Bacillus licheniformis Alleviates Clostridium perfringens-Induced Intestinal Injury in Mice Model by Modulating Inflammation, Apoptosis, and Cecal Microbial–Metabolic Responses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
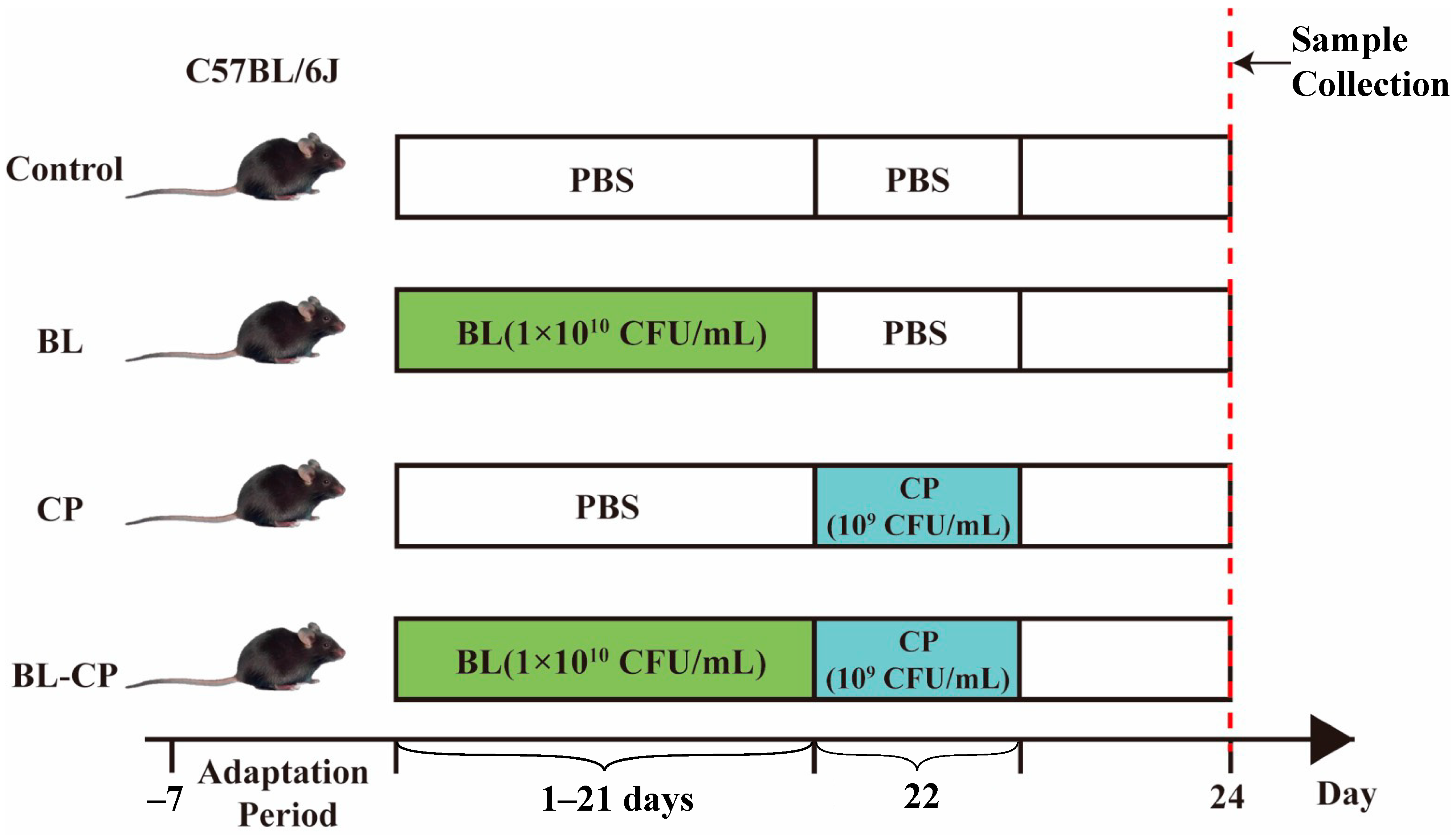
2.1. Animals and Experimental Design
2.2. Body Weight and Immune Organ Indices
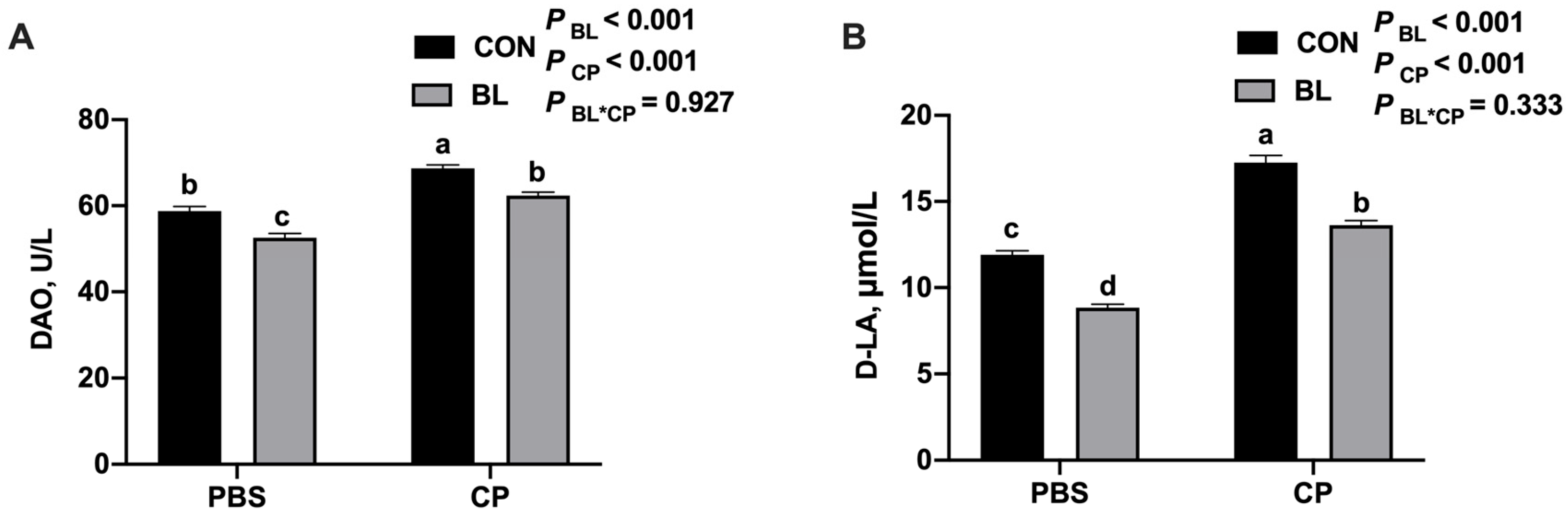
2.3. Serum Biochemical Analysis
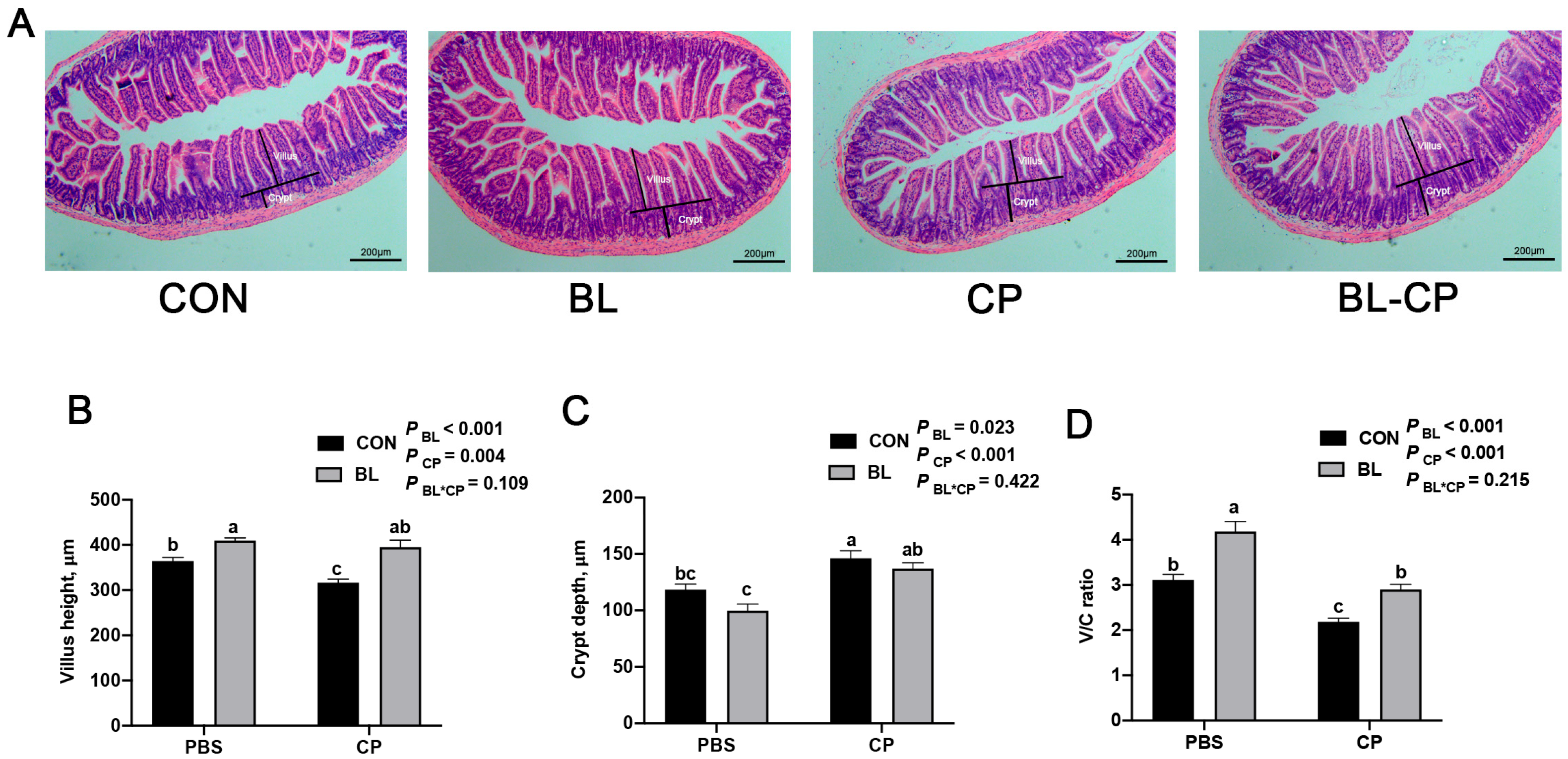
2.4. Ileum Morphology
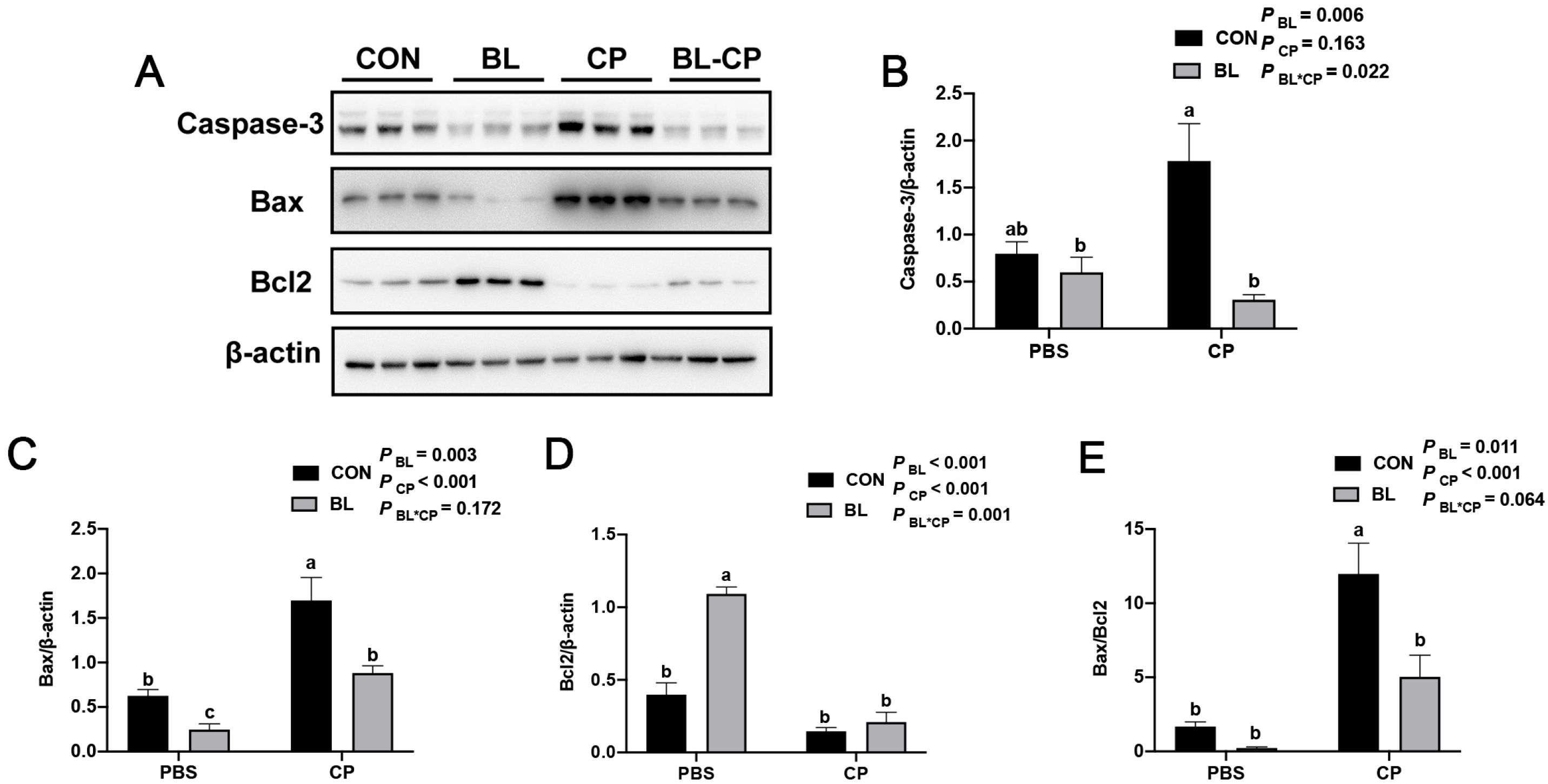
2.5. Western Blot Analysis
2.6. Volatile Fatty Acid (VFA) Analysis
2.7. Cecal Microbiota Analysis
2.8. Cecal Metabolome Analysis
2.9. Statistical Analysis
3. Results
3.1. B. licheniformis Alleviates Intestinal Injury Caused by C. perfringens Infection
3.2. B. licheniformis Reduces Systemic Inflammation Induced by C. perfringens
3.3. B. licheniformis Preserves Intestinal Morphology Disrupted by C. perfringens
3.4. B. licheniformis Improves Intestinal Barrier Function
3.5. B. licheniformis Inhibits Ileal Epithelial Apoptosis
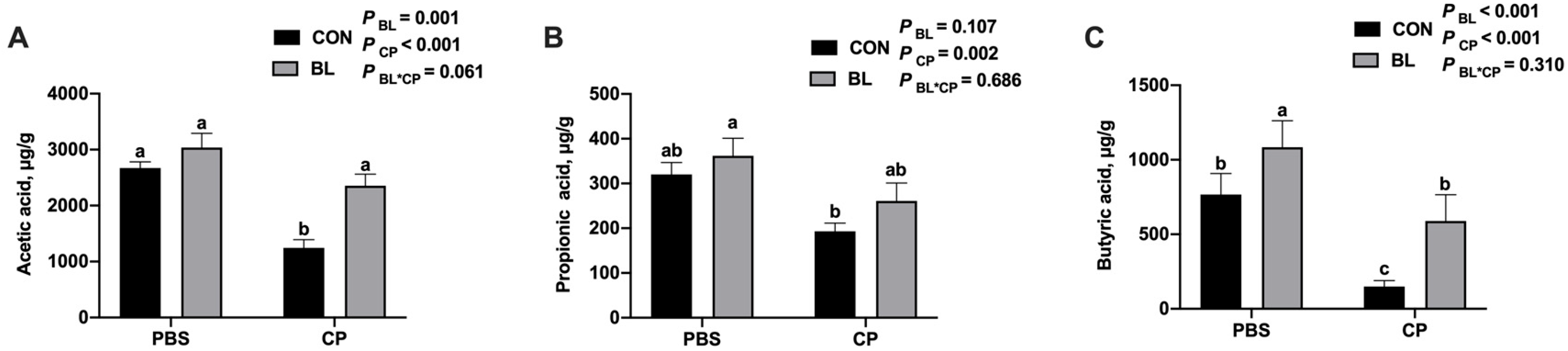
3.6. B. licheniformis Modulates Cecal Short-Chain Fatty Acid Production
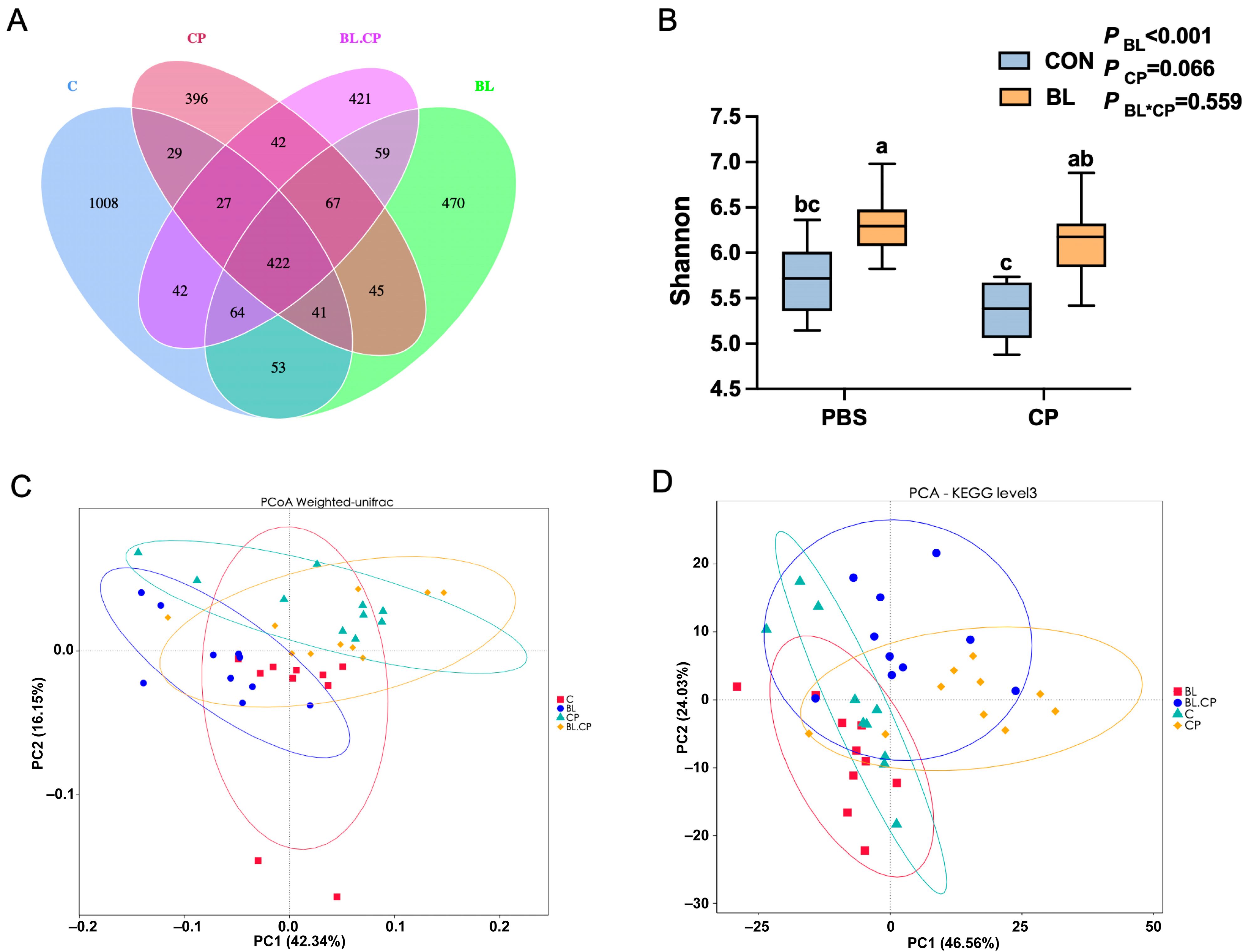
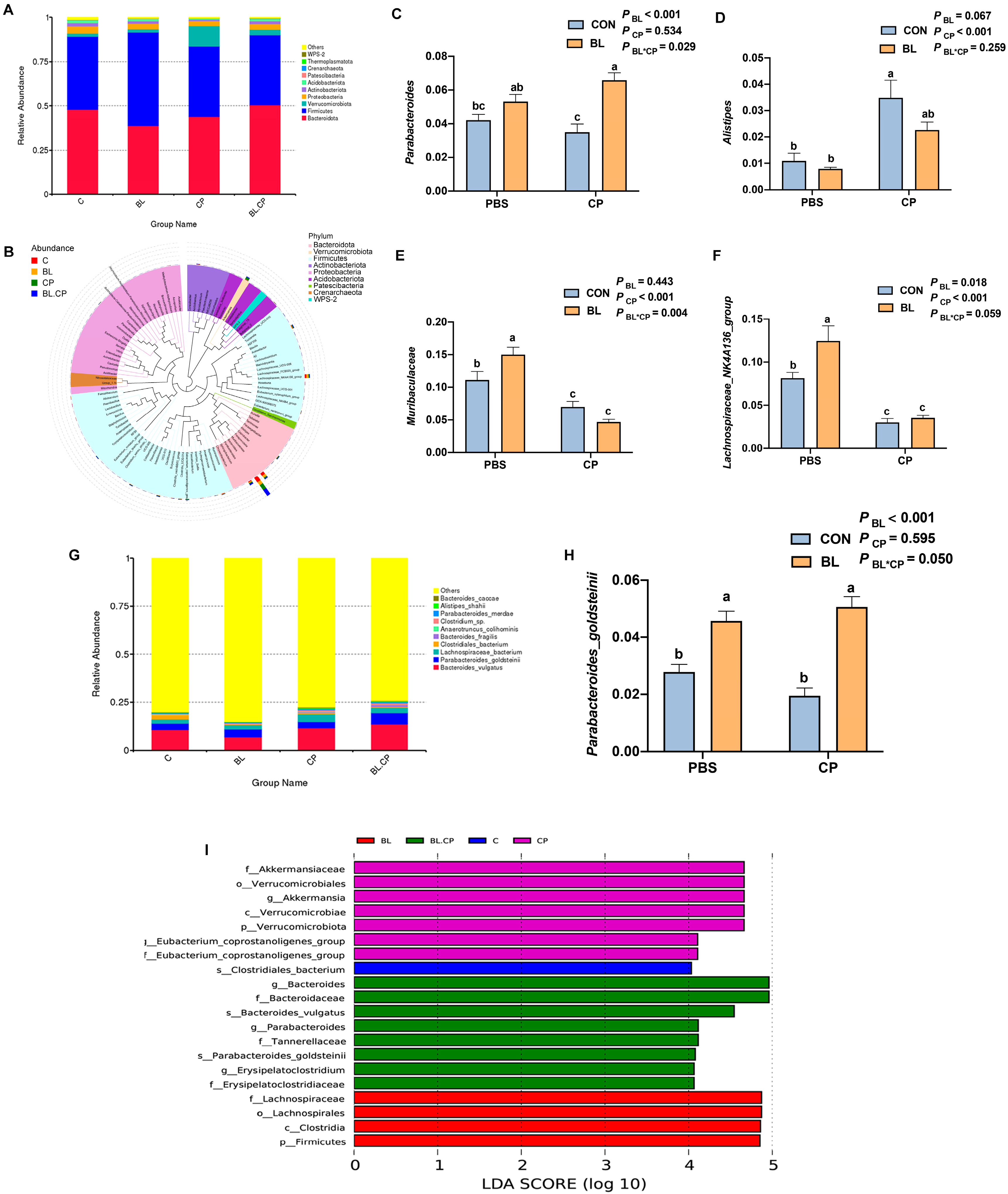
3.7. B. licheniformis Reshapes Gut Microbial Composition and Diversity
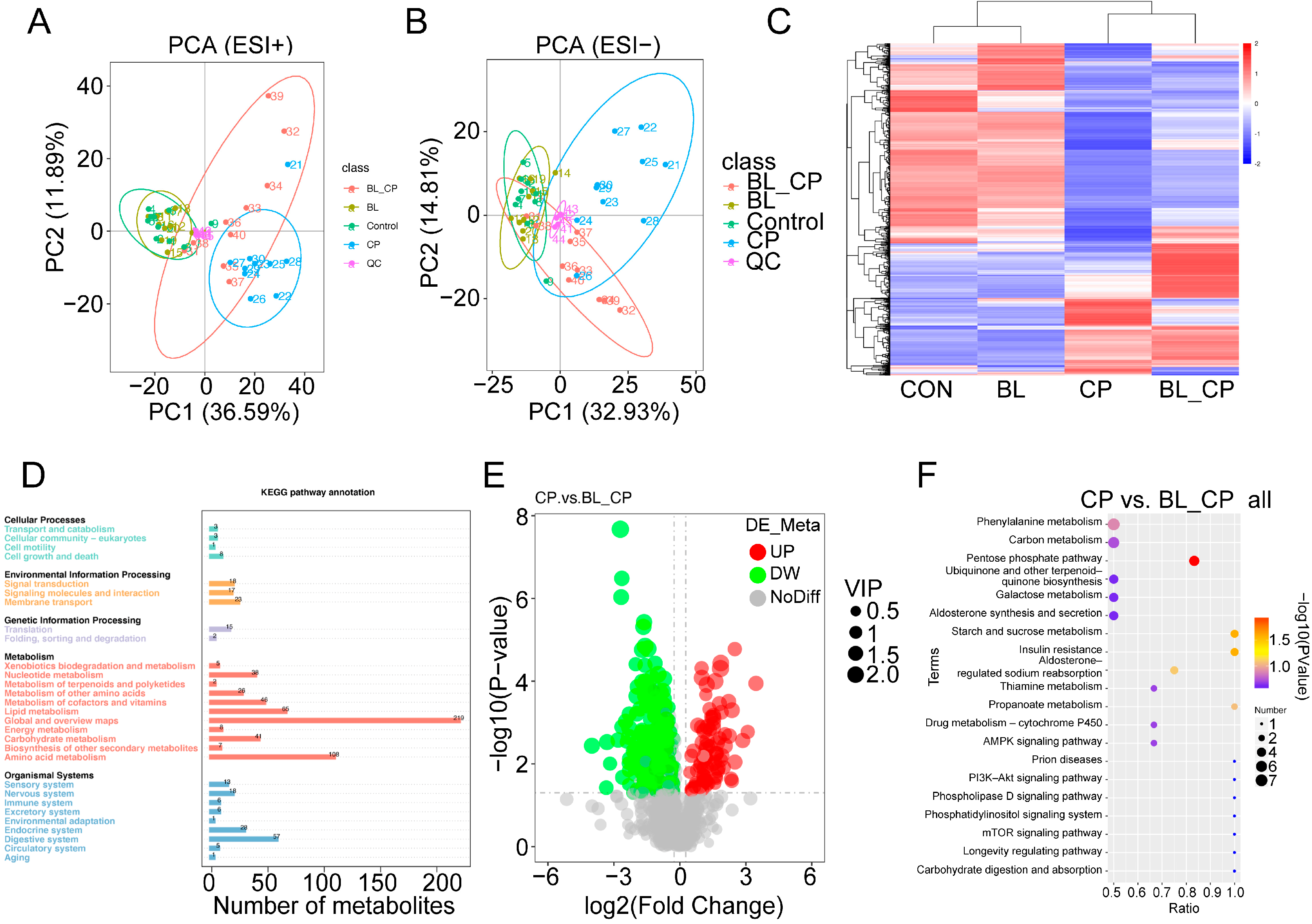
3.8. B. licheniformis Alters Cecal Metabolic Profiles During Infection
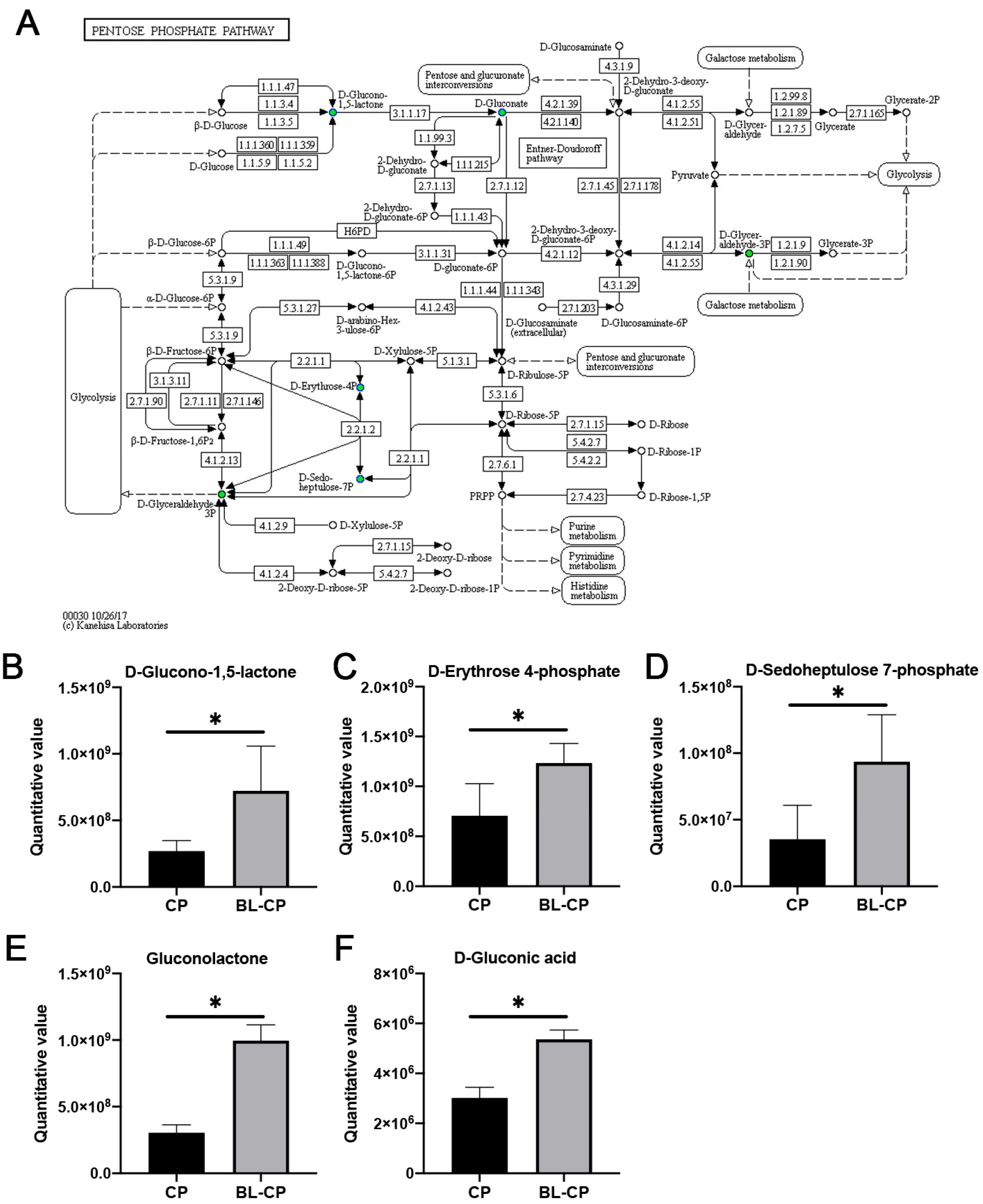
3.9. B. licheniformis Activates the Pentose Phosphate Pathway in the Gut
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B. licheniformis | Bacillus licheniformis |
| C. perfringens | Clostridium perfringens |
| DAO | Diamine oxidase |
| D-LA | D-lactic acid |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-alpha |
| VFAs | Volatile fatty acids |
| SCFA | Short-chain fatty acid |
| HE | Hematoxylin and eosin |
| ASVs | Amplicon sequence variants |
| PCoA | Principal coordinates analysis |
| NMDS | Non-metric multidimensional scaling |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PLS-DA | Partial least squares discriminant analysis |
| PPP | Pentose phosphate pathway |
| PVDF | Polyvinylidene difluoride |
| Bcl-2 | B-cell lymphoma-2 |
| Bax | Bcl-2-associated X protein |
References
- Tamura, A.; Tsukita, S. Paracellular barrier and channel functions of TJ claudins in organizing biological systems: Advances in the field of barriology revealed in knockout mice. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 36, pp. 177–185. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A.; Duparc, T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharmacol. 2013, 13, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, S.; Liu, S.; Jin, M.; Wang, Y.; Zong, X. Comparative multiomics analyses reveal the breed effect on the colonic host–microbe interactions in pig. iMetaOmics 2024, 1, e8. [Google Scholar] [CrossRef]
- Rood, J.I. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 1998, 52, 333–360. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-X.; Porter Corrine, J.; Hardy Simon, P.; Steer, D.; Smith, A.I.; Quinsey Noelene, S.; Hughes, V.; Cheung Jackie, K.; Keyburn Anthony, L.; Kaldhusdal, M.; et al. Structural and Functional Analysis of the Pore-Forming Toxin NetB from Clostridium perfringens. mBio 2013, 4, 10–1128. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Rudeaux, F.; Kim, I.H. Efficacy of dietary Bacillus subtilis and Bacillus licheniformis supplementation continuously in pullet and lay period on egg production, excreta microflora, and egg quality of Hyline-Brown birds. Poult. Sci. 2019, 98, 4722–4728. [Google Scholar] [CrossRef]
- Knap, I.; Lund, B.; Kehlet, A.B.; Hofacre, C.; Mathis, G. Bacillus licheniformis Prevents Necrotic Enteritis in Broiler Chickens. Avian Dis. 2010, 54, 931–935. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Yu, S.H.; Wu, S.G.; Yoon, I.; Quigley, J.; Gao, Y.P.; Qi, G.H. Effects of Yeast Culture in Broiler Diets on Performance and Immunomodulatory Functions. Poult. Sci. 2008, 87, 1377–1384. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, D.; Wang, H.; Qing, X.; Sun, N.; Xin, J.; Luo, M.; Khalique, A.; Pan, K.; Shu, G.; et al. Dietary Probiotic Bacillus licheniformis H2 Enhanced Growth Performance, Morphology of Small Intestine and Liver, and Antioxidant Capacity of Broiler Chickens Against Clostridium perfringens-Induced Subclinical Necrotic Enteritis. Probiotics Antimicrob. Proteins 2020, 12, 883–895. [Google Scholar] [CrossRef]
- Teo, A.Y.L.; Tan, H.M. Effect of Bacillus subtilis PB6 (CloSTAT) on Broilers Infected with a Pathogenic Strain of Escherichia coli. J. Appl. Poultry Res. 2006, 15, 229–235. [Google Scholar] [CrossRef]
- Jayaraman, S.; Thangavel, G.; Kurian, H.; Mani, R.; Mukkalil, R.; Chirakkal, H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013, 92, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Dai, Z.; Cao, G.; Cui, Z.; Zhang, R.; Xu, Y.; Wu, Y.; Yang, C. Protective effects of Bacillus licheniformis on growth performance, gut barrier functions, immunity and serum metabolome in lipopolysaccharide-challenged weaned piglets. Front. Immunol. 2023, 14, 1140564. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Hernandez-Velasco, X.; Kallapura, G.; Menconi, A.; Pumford, N.R.; Morgan, M.J.; Layton, S.L.; Bielke, L.R.; Hargis, B.M.; Téllez, G. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult. Sci. 2014, 93, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Kim, I.H. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult. Sci. 2014, 93, 364–370. [Google Scholar] [CrossRef]
- Sun, W.; Chen, W.; Meng, K.; Cai, L.; Li, G.; Li, X.; Jiang, X. Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology 2023, 12, 238. [Google Scholar] [CrossRef]
- Zeng, Z.; Yue, W.; Kined, C.; Wang, P.; Liu, R.; Liu, J.; Chen, X. Bacillus licheniformis reverses the environmental ceftriaxone sodium-induced gut microbial dysbiosis and intestinal inflammation in mice. Ecotoxicol. Environ. Saf. 2023, 257, 114890. [Google Scholar] [CrossRef]
- Xiao, X.; Qin, S.; Cui, T.; Liu, J.; Wu, Y.; Zhong, Y.; Yang, C. Bacillus licheniformis suppresses Clostridium perfringens infection via modulating inflammatory response, antioxidant status, inflammasome activation and microbial homeostasis in broilers. Poult. Sci. 2024, 103, 104222. [Google Scholar] [CrossRef]
- Han, Y.; Xu, X.; Wang, J.; Cai, H.; Li, D.; Zhang, H.; Yang, P.; Meng, K. Dietary Bacillus licheniformis shapes the foregut microbiota, improving nutrient digestibility and intestinal health in broiler chickens. Front. Microbiol. 2023, 14, 1113072. [Google Scholar] [CrossRef]
- Zhou, Q.; Dai, W.; Bao, Y.; Chen, J.; Han, X.; Liu, C.; Hou, M.; Yao, H.; Hao, C.; Li, S.; et al. Baseline gut microbiome impacts probiotics Bacillus licheniformis CMCC63516 in modulating the gut microbiome and preventing antibiotic-associated diarrhea: A double-blind, randomized controlled trial. Clin. Transl. Med. 2023, 13, e1184. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, X.; Shen, C.; Ao, Z.; Cao, X.; Song, C.; Mehmood, M.A.; Wu, T.; Mei, J.; He, M.; et al. Bacillus licheniformis-based intensive fermentation of Tibetan tea improved its bioactive compounds and reinforced the intestinal barrier in mice. Front. Microbiol. 2024, 15, 1376757. [Google Scholar] [CrossRef]
- Yaderets, V.; Karpova, N.; Glagoleva, E.; Shibaeva, A.; Dzhavakhiya, V. Bacillus subtilis RBT-7/32 and Bacillus licheniformis RBT-11/17 as New Promising Strains for Use in Probiotic Feed Additives. Microorganisms 2023, 11, 2729. [Google Scholar] [CrossRef] [PubMed]
- Palkovicsné Pézsa, N.; Kovács, D.; Rácz, B.; Farkas, O. Effects of Bacillus licheniformis and Bacillus subtilis on Gut Barrier Function, Proinflammatory Response, ROS Production and Pathogen Inhibition Properties in IPEC-J2—Escherichia coli/Salmonella Typhimurium Co-Culture. Microorganisms 2022, 10, 936. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, A.; Zaneb, H.; Nasir, A.; Adil, M.; Ali, H.; Muhammad, N.; Rehman, T.; Rehman, A.; Rehman, H. Effects of Bacillus subtilis on performance, immune system and gut in Salmonella-challenged broilers. S. Afr. J. Anim. Sci. 2020, 50. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Chen, Y.; Yu, C.; Azevedo, P.; Gong, J.; Yang, C. Bacillus licheniformis PF9 improves barrier function and alleviates inflammatory responses against enterotoxigenic Escherichia coli F4 infection in the porcine intestinal epithelial cells. J. Anim. Sci. Biotechnol. 2022, 13, 86. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, B.; Wang, Q.; Tang, L.; Zou, P.; Zeng, Z.; Zhang, H.; Gong, L.; Li, W. Protective Effects of Lactobacillus plantarum Lac16 on Clostridium perfringens Infection-Associated Injury in IPEC-J2 Cells. Int. J. Mol. Sci. 2021, 22, 12388. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Chen, J.; Xu, Z.; Wang, S.; Xia, X.; Liu, D.; Wang, S.; Xie, C.; Wu, J.; et al. Preventive Effects of Bacillus licheniformis on Heat Stroke in Rats by Sustaining Intestinal Barrier Function and Modulating Gut Microbiota. Front. Microbiol. 2021, 12, 630841. [Google Scholar] [CrossRef]
- HS, R.; Halami, P.M. The Combined Effect of Potential Probiotic Bacillus licheniformis MCC 2514 and Bifidobacterium breve NCIM 5671 Towards Anti-inflammatory Activity on HT-29 Cell Lines. Probiotics Antimicrob. Proteins 2023, 15, 351–362. [Google Scholar] [CrossRef]
- Guo, F.; Wang, F.; Ma, H.; Ren, Z.; Yang, X.; Yang, X. Study on the interactive effect of deoxynivalenol and Clostridium perfringens on the jejunal health of broiler chickens. Poult. Sci. 2021, 100, 100807. [Google Scholar] [CrossRef]
- Du, E.; Wang, W.; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19. [Google Scholar] [CrossRef]
- El Aidy, S.; van den Bogert, B.; Kleerebezem, M. The small intestine microbiota, nutritional modulation and relevance for health. Curr. Opin. Biotechnol. 2015, 32, 14–20. [Google Scholar] [CrossRef]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Q.; Ci, X.; Chen, S.; Xie, Z.; Li, H.; Zhang, H.; Chen, F.; Xie, Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020, 99, 6606–6618. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb. Cell Factories 2019, 18, 112. [Google Scholar] [CrossRef]
- Martin Manuel, P.; Elena, B.; Carolina, M.G.; Gabriela, P. Oral probiotics supplementation can stimulate the immune system in a stress process. J. Nutr. Intermed. Metab. 2017, 8, 29–40. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Bjarnason, I.; MacPherson, A.; Hollander, D. Intestinal permeability: An overview. Gastroenterology 1995, 108, 1566–1581. [Google Scholar] [CrossRef]
- Turner, J.R.; Buschmann, M.M.; Romero-Calvo, I.; Sailer, A.; Shen, L. The role of molecular remodeling in differential regulation of tight junction permeability. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 36, pp. 204–212. [Google Scholar] [CrossRef]
- Cai, C.; Li, W.; Chen, J.; Li, X.; Chen, S. Diamine oxidase as a marker for diagnosis of superior mesenteric arterial occlusion. Hepatogastroenterology 2012, 59, 155–158. [Google Scholar] [CrossRef]
- Gilani, S.; Howarth, G.S.; Kitessa, S.M.; Tran, C.D.; Forder, R.E.A.; Hughes, R.J. New biomarkers for increased intestinal permeability induced by dextran sodium sulphate and fasting in chickens. J. Anim. Physiol. Anim. Nutr. 2017, 101, e237–e245. [Google Scholar] [CrossRef]
- Xu, B.; Liang, S.; Zhao, J.; Li, X.; Guo, J.; Xin, B.; Li, B.; Huo, G.; Ma, W. Bifidobacterium animalis subsp. lactis XLTG11 improves antibiotic-related diarrhea by alleviating inflammation, enhancing intestinal barrier function and regulating intestinal flora. Food Funct. 2022, 13, 6404–6418. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S. Modulating Bcl-2 Family Proteins and Caspase-3 in Induction of Apoptosis by Paeoniflorin in Human Cervical Cancer Cells. Phytother. Res. 2011, 25, 1551–1557. [Google Scholar] [CrossRef]
- Huang, J.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wu, B. The Association between Splenocyte Apoptosis and Alterations of Bax, Bcl-2 and Caspase-3 mRNA Expression, and Oxidative Stress Induced by Dietary Nickel Chloride in Broilers. Int. J. Environ. Res. Public Health 2013, 10, 7310–7326. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.S.; Kim, S.H. Treadmill exercise ameliorates symptoms of Alzheimer disease through suppressing microglial activation-induced apoptosis in rats. J. Exerc. Rehabil. 2016, 12, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.-C.; Youle, R.J. Mitochondria in Apoptosis: Bcl-2 Family Members and Mitochondrial Dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.-h. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother 2020, 125, 109914. [Google Scholar] [CrossRef]
- Gong, L.; Wang, B.; Zhou, Y.; Tang, L.; Zeng, Z.; Zhang, H.; Li, W. Protective Effects of Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 Against Clostridium perfringens Infection in Broilers. Front. Immunol. 2021, 11, 628374. [Google Scholar] [CrossRef]
- Sergey, R.K.; Christine, F.F.; Wei Yun, Z.; Barbara, A.W.; Jeannette, K.; Wolfgang-Bernhard, S.; Willem, M.d.V.; Antoon, D.L.A.; Hauke, S. Microbial diversity studies of the porcine gastrointestinal ecosystem during weaning transition. Anim. Res. 2004, 53, 317–324. [Google Scholar]
- Shen, B.; Hu, J.; Song, H.; Wang, Z.; Fan, J.; Sun, Y.; Wang, Q. Antibiotics exacerbated colitis by affecting the microbiota, Treg cells and SCFAs in IL10-deficient mice. Biomed. Pharmacother. 2019, 114, 108849. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Luo, Z.; Liu, D. Supplemental Bacillus subtilis PB6 Improves Growth Performance and Gut Health in Broilers Challenged with Clostridium perfringens. J. Immunol. Res. 2021, 2021, 2549541. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, X.; Wang, C.; Kulandaivelu, J.; Jiang, G. Enhanced anaerobic digestion of primary sludge with additives: Performance and mechanisms. Bioresour. Technol. 2020, 316, 123970. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mei, R.; Wilson, F.P.; Yuan, H.; Bocher, B.T.W.; Liu, W.-T. Ecogenomics-Based Mass Balance Model Reveals the Effects of Fermentation Conditions on Microbial Activity. Front. Microbiol. 2020, 11, 595036. [Google Scholar] [CrossRef] [PubMed]
- Musa, B.B.; Duan, Y.; Khawar, H.; Sun, Q.; Ren, Z.; Elsiddig Mohamed, M.A.; Abbasi, I.H.R.; Yang, X. Bacillus subtilis B21 and Bacillus licheniformis B26 improve intestinal health and performance of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1039–1049. [Google Scholar] [CrossRef]
- Liu, X.; Yan, H.; Lv, L.; Xu, Q.; Yin, C.; Zhang, K.; Wang, P.; Hu, J. Growth Performance and Meat Quality of Broiler Chickens Supplemented with Bacillus licheniformis in Drinking Water. Asian-Australas. J. Anim. Sci. 2012, 25, 682–689. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Wang, C.C.; Wu, H.; Lin, F.H.; Gong, R.; Xie, F.; Peng, Y.; Feng, J.; Hu, C.H. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 2017, 24, 40–46. [Google Scholar] [CrossRef]
- Oakley, B.B.; Buhr, R.J.; Ritz, C.W.; Kiepper, B.H.; Berrang, M.E.; Seal, B.S.; Cox, N.A. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet. Res. 2014, 10, 282. [Google Scholar] [CrossRef]
- Jiang, Z.; Su, W.; Yang, M.; Fu, J.; Gong, T.; Li, W.; Wen, C.; Wang, X.; Wang, F.; Jin, M.; et al. Integrated multi-omics reveals the Bacillus amyloliquefaciens BA40 against Clostridium perfringens infection in weaned piglets. J. Adv. Res. 2025. [Google Scholar] [CrossRef]


| Number | Metabolites | Formula | Category | Mass (M/Z) | RT (Min) | CON vs. BL | CP vs. BL-CP | ||
|---|---|---|---|---|---|---|---|---|---|
| Trend | p Value | Trend | p Value | ||||||
| 1 | 12-Oxo-phytodienoic acid | C18H28O3 | Fatty Acyls | 293.2114 | 6.233 | up | 0.0004 | up | 0.0223 |
| 2 | 19(R)-hydroxy Prostaglandin A2 | C20H30O5 | Fatty Acyls | 331.1920 | 6.951 | up | 0.0011 | up | 0.0028 |
| 3 | Docosatrienoic acid | C22H38O2 | Fatty Acyls | 333.2804 | 11.139 | down | 0.0198 | down | 0.0017 |
| 4 | 10-Undecenoic acid | C11H20O2 | Fatty Acyls | 185.1539 | 6.417 | down | 0.0292 | down | 0.0001 |
| 5 | Deoxycorticosterone | C21H30O3 | Steroids and steroid derivatives | 699.4053 | 5.368 | up | 0.0050 | up | 0.0017 |
| 6 | 7-Ketodeoxycholic acid | C24H38O5 | Steroids and steroid derivatives | 405.2649 | 6.586 | up | 0.0095 | down | 0.0005 |
| 7 | 17α-Ethynylestradiol | C20H24O2 | Steroids and steroid derivatives | 297.1853 | 6.594 | up | 0.0266 | down | 0.0021 |
| 8 | Taurodeoxycholic acid sodium salt | C26H44NNaO6S | Steroids and steroid derivatives | 522.2875 | 8.99 | up | 0.0287 | down | 0.0257 |
| 9 | 11-Oxoetiocholanolone | C19H28O3 | Steroids and steroid derivatives | 287.2006 | 7.303 | up | 0.0336 | down | 0.0057 |
| 10 | Cortisol | C21H30O5 | Steroids and steroid derivatives | 363.2171 | 6.229 | up | 0.0467 | down | 0.0358 |
| 11 | LysoPE 16:0 | C21H44NO7P | Glycerophospholipids | 452.2792 | 8.566 | up | 0.0288 | up | 0.0033 |
| 12 | LysoPC 12:1 | C20H36NO7P | Glycerophospholipids | 432.2153 | 7.81 | down | 0.0450 | down | 0.0000 |
| 13 | LysoPC 14:0 | C22H46NO7P | Glycerophospholipids | 512.2999 | 8.565 | up | 0.0038 | up | 0.0004 |
| 14 | LPG 4:0 | C10H21O9P | Glycerophospholipids | 315.0857 | 5.237 | down | 0.0472 | up | 0.0167 |
| 15 | Diosgenin | C27H42O3 | Prenol lipids | 397.3107 | 8.221 | up | 0.0094 | down | 0.0201 |
| 16 | Hippuric acid | C9H9NO3 | Benzene and substituted derivatives | 178.0515 | 5.402 | down | 0.0004 | up | 0.0273 |
| 17 | 4-Ethylbenzaldehyde | C9H10O | Benzene and substituted derivatives | 135.0806 | 6.756 | up | 0.0398 | down | 0.0049 |
| 18 | Eugenol | C10H12O2 | Phenols | 181.0875 | 5.723 | up | 0.0192 | up | 0.0000 |
| 19 | tert-Butyl N-[1-(aminocarbonyl)-3-methylbutyl] carbamate | C11H22N2O3 | Carboxylic acids and derivatives | 231.1708 | 5.138 | down | 0.0012 | up | 0.0030 |
| 20 | Creatinine | C4H7N3O | Carboxylic acids and derivatives | 114.0664 | 1.316 | up | 0.0151 | up | 0.0053 |
| 21 | Cyanuric acid | C3H3N3O3 | Triaziness | 128.0082 | 2.424 | down | 0.0183 | up | 0.0001 |
| 22 | Neopterin | C9H11N5O4 | Pteridines and derivatives | 252.0731 | 1.389 | up | 0.0187 | up | 0.0013 |
| 23 | Isophorone | C9H14O | Organooxygen compounds | 139.1119 | 6.08 | down | 0.0470 | up | 0.0003 |
| 24 | Verbascose | C30H52O26 | Organooxygen compounds | 827.2662 | 1.409 | down | 0.0250 | up | 0.0017 |
| 25 | 4-Methoxycinnamal-dehyde | C10H10O2 | Cinnamaldehydes | 163.0756 | 5.985 | down | 0.0053 | down | 0.0125 |
| 26 | Aflatoxin G2 | C17H14O7 | Coumarins and derivatives | 329.0698 | 6.403 | down | 0.0416 | down | 0.0027 |
| 27 | Thymidine 5′-monophosphate | C10H15N2O8P | Pyrimidine nucleotides | 321.0496 | 2.841 | up | 0.0148 | up | 0.0215 |
| 28 | Normorphine | C16H17NO3 | Morphinans | 272.1286 | 5.987 | down | 0.0190 | down | 0.0008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Zhang, M.; Xu, H.; Yu, X.; Hu, Y.; Xu, Y.; Xiao, X.; Yang, C. Bacillus licheniformis Alleviates Clostridium perfringens-Induced Intestinal Injury in Mice Model by Modulating Inflammation, Apoptosis, and Cecal Microbial–Metabolic Responses. Animals 2025, 15, 1409. https://doi.org/10.3390/ani15101409
Zhong Y, Zhang M, Xu H, Yu X, Hu Y, Xu Y, Xiao X, Yang C. Bacillus licheniformis Alleviates Clostridium perfringens-Induced Intestinal Injury in Mice Model by Modulating Inflammation, Apoptosis, and Cecal Microbial–Metabolic Responses. Animals. 2025; 15(10):1409. https://doi.org/10.3390/ani15101409
Chicago/Turabian StyleZhong, Yifan, Meiting Zhang, Haocheng Xu, Xiaorong Yu, Yashi Hu, Yangyi Xu, Xiao Xiao, and Caimei Yang. 2025. "Bacillus licheniformis Alleviates Clostridium perfringens-Induced Intestinal Injury in Mice Model by Modulating Inflammation, Apoptosis, and Cecal Microbial–Metabolic Responses" Animals 15, no. 10: 1409. https://doi.org/10.3390/ani15101409
APA StyleZhong, Y., Zhang, M., Xu, H., Yu, X., Hu, Y., Xu, Y., Xiao, X., & Yang, C. (2025). Bacillus licheniformis Alleviates Clostridium perfringens-Induced Intestinal Injury in Mice Model by Modulating Inflammation, Apoptosis, and Cecal Microbial–Metabolic Responses. Animals, 15(10), 1409. https://doi.org/10.3390/ani15101409






