Cattle Zoonotic and Non-Zoonotic Tick-Borne Pathogens in Europe—A Retrospective Analysis of the Past 15 Years
Simple Summary
Abstract
1. Introduction
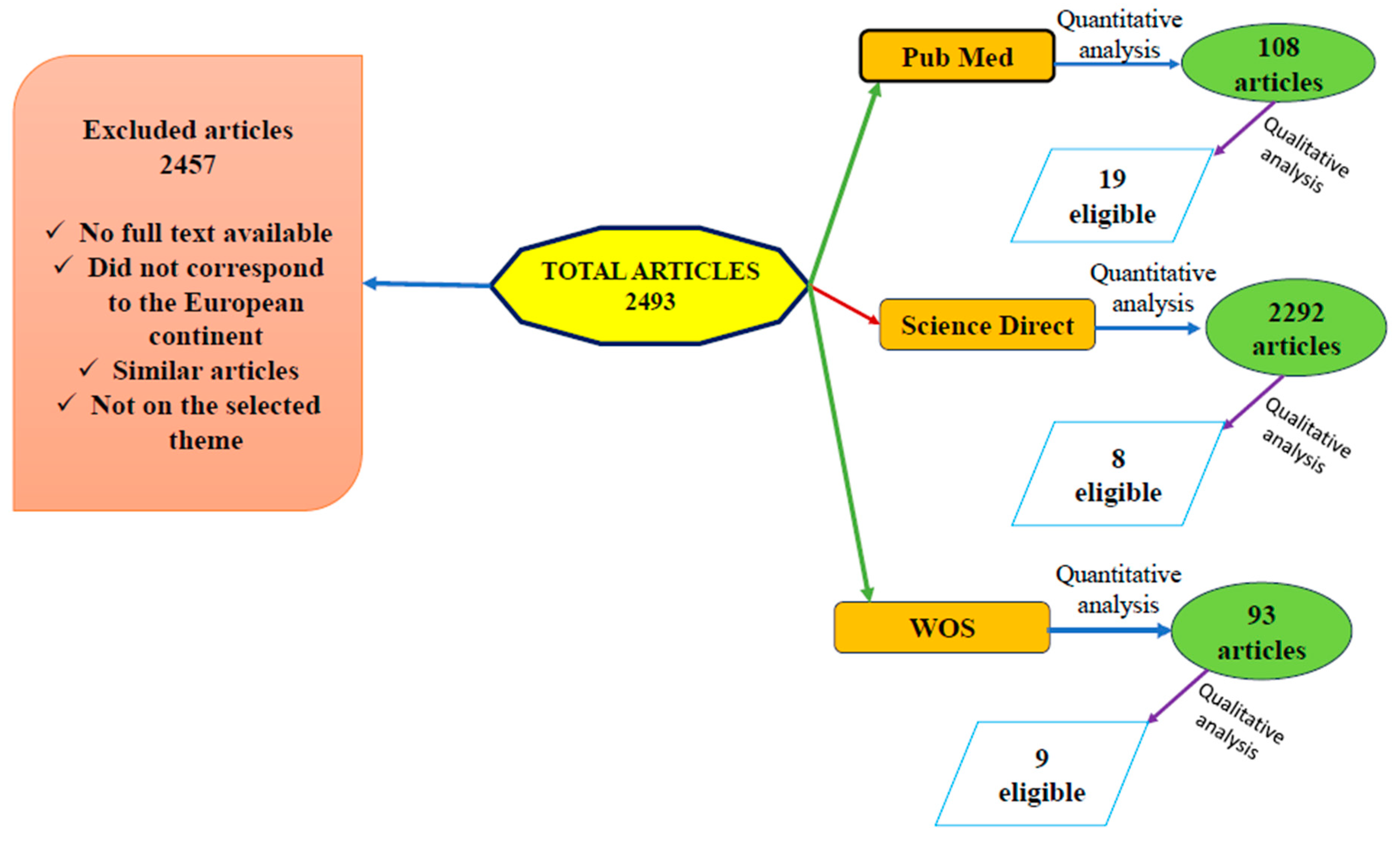
2. Materials and Methods
Literature Survey
- Timeframe: 2009–2024;
- Identification of tick species involved in disease transmission and the dissemination of etiological agents across Europe;
- Diagnostic methods employed;
- Pathogenic species frequently reported in cattle;
- Pathogenic species commonly identified in invertebrate hosts.
3. Results
3.1. Diagnostic Methods
3.2. Species of Ticks
- ✓
- ✓
- Borrelia miyamotoi, first isolated in 1995 in Japan from the species Ixodes persulcatus, was later detected for the first time in the tick species Dermacentor reticulatus [40].
- ✓
- ✓
- ✓
- ✓
- ✓
- ✓
- ✓
- For Babesia spp., R. turanicus/D. marginatus, and Hyalomma lusitanicum, tick species could be the most important vectors [43].
- ✓
- ✓
- Babesia bovis was detected in Rhipicephalus turanicus, Rh. bursa, H.excavatum, and H. anatolicum ticks [82].
- ✓
- ✓
- ✓
- ✓
3.3. Tick-Borne Pathogens
3.3.1. Anaplasma spp.
3.3.2. Babesia spp.
3.3.3. Theileria spp.
3.3.4. Borrelia spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Springer, A.; Glass, A.; Topp, A.K.; Strube, C. Zoonotic Tick-Borne Pathogens in Temperate and Cold Regions of Europe—A Review on the Prevalence in Domestic Animals. Front. Vet. Sci. 2020, 7, 604910. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 9 April 2024).
- Palomar, A.M.; Molina, I.; Bocanegra, C.; Portillo, A.; Salvador, F.; Moreno, M.; Oteo, J.A. Old zoonotic agents and novel variants of tick-borne microorganisms from Benguela (Angola), July 2017. Parasites Vectors 2022, 15, 140. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.N.F. The concept of One Health applied to the problem of zoonotic diseases. Rev. Med. Virol. 2022, 32, e2326. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.J.; Gunn, A. The One Health concept. Br. J. Biomed. Sci. 2024, 81, 12366. [Google Scholar] [CrossRef]
- Johnson, N.; Phipps, L.P.; Hansford, K.M.; Folly, A.J.; Fooks, A.R.; Medlock, J.M.; Mansfield, K.L. One Health Approach to Tick and Tick-Borne Disease Surveillance in the United Kingdom. Int. J. Environ. Res. Public Health 2022, 19, 5833. [Google Scholar] [CrossRef]
- Ioniţă, M.; Mitrea, I.L. Actualități privind bolile transmise de vectori: Riscuri pentru sănătatea omului și a animalelor (I. boli transmise de căpușe). Rev. Rom. Med. Vet. 2017, 27, 8–14. [Google Scholar]
- Mapholi, N.O.; Marufu, M.C.; Maiwashe, A.; Banga, C.B.; Muchenje, V.; MacNeil, M.D.; Chimonyo, M.; Dzama, K. Towards a genomics approach to tick (Acari: Ixodidae) control in cattle: A review. Ticks Tick-Borne Dis. 2014, 5, 475–483. [Google Scholar] [CrossRef]
- Johnson, N.; Paul Phipps, L.; McFadzean, H.; Barlow, A.M. An outbreak of bovine babesiosis in February, 2019, triggered by above average winter temperatures in southern England and co-infection with Babesia divergens and Anaplasma phagocytophilum. Parasites Vectors 2020, 13, 305. [Google Scholar] [CrossRef]
- Fesseha, H.; Mathewos, M.; Eshetu, E.; Tefera, B. Babesiosis in cattle and ixodid tick distribution in Dasenech and Salamago Districts, southern Ethiopia. Sci. Rep. 2022, 12, 6385. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Adjou Moumouni, P.F.; Mohammed-Geba, K.; Sheir, S.K.; Hashem, I.S.; Cao, S.; Terkawi, M.A.; Kamyingkird, K.; Nishikawa, Y.; Suzuki, H.; et al. Molecular and serological prevalence of Babesia bigemina and Babesia bovis in cattle and water buffalos under small-scale dairy farming in Beheira and Faiyum Provinces, Egypt. Vet. Parasitol. 2013, 198, 187–192. [Google Scholar] [CrossRef]
- Elsify, A.; Sivakumar, T.; Nayel, M.; Salama, A.; Elkhtam, A.; Rizk, M.; Omar, M.; Sultan, K.; Elsayed, S.; Igarashi, I.; et al. An epidemiological survey of bovine Babesia and Theileria parasites in cattle, buffaloes, and sheep in Egypt. Parasitol. Int. 2014, 64, 79–85. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Hans Dautel, K.O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick-Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.M.; Marth, E.H. Borrelia burgdorferi: Another cause of foodborne illness? Int. J. Food Microbiol. 1991, 14, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Moga Mânzat, R. Bacterioze; Publisher Brumar: Timişoara, Romania, 2015; pp. 252–253. [Google Scholar]
- Young, K.M.; Corrin, T.; Wilhelm, B.; Uhland, C.; Greig, J.; Mascarenhas, M.; Waddell, L.A. Zoonotic Babesia: A scoping review of the global evidence. PLoS ONE 2019, 14, e0226781. [Google Scholar] [CrossRef] [PubMed]
- Rożej-Bielicka, W.; Stypułkowska-Misiurewicz, H.; Gołąb, E. Human babesiosis. Przegląd Epidemiol. 2015, 69, 489–608. [Google Scholar]
- Fernández, N.; Revuelta, B.; Aguilar, I.; Soares, J.F.; Zintl, A.; Gray, J.; Montero, E.; Gonzalez, L.M. Babesia and Theileria identification in adult ixodid ticks from Tapada Nature Reserve, Portugal. Pathogens 2022, 11, 222. [Google Scholar] [CrossRef]
- Springer, A.; Glass, A.; Probst, J.; Strube, C. Tick-borne zoonoses and commonly used diagnostic methods in human and veterinary medicine. Parasitol. Res. 2021, 120, 4075–4090. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Altay, K.; Erol, U.; Şahin, O.F. Anaplasma capra: A new emerging tick-borne zoonotic pathogen. Vet. Res. Commun. 2024, 48, 1329–1340. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Lyme Disease. Available online: https://www.cdc.gov/lyme/about/index.html (accessed on 4 May 2025).
- Tilly, K.; Rosa, P.A.; Stewart, P.E. Biology of infection with Borrelia burgdorferi. Infect. Dis. Clin. N. Am. 2008, 22, 217–234. [Google Scholar] [CrossRef]
- Giubega, S.; Dreghiciu, C.; Ilie, M.S.; Dărăbuş, G. Diagnostic methods used for the detection of Theileria equi: Review of the last decade. Cluj Vet. J. 2022, 27, 16–24. [Google Scholar] [CrossRef]
- Wikipedia Enciclopedia Liberă. Available online: https://www.wikiwand.com/ro/Europa (accessed on 15 June 2024).
- Cattle-Definition-Meaning-Description & Breeds. Available online: https://www.animalscience.care/ (accessed on 15 June 2024).
- Mapchart. Available online: https://www.mapchart.net/detworld.html (accessed on 15 June 2024).
- Zintl, A.; McGrath, G.; O’Grady, L.; Fanning, J.; Downing, K.; Roche, D.; Casey, M.; Gray, J.S. Changing incidence of bovine babesiosis in Ireland. Ir. Vet. J. 2014, 67, 19. [Google Scholar] [CrossRef] [PubMed]
- McFadzean, H.; Johnson, N.; Phipps, L.P.; Swinson, V.; Boden, L.A. Surveillance and Risk Analysis for Bovine Babesiosis in England and Wales to Inform Disease Distribution. Animals 2023, 13, 2118. [Google Scholar] [CrossRef]
- Bajer, A.; Beck, A.; Beck, R.; Behnke, J.M.; Dwużnik-Szarek, D.; Eichenberger, R.M.; Farkas, R.; Fuehrer, H.-P.; Heddergott, M.; Jokelainen, P.; et al. Babesiosis in Southeastern, Central and Northeastern Europe: An Emerging and Re-Emerging Tick-Borne Disease of Humans and Animals. Microorganisms 2022, 10, 945. [Google Scholar] [CrossRef]
- Staniec, M.; Adaszek, Ł.; Winiarczyk, M.; Skrzypczak, M.; Nowakiewicz, A.; Buczek, K.; Winiarczyk, S. Detection of Babesia occultans protozoa in cattle from territory of eastern Poland. Nachweis des Protozoons Babesia occultans bei Rindern in einem Gebiet in Ostpolen. Tierärztliche Praxis Ausgabe G Großtiere/Nutztiere 2018, 46, 257–259. [Google Scholar] [CrossRef]
- Mysterud, A.; Stigum, V.M.; Seland, I.V.; Herland, A.; Easterday, W.R.; Jore, S.; Østerås, O.; Viljugrein, H. Tick abundance, pathogen prevalence, and disease incidence in two contrasting regions at the northern distribution range of Europe. Parasites Vectors 2018, 11, 309. [Google Scholar] [CrossRef]
- Andersson, M.O.; Víchová, B.; Tolf, C.; Krzyzanowska, S.; Waldenström, J.; Karlsson, M.E. Co-infection with Babesia divergens and Anaplasma phagocytophilum in cattle (Bos taurus), Sweden. Ticks Tick-Borne Dis. 2017, 8, 933–935. [Google Scholar] [CrossRef]
- Dugat, T.; Leblond, A.; Keck, N.; Lagrée, A.C.; Desjardins, I.; Joulié, A.; Pradier, S.; Durand, B.; Boulouis, H.J.; Haddad, N. One particular Anaplasma phagocytophilum ecotype infects cattle in the Camargue, France. Parasites Vectors 2017, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Omeragić, J.; Šerić-Haračić, S.; Klarić Soldo, D.; Kapo, N.; Fejzić, N.; Škapur, V.; Medlock, J. Distribution of ticks in Bosnia and Herzegovina. Ticks Tick-Borne Dis. 2022, 13, 101870. [Google Scholar] [CrossRef]
- Hvidsten, D.; Frafjord, K.; Gray, J.S.; Henningsson, A.J.; Jenkins, A.; Kristiansen, B.E.; Lager, M.; Rognerud, B.; Slåtsve, A.M.; Stordal, F.; et al. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick-Borne Dis. 2020, 11, 101388. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Species diversity and abundance of ticks in three habitats in southern Italy. Ticks Tick-Borne Dis. 2013, 4, 251–255. [Google Scholar] [CrossRef]
- Zhou, M.; Cao, S.; Sevinc, F.; Sevinc, M.; Ceylan, O.; Moumouni, P.F.A.; Jirapattharasate, C.; Liu, M.; Wang, G.; Iguchi, A.; et al. Molecular detection and genetic identification of Babesia bigemina, Theileria annulata, Theileria orientalis and Anaplasma marginale in Turkey. Ticks Tick-Borne Dis. 2016, 7, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.G.; Henriques, G.; Sánchez, C.; Marques, P.X.; Suarez, C.E.; Oliva, A. First survey for Babesia bovis and Babesia bigemina infection in cattle from Central and Southern regions of Portugal using serological and DNA detection methods. Vet. Parasitol. 2009, 166, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.; Krücken, J.; McKay-Demeler, J.; Pachnicke, S.; Krieger, K.; von Samson-Himmelstjerna, G. Dermacentor reticulatus in Berlin/Brandenburg (Germany): Activity patterns and associated pathogens. Ticks Tick-Borne Dis. 2019, 10, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Bursakov, S.A.; Kovalchuk, S.N. Co-infection with tick-borne disease agents in cattle in Russia. Ticks Tick-Borne Dis. 2019, 10, 709–713. [Google Scholar] [CrossRef]
- Sprong, H.; Moonen, S.; van Wieren, S.E.; Hofmeester, T.R. Effects of cattle grazing on Ixodes ricinus-borne disease risk in forest areas of the Netherlands. Ticks Tick-Borne Dis. 2020, 11, 101355. [Google Scholar] [CrossRef]
- Torina, A.; Alongi, A.; Scimeca, S.; Vicente, J.; Caracappa, S.; De La Fuente, J. Prevalence of Tick-Borne Pathogens in Ticks in Sicily. Transbound. Emerg. Dis. 2010, 57, 46–48. [Google Scholar] [CrossRef]
- Adjadj, N.R.; Cargnel, M.; Ribbens, S.; Quinet, C.; Malandrin, L.; Mignon, B.; Mori, M. Prevalence of Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Rickettsia spp. and Babesia spp. in cattle serum and questing ticks from Belgium. Ticks Tick-Borne Dis. 2023, 14, 102146. [Google Scholar] [CrossRef]
- Cicculli, V.; Capai, L.; Quilichini, Y.; Masse, S.; Fernández-Alvarez, A.; Minodier, L.; Bompard, P.; Charrel, R.; Falchi, A. Molecular investigation of tick-borne pathogens in ixodid ticks infesting domestic animals (cattle and sheep) and small rodents (black rats) of Corsica, France. Ticks Tick-Borne Dis. 2019, 10, 606–613. [Google Scholar] [CrossRef]
- Gomes, J.; Salgueiro, P.; Inácio, J.; Amaro, A.; Pinto, J.; Tait, A.; Shiels, B.; Pereira da Fonseca, I.; Santos-Gomes, G.; Weir, W. Population diversity of Theileria annulata in Portugal. Infect. Genet. Evol. 2016, 42, 14–19. [Google Scholar] [CrossRef]
- Gandy, S.; Medlock, J.; Cull, B.; Smith, R.; Gibney, Z.; Sewgobind, S.; Parekh, I.; Harding, S.; Johnson, N.; Hansford, K. Detection of Babesia species in questing Ixodes ricinus ticks in England and Wales. Ticks Tick-Borne Dis. 2024, 15, 102291. [Google Scholar] [CrossRef] [PubMed]
- Palomar, A.M.; Portillo, A.; Santibáñez, P.; Mazuelas, D.; Roncero, L.; García-Álvarez, L.; Santibáñez, S.; Gutiérrez, Ó.; Oteo, J.A. Detection of tick-borne Anaplasma bovis, Anaplasma phagocytophilum and Anaplasma centrale in Spain. Med. Vet. Entomol. 2015, 29, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Takács, N.; Kontschán, J.; György, Z.; Micsutka, A.; Iceton, S.; Flaisz, B.; Farkas, R.; Hofmann-Lehmann, R. Diversity of Haemaphysalis-associated piroplasms of ruminants in Central-Eastern Europe, Hungary. Parasites Vectors 2015, 8, 627. [Google Scholar] [CrossRef]
- Toma, L.; Di Luca, M.; Mancini, F.; Severini, F.; Mariano, C.; Nicolai, G.; Laghezza Masci, V.; Ciervo, A.; Fausto, A.M.; Cacciò, S.M. Molecular characterization of Babesia and Theileria species in ticks collected in the outskirt of Monte Romano, Lazio Region, Central Italy. Annali Dell’istituto Superiore Di Sanità 2017, 53, 30–34. [Google Scholar] [PubMed]
- Calleja-Bueno, L.; Sainz, Á.; García-Sancho, M.; González-Martín, J.V.; Díaz-Regañón, D.; Rodríguez-Franco, F.; Agulla, B.; Tormo, B.; Villaescusa, A. First detection of Anaplasma phagocytophilum and Babesia divergens and high infection rates of Anaplasma marginale and Babesia bigemina in cattle in extensive grazing systems of Central Spain. Transbound. Emerg. Dis. 2022, 69, e1090–e1100. [Google Scholar] [CrossRef]
- Springer, A.; Jordan, D.; Höltershinken, M.; Barutzki, D.; Strube, C. Endemisation and management of Babesia divergens on a beef production farm. Curr. Res. Parasitol. Vector-Borne Dis. 2024, 6, 100188. [Google Scholar] [CrossRef]
- Grech-Angelini, S.; Stachurski, F.; Lancelot, R.; Boissier, J.; Allienne, J.F.; Marco, S.; Maestrini, O.; Uilenberg, G. Ticks (Acari: Ixodidae) infesting cattle and some other domestic and wild hosts on the French Mediterranean island of Corsica. Parasites Vectors 2016, 9, 582. [Google Scholar] [CrossRef]
- Springer, A.; Höltershinken, M.; Lienhart, F.; Ermel, S.; Rehage, J.; Hülskötter, K.; Lehmbecker, A.; Wohlsein, P.; Barutzki, D.; Gietl, C.; et al. Emergence and Epidemiology of Bovine Babesiosis Due to Babesia divergens on a Northern German Beef Production Farm. Front. Vet. Sci. 2020, 7, 649. [Google Scholar] [CrossRef]
- Fedorina, E.A.; Arkhipova, A.L.; Kosovskiy, G.Y.; Kovalchuk, S.N. Molecular survey and genetic characterization of Anaplasma marginale isolates in cattle from two regions of Russia. Ticks Tick-Borne Dis. 2019, 10, 251–257. [Google Scholar] [CrossRef]
- Calleja-Bueno, L.; Sainz, Á.; García-Sancho, M.; Rodríguez-Franco, F.; González-Martín, J.V.; Villaescusa, A. Molecular, epidemiological, haematological and biochemical evaluation in asymptomatic Theileria annulata infected cattle from an endemic region in Spain. Ticks Tick-Borne Dis. 2017, 8, 936–941. [Google Scholar] [CrossRef]
- Zając, Z.; Woźniak, A.; Kulisz, J. Infestation of dairy cows by ticks Dermacentor reticulatus (Fabricius, 1794) and Ixodes ricinus (Linnaeus, 1758) in eastern Poland. Ann. Parasitol. 2020, 66, 87–96. [Google Scholar]
- Ferrolho, J.; Antunes, S.; Santos, A.S.; Velez, R.; Padre, L.; Cabezas-Cruz, A.; Santos-Silva, M.M.; Domingos, A. Detection and phylogenetic characterization of Theileria spp. and Anaplasma marginale in Rhipicephalus bursa in Portugal. Ticks Tick-Borne Dis. 2016, 7, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Pienaar, R.; Latif, A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 2015, 4, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Knorr, S.; Reissert-Oppermann, S.; Tomás-Cortázar, J.; Barriales, D.; Azkargorta, M.; Iloro, I.; Elortza, F.; Pinecki-Socias, S.; Anguita, J.; Hovius, J.W.; et al. Identification and Characterization of Immunodominant Proteins from Tick Tissue Extracts Inducing a Protective Immune Response against Ixodes ricinus in Cattle. Vaccines 2021, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- National Human Genome Research Institute. Available online: https://www.genome.gov/genetics-glossary/Polymerase-Chain-Reaction (accessed on 25 August 2024).
- Schwartz, I.; Wormser, G.P.; Schwartz, J.J.; Cooper, D.; Weiss, G.H.; Gazumyan, A.; Berger, B.W.; Edelman, R. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J. Clin. Microbiol. 1992, 30, 3082–3088. [Google Scholar] [CrossRef]
- Johnson, B.J.; Happ, C.M.; Mayer, L.W.; Piesman, J. Detection of Borrelia burgdorferi in ticks by species-specific amplification of the flagellin gene. Am. J. Trop. Med. Hyg. 1992, 47, 730–741. [Google Scholar] [CrossRef]
- Hojgaard, A.; Osikowicz, L.M.; Eisen, L.; Eisen, R.J. Evaluation of a novel multiplex PCR amplicon sequencing assay for detection of human pathogens in Ixodes ticks. Ticks Tick-Borne Dis. 2020, 11, 101504. [Google Scholar] [CrossRef]
- EMBL’s European Bioinformatics Institute. Available online: https://www.ebi.ac.uk/training/online/courses/functional-genomics-ii-common-technologies-and-data-analysis-methods/real-time-pcr/ (accessed on 25 August 2024).
- Hu, K.; Liu, T.; Xu, W.; Zhang, Y.; Wang, L.; Li, J. Real-time quantitative reverse transcription PCR assay for the detection of Nuomin virus—An emerging tick-borne virus. J. Virol. Methods 2024, 330, 115032. [Google Scholar] [CrossRef]
- Chen, Z.; Halford, N.G.; Liu, C. Real-Time Quantitative PCR: Primer Design, Reference Gene Selection, Calculations and Statistics. Metabolites 2023, 13, 806. [Google Scholar] [CrossRef]
- Merck. Available online: https://www.sigmaaldrich.com/RO/en/technical-documents/protocol/genomics/pcr/kapa-multiplex-rtpcr?srsltid=AfmBOoqix_8Fmgp6dovPgMTRrATqsAexLKy5xp_IrNOSJb7drVPzI560 (accessed on 25 August 2024).
- Hojgaard, A.; Lukacik, G.; Piesman, J. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti with two different multiplex PCR assays. Ticks Tick-Borne Dis. 2014, 5, 349–351. [Google Scholar] [CrossRef]
- Cold Spring Harbor Protocols. Available online: https://cshprotocols.cshlp.org/content/2019/2/pdb.prot095182 (accessed on 25 August 2024).
- Wills, M.K.B.; Kirby, A.M.; Lloyd, V.K. Detecting the Lyme disease spirochete, Borrelia burgdorferi, in ticks using nested PCR. J. Vis. Exp. 2018, 132, 56471. [Google Scholar] [CrossRef]
- Teal, A.E.; Habura, A.; Ennis, J.; Keithly, J.S.; Madison-Antenucci, S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J. Clin. Microbiol. 2012, 50, 903–908. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, J.; Li, X.; Chen, L.; Zhao, Y.; Zhou, X.; Liu, Q. Nested qPCR assay to detect Babesia duncani infection in hamsters and humans. Parasitol. Res. 2022, 121, 3603–3610. [Google Scholar] [CrossRef] [PubMed]
- Medline Plus. Available online: https://medlineplus.gov/ency/article/003332.htm (accessed on 25 August 2024).
- Kohl, T.O.; Ascoli, C.A. Direct competitive enzyme-linked immunosorbent assay (ELISA). Cold Spring Harb. Protoc. 2017, 2017, pdb.prot093740. [Google Scholar] [CrossRef] [PubMed]
- Sino Biological. Available online: https://www.sinobiological.com/category/indirect-elisa, (accessed on 25 August 2024).
- Shah, K.; Maghsoudlou, P. Enzyme-linked immunosorbent assay (ELISA): The basics. Br. J. Hosp. Med. 2016, 77, C98–C101. [Google Scholar] [CrossRef]
- Researchgate. Available online: https://www.researchgate.net/publication/329488369_Indirect_Fluoressnt_Antibody_Test_IFAT (accessed on 25 August 2024).
- Boscolo, M.; Gobbo, M.; Mantovani, W.; Degani, M.; Anselmi, M.; Monteiro, G.B.; Marocco, S.; Angheben, A.; Mistretta, M.; Santacatterina, M.; et al. Evaluation of an indirect immunofluorescence assay for strongyloidiasis as a tool for diagnosis and follow-up. Clin. Vaccine Immunol. 2007, 14, 129–133. [Google Scholar] [CrossRef]
- Lira-Amaya, J.J.; Martínez-García, G.; Santamaria-Espinosa, R.M.; Castañeda-Arriola, R.O.; Ojeda-Carrasco, J.J.; Ávila-Ramírez, G.; Figueroa-Millán, J.V. Comparative study of indirect fluorescent antibody, ELISA, and immunochromatography tests for serological diagnosis of bovine babesiosis caused by Babesia bovis. Animals 2021, 11, 3358. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.; Weakley, M.; Do, T.; Mir, S. Current and future molecular diagnostics of tick-borne diseases in cattle. Vet. Sci. 2022, 9, 241. [Google Scholar] [CrossRef]
- Shengwei, J.; Onur, C.; Zhuowei, M.; Eloiza, M.G.; Iqra, Z.; Hang, L.; Yae, H.; Mutlu, S.; Tatsunori, M.; Aiko, I.; et al. Protozoan and Rickettsial Pathogens in Ticks Collected from Infested Cattle from Turkey. Pathogens 2022, 11, 500. [Google Scholar] [CrossRef]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51 Pt 6, 2145–2165. [Google Scholar]
- Seo, M.G.; Ouh, I.O.; Lee, H.; Geraldino, P.J.L.; Rhee, M.H.; Kwon, O.D.; Kwak, D. Differential identification of Anaplasma in cattle and potential of cattle to serve as reservoirs of Anaplasma capra, an emerging tick-borne zoonotic pathogen. Vet. Microbiol. 2018, 226, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Aubry, P.; Geale, D.W. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S.A. The natural history of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.L.; Madruga, C.R.; Araújo, F.R.; Menk, C.F.; de Almeida, M.A.O.; Melo, E.P.; Kessler, R.H. Serological survey of Babesia bovis, Babesia bigemina, and Anaplasma marginale antibodies in cattle from the semi-arid region of the state of Bahia, Brazil, by enzyme-linked immunosorbent assays. Memórias do Instituto Oswaldo Cruz 2005, 100, 513–517. [Google Scholar] [CrossRef]
- Benavides, M.V.; Sacco, A.M.S. Differential Bos taurus cattle response to Babesia bovis infection. Vet. Parasitol. 2007, 150, 54–64. [Google Scholar] [CrossRef]
- Kessler, R.H.; Schenk, M.A.M. Carrapato, Tristeza Parasitária e Tripanossomose dos Bovinos; Embrapa Gado de Corte: Campo Grande, Brazil, 1998; p. 157. [Google Scholar]
- Tsai, Y.L.; Chomel, B.B.; Chang, C.C.; Kass, P.H.; Conrad, P.A.; Chuang, S.T. Bartonella and Babesia infections in cattle and their ticks in Taiwan. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 179–187. [Google Scholar] [CrossRef]
- Cernăianu, C. Piroplasme şi Piroplasmoze; Published Academiei Republicii Populare Romîne: Timisoara, Romania, 1958. [Google Scholar]
- Jacob, S.S.; Sengupta, P.P.; Paramanandham, K.; Suresh, K.P.; Chamuah, J.K.; Rudramurthy, G.R.; Roy, P. Bovine babesiosis: An insight into the global perspective on the disease distribution by systematic review and meta-analysis. Vet. Parasitol. 2020, 283, 109136. [Google Scholar] [CrossRef]
- Dărăbuş, G.; Oprescu, I.; Morariu, S.; Mederle, N.; Ilie, M.S.; Imre, M. Parazitologie şi Boli Parazitare; Published Mirton: Timisoara, Romania, 2023. [Google Scholar]
- Patial, V.; Gupta, T.; Angaria, S.; Bali, D.; Katoch, A.; Gautam, M.; Singh, N.K.; Sharma, M.; Chahota, R. Theileria orientalis outbreak in an organized cattle breeding farm. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100572. [Google Scholar] [CrossRef]
- Bonnet, S.; Michelet, L.; Moutailler, S.; Cheval, J.; Hébert, C.; Vayssier-Taussat, M.; Eloit, M. Identification of parasitic communities within European ticks using next-generation sequencing. PLoS Negl. Trop. Dis. 2014, 8, e2753. [Google Scholar] [CrossRef]
- Ioniţă, M.; Mitrea, I.L.; Pfister, K.; Hamel, D.; Silaghi, C. Molecular evidence for bacterial and protozoan pathogens in hard ticks from Romania. Vet. Parasitol. 2013, 196, 71–76. [Google Scholar] [CrossRef]
- Gomes, J.; Soares, R.; Santos, M.; Santos-Gomes, G.; Botelho, A.; Amaro, A.; Inácio, J. Detection of Theileria and Babesia infections amongst asymptomatic cattle in Portugal. Ticks Tick-Borne Dis. 2013, 4, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Nasreldin, N.; Ewida, R.M.; Hamdon, H.; Elnaker, Y.F. Molecular diagnosis and biochemical studies of tick-borne diseases (anaplasmosis and babesiosis) in Aberdeen Angus Cattle in New Valley, Egypt. Vet. World 2020, 13, 1884–1891. [Google Scholar] [CrossRef]
- Agina, O.A.; Shaari, M.R.; Isa, N.M.M.; Ajat, M.; Zamri-Saad, M.; Hamzah, H. Clinical Pathology, Immunopathology and Advanced Vaccine Technology in Bovine Theileriosis: A Review. Pathogens 2020, 9, 697. [Google Scholar] [CrossRef]
- Bishop, R.; Musoke, A.; Morzaria, S.; Gardner, M.; Nene, V. Theileria: Intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology 2004, 129 (Suppl. S1), S271–S283. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, S.; Jia, L.; Xue, S.; Yu, L.; Kamyingkird, K.; Moumouni, P.F.; Moussa, A.A.; Zhou, M.; Zhang, Y.; et al. Molecular detection of Theileria species in sheep from northern China. J. Vet. Med. Sci. 2013, 75, 1227–1230. [Google Scholar] [CrossRef]
- Anupama, R.; Srinivasan, S.R.; Parthiban, M. Molecular studies on theileriosis and identification of Theileria orientalis in India using PCR. Indian Vet. J. 2015, 92, 9–11. [Google Scholar]
- Mohd Hasan, L.I.; Kho, K.L.; Koh, F.X.; Hassan Nizam, Q.N.; Tay, S.T. Molecular evidence of hemoplasmas in Malaysian cattle and ticks. Trop. Biomed. 2017, 34, 668–674. [Google Scholar] [PubMed]
- Ola-Fadunsin, S.D.; Maizatul, A.M.; Ibrahim, A.R.; Alizawathy, A.; Chandrawathani, P.; Jesse, F.F.A.; Sani, R.A.; Sharma, R.S.K. Molecular Prevalence and Species Co-Infection of Bovine Haemoparasites in Peninsular Malaysia. Malays. J. Vet. Res. 2017, 8, 13–22. [Google Scholar]
- Sivakumar, T.; Fujita, S.; Tuvshintulga, B.; Kothalawala, H.; Silva, S.S.P.; Yokoyama, N. Discovery of a new Theileria sp. closely related to Theileria annulata in cattle from Sri Lanka. Sci. Rep. 2019, 9, 16132. [Google Scholar] [CrossRef]
- Watts, J.G.; Playford, M.C.; Hickey, K.L. Theileria orientalis: A review. N. Z. Vet. J. 2016, 64, 3–9. [Google Scholar] [CrossRef]
- Maggi, R.G. Animal Health: Ectoparasites. In Encyclopedia of Agriculture and Food Systems, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 5, pp. 315–326. [Google Scholar]
- Kovalchuk, S.N. Molecular characterization and phylogenetic study of Theileria sp. parasites detected in cattle from the Moscow region of Russia. Ticks Tick-Borne Dis. 2022, 13, 101835. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Hayashida, K.; Sugimoto, C.; Yokoyama, N. Evolution and genetic diversity of Theileria. Infect. Genet. Evol. 2014, 27, 250–263. [Google Scholar] [CrossRef]
- Dobbelaere, D.A.; Küenzi, P. The strategies of the Theileria parasite: A new twist in host-pathogen interactions. Curr. Opin. Immunol. 2004, 16, 524–530. [Google Scholar] [CrossRef]
- Sugimoto, C.; Fujisaki, K. Non-Transforming Theileria Parasites of Ruminants. In Theileria; Dobbelaere, D.A.E., McKeever, D.J., Eds.; World Class Parasites; Springer: Boston, MA, USA, 2002; Volume 3, pp. 93–106. [Google Scholar]
- Aparna, M.; Ravindran, R.; Vimalkumar, M.B.; Lakshmanan, B.; Rameshkumar, P.; Kumar, K.G.; Promod, K.; Ajithkumar, S.; Ravishankar, C.; Devada, K.; et al. Molecular characterization of Theileria orientalis causing fatal infection in crossbred adult bovines of South India. Parasitol. Int. 2011, 60, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Colunga-Salas, P.; Sánchez-Montes, S.; León-Paniagua, L.; Becker, I. Borrelia in neotropical bats: Detection of two new phylogenetic lineages. Ticks Tick-Borne Dis. 2021, 12, 101642. [Google Scholar] [CrossRef] [PubMed]
- Margos, G.; Vollmer, S.A.; Ogden, N.H.; Fish, D. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect. Genet. Evol. 2011, 11, 1545–1563. [Google Scholar] [CrossRef]
- Furuno, K.; Lee, K.; Itoh, Y.; Suzuki, K.; Yonemitsu, K.; Kuwata, R.; Shimoda, H.; Watarai, M.; Maeda, K.; Takano, A. Epidemiological study of relapsing fever borreliae detected in Haemaphysalis ticks and wild animals in the western part of Japan. PLoS ONE 2017, 12, e0174727. [Google Scholar] [CrossRef]
- Rodino, K.G.; Theel, E.S.; Pritt, B.S. Tick-Borne Diseases in the United States. Clin. Chem. 2020, 66, 537–548. [Google Scholar] [CrossRef]
- Belongia, E.A. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector-Borne Zoonotic Dis. 2002, 2, 265–273. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes Tick-Borne Pathogens: Ecological, Epidemiological, and Clinical Consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef]
- Cutler, S.J.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. Tick-borne diseases and co-infection: Current considerations. Ticks Tick-Borne Dis. 2021, 12, 101607. [Google Scholar] [CrossRef] [PubMed]
| Disease | Pathogen Name | Available Diagnostic Test | Geographical Area | Prevalence | References |
|---|---|---|---|---|---|
| Anaplasmosis | A. phagocytophilum * | Giemsa Staining Method, PCR | Great Britain | 22.9% | [29,81] |
| A. phagocytophilum ** | PCR | Norway | 1.0–13.5% | [32] | |
| A. phagocytophilum * | PCR | Sweden | 23.9% | [33] | |
| A. phagocytophilum * | PCR | France | 0.6% | [34] | |
| A. phagocytophilum ** | PCR | France | 1.75% | [34] | |
| A. phagocytophilum ** | PCR | Germany | 0.05% | [40] | |
| A. bovis * | Croatia | [30] | |||
| Anaplasma spp. * | ELISA | Belgium | 15.6% | [44] | |
| A. phagocytophilum * | IFAT | Belgium | 34.2% | [44] | |
| A. phagocytophilum ** | RT-PCR | Belgium | 0.5% | [44] | |
| A. phagocytophilum ** | PCR | Spain | 5.6% | [48] | |
| A. centrale ** | PCR | Spain | 1.4% | [48] | |
| A. marginale * | RT-PCR | Russia | 42% | [55] | |
| A. marginale ** | PCR | Portugal | 2.6% | [58] | |
| A. marginale ** | PCR | France | 2% | [45] | |
| A. marginale * | PCR | France | 6% | [45] | |
| Babesiosis | B. divergens * | Giemsa Staining Method, PCR | Great Britain | 57.1% | [29,81] |
| B. divergens * | PCR | Bosnia and Herzegovina | [30] | ||
| B. divergens * | Austria | [30] | |||
| B. divergens * | Germany | 13.0–21.0% | [30] | ||
| B. divergens * | PCR | Sweden | 53.5% | [33] | |
| B. major * | Germany | [30] | |||
| B. divergens * | Hungary | [30] | |||
| Babesia spp * | PCR | Poland | 10.4% | [30] | |
| B. major ** | PCR | Switzerland | [30] | ||
| B. occultans * | RT-PCR | Poland | 10.4% | [31] | |
| B. bigemina * | PCR | Turkey | 11.2% | [38] | |
| B. bovis * | iELISA | Portugal | 79% | [39] | |
| B. bovis * | PCR | Portugal | 71% | [39] | |
| B. bigemina * | iELISA | Portugal | 52% | [39] | |
| B. bigemina * | PCR | Portugal | 34% | [39] | |
| Babesia spp. * | IFAT | Belgium | 3.4% | [44] | |
| Babesia spp. ** | PCR | England and Wales | 0.38% | [47] | |
| B. divergens * | RT-PCR | Germany | 6.5% | [52] | |
| B. divergens ** | RT-PCR | Germany | 0.9% | [52] | |
| B. microti ** | PCR | Germany | 0.49% | [54] | |
| B. venatorum ** | PCR | Germany | 0.42% | [54] | |
| B. capreoli ** | PCR | Germany | 0.07% | [54] | |
| Theileriosis | T. orientalis * | Croatia | [30] | ||
| T. sergenti * | PCR | Serbia | 3.7% | [30] | |
| Theileria spp. * | PCR | Serbia | 3.7% | [30] | |
| T. buffeli * | PCR | Serbia | 3.7% | [30] | |
| T. orientalis * | Hungary | [30] | |||
| T. orientalis * | PCR | Turkey | 5.6% | [38] | |
| T. annulata * | PCR | Turkey | 18.9% | [38] | |
| T. annulata * | PCR | Spain | 22.4% | [56] | |
| T. annulata ** | PCR | Portugal | 0.37% | [58] | |
| T. annulata * | PCR | Portugal | 30% | [46] | |
| T. orientalis * | PCR | Hungary | [49] | ||
| T. annulata * | PCR | Spain | 22.4% | [56] | |
| Borreliosis | B. burgdorferi (s.l.) ** | PCR | Norway | 0.17–24.2% | [32] |
| Borrelia spp. * | ELISA | Belgium | 12.9% | [44] | |
| B. burgdorferi sensu lato ** | RT-PCR | Belgium | 13.8% | [44] | |
| B. afzelii ** | RT-PCR | Belgium | 65.7% | [44] | |
| B. garinii ** | RT-PCR | Belgium | 17.1% | [44] | |
| B. burgdorferi ** | PCR | France | [45] | ||
| B. miyamotoi ** | PCR | Germany | 0.25% | [40] | |
| B. afzelii ** | PCR | Germany | [40] |
| Parasitic/Bacterial Pathogen Agent | Vectors | References |
|---|---|---|
| B. burgdorferi sensu lato | I. ricinus, H. marginatum | [7,32,36,42,45,54] |
| B. miyamotoi | I. persulcatus, D. reticulatus | [40] |
| Anaplasma spp. | R. turanicus | [43] |
| A. phagocytophilum | I. ricinus, H. punctata, R. pusillus, D. marginatus, H. marginatum, and Rhipicephalus spp. | [7,9,32,34,42,44] |
| A. bovis | H. punctata | [44] |
| A. marginale | R. bursa, R. turanicus, H. excavatum | [58,82] |
| B. divergens, B. venatorum, B. divergens/capreoli, B. odocoilei-like | I. ricinus | [9,28,29,47] |
| B. venatorum | I. ricinus | [42] |
| Babesia spp. | R. turanicus/D. marginatus, H. lusitanicum | [50] |
| B. bovis | R. turanicus, R. bursa, H. excavatum, H. anatolicum | [82] |
| B. occultans | H. excavatum | [82] |
| Theileria spp. | R. turanicus/D. marginatus, H. lusitanicum | [43] |
| Th. annulata | R. bursa, R. turanicus, H. excavatum | [58,82] |
| Th. sergenti/buffeli/orientalis | R. annulatus | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, D.; Dreghiciu, I.C.; Oprescu, I.; Imre, M.; Florea, T.; Plesko, A.; Morariu, S.; Ilie, M.S. Cattle Zoonotic and Non-Zoonotic Tick-Borne Pathogens in Europe—A Retrospective Analysis of the Past 15 Years. Animals 2025, 15, 1408. https://doi.org/10.3390/ani15101408
Hoffman D, Dreghiciu IC, Oprescu I, Imre M, Florea T, Plesko A, Morariu S, Ilie MS. Cattle Zoonotic and Non-Zoonotic Tick-Borne Pathogens in Europe—A Retrospective Analysis of the Past 15 Years. Animals. 2025; 15(10):1408. https://doi.org/10.3390/ani15101408
Chicago/Turabian StyleHoffman, Diana, Ioan Cristian Dreghiciu, Ion Oprescu, Mirela Imre, Tiana Florea, Anamaria Plesko, Sorin Morariu, and Marius Stelian Ilie. 2025. "Cattle Zoonotic and Non-Zoonotic Tick-Borne Pathogens in Europe—A Retrospective Analysis of the Past 15 Years" Animals 15, no. 10: 1408. https://doi.org/10.3390/ani15101408
APA StyleHoffman, D., Dreghiciu, I. C., Oprescu, I., Imre, M., Florea, T., Plesko, A., Morariu, S., & Ilie, M. S. (2025). Cattle Zoonotic and Non-Zoonotic Tick-Borne Pathogens in Europe—A Retrospective Analysis of the Past 15 Years. Animals, 15(10), 1408. https://doi.org/10.3390/ani15101408









