougall, C.A.; Lowenthal, J.W.; Jilbert, A.R.; Kotlarski, I. Structural and functional homology between duck and chicken interferon-gamma. Dev. Comp. Immunol. 2001, 25, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Chuensirikulchai, K.; Pata, S.; Laopajon, W.; Takheaw, N.; Kotemul, K.; Jindaphun, K.; Khummuang, S.; Kasinrerk, W. Identification of different functions of CD8(+) T cell subpopulations by a novel monoclonal antibody. Immunology 2024, 173, 321–338. [Google Scholar] [CrossRef]
- Dai, M.; Xu, C.; Chen, W.; Liao, M. Progress on chicken T cell immunity to viruses. Cell. Mol. Life Sci. 2019, 76, 2779–2788. [Google Scholar] [CrossRef]
- Qi, S.; Shi, H.; Liu, L.; Zhou, L.; Zhang, Z. Dynamic visualization of the whole process of cytotoxic T lymphocytes killing B16 tumor cells in vitro. J. Biomed. Opt. 2019, 24, 051413. [Google Scholar] [CrossRef]
- Shafqat, A.; Omer, M.H.; Ahmad, O.; Niaz, M.; Abdulkader, H.S.; Shafqat, S.; Mushtaq, A.H.; Shaik, A.; Elshaer, A.N.; Kashir, J.; et al. SARS-CoV-2 epitopes inform future vaccination strategies. Front. Immunol. 2022, 13, 1041185. [Google Scholar] [CrossRef]
- Zhao, D.; Han, K.; Zhang, L.; Wang, H.; Tian, Y.; Huang, X.; Liu, Q.; Yang, J.; Liu, Y.; Li, Y. Identification and immuno-genic evaluation of T cell epitopes based on tembusu virus envelope protein in ducks. Virus Res. 2018, 257, 74–81. [Google Scholar] [CrossRef]
- Stewart, J.A.; Yang, H.; Hahn, E.H.; Tompkins, W.A. T cell-dependent and -independent stimulation of hamster spleen NK-like cells with concanavalin A. J. Leukoc. Biol. 1985, 37, 75–86. [Google Scholar] [CrossRef]
- Li, Z.L.; Fu, G.H.; Feng, Z.H.; Chen, J.H.; Shi, S.H.; Liu, R.C.; Cheng, L.F.; Chen, H.M.; Wang, C.H.; Yu, H. Evaluation of a novel inactivated vaccine against duck circovirus in muscovy ducks. Vet. Microbiol. 2020, 241, 108574. [Google Scholar]
- Zhao, Y.; Chen, P.; Hu, Y.; Liu, J.; Jiang, Y.; Zeng, X.; Deng, G.; Shi, J.; Li, Y.; Tian, G.; et al. Recombinant duck enteritis virus bearing the hemagglutinin genes of H5 and H7 influenza viruses is an ideal multivalent live vaccine in ducks. Emerg. Microbes Infect. 2024, 13, 2284301. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Song, W.; Chen, J.; Jin, C.; Lin, J.; Liao, M.; Dai, M. Development of a Monoclonal Antibody Against Duck IFN-γ Protein and the Application for Intracellular Cytokine Staining. Animals 2025, 15, 815. https://doi.org/10.3390/ani15060815
Chen Y, Song W, Chen J, Jin C, Lin J, Liao M, Dai M. Development of a Monoclonal Antibody Against Duck IFN-γ Protein and the Application for Intracellular Cytokine Staining. Animals. 2025; 15(6):815. https://doi.org/10.3390/ani15060815
Chicago/Turabian StyleChen, Yingyi, Wei Song, Junqiang Chen, Chenyang Jin, Jiewei Lin, Ming Liao, and Manman Dai. 2025. "Development of a Monoclonal Antibody Against Duck IFN-γ Protein and the Application for Intracellular Cytokine Staining" Animals 15, no. 6: 815. https://doi.org/10.3390/ani15060815
APA StyleChen, Y., Song, W., Chen, J., Jin, C., Lin, J., Liao, M., & Dai, M. (2025). Development of a Monoclonal Antibody Against Duck IFN-γ Protein and the Application for Intracellular Cytokine Staining. Animals, 15(6), 815. https://doi.org/10.3390/ani15060815





