Transcriptomic Profiling of Hypoxia-Adaptive Responses in Tibetan Goat Fibroblasts
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Ear Tissues from Tibetan Goat and Yunling Goat in Yunnan Province
2.2. Isolation, Culture and Identification of Goat Fibroblast Cells
2.3. Hypoxic and Normoxic Treatment of Cell Cultures
2.4. Sample Collection, RNA Extraction and RNA Sequencing
2.5. RNA-Seq Analysis
2.6. Machine Learning Analysis
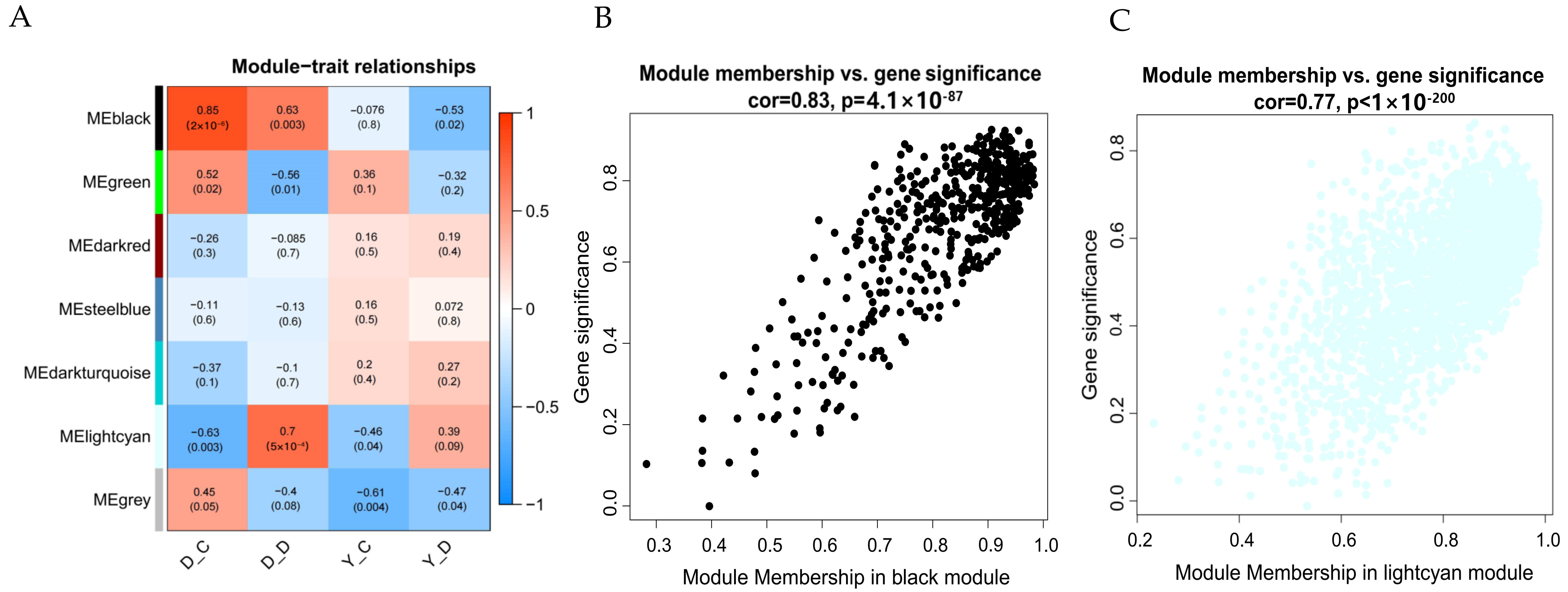
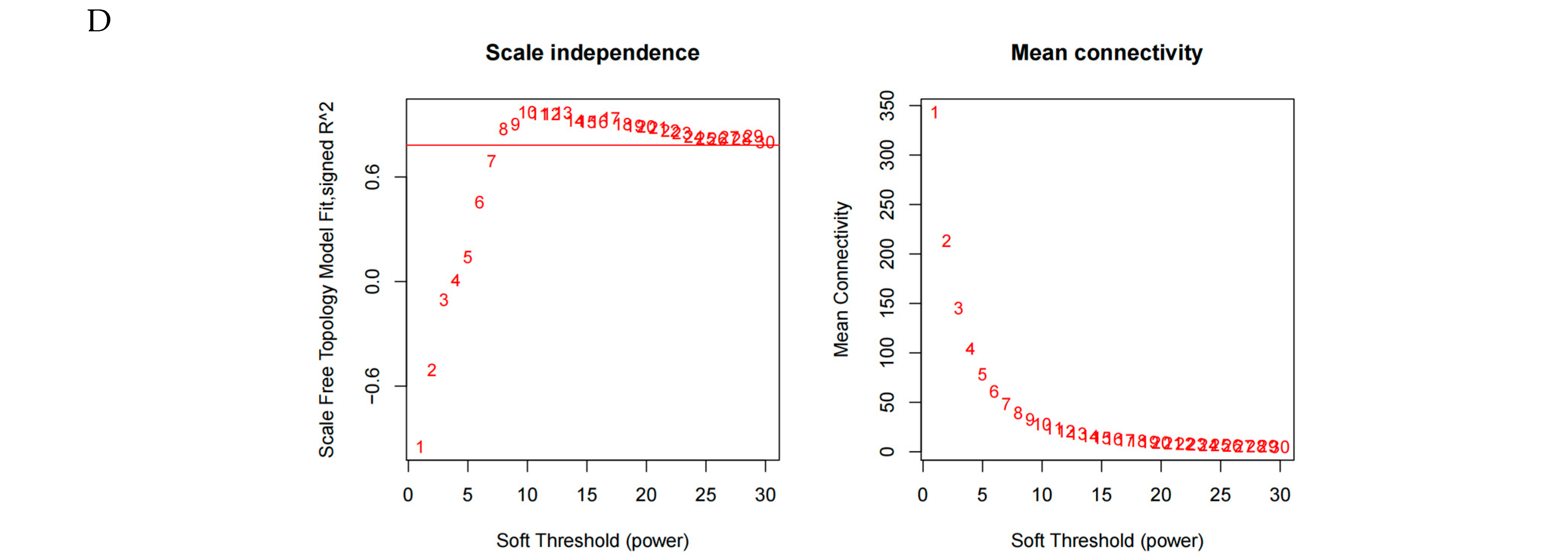
2.7. Weighted Gene Co-Expression Network Analysis (WGCNA)
2.8. Protein–Protein Interaction Network Analysis
2.9. Quantitative RT-PCR Analysis
3. Results
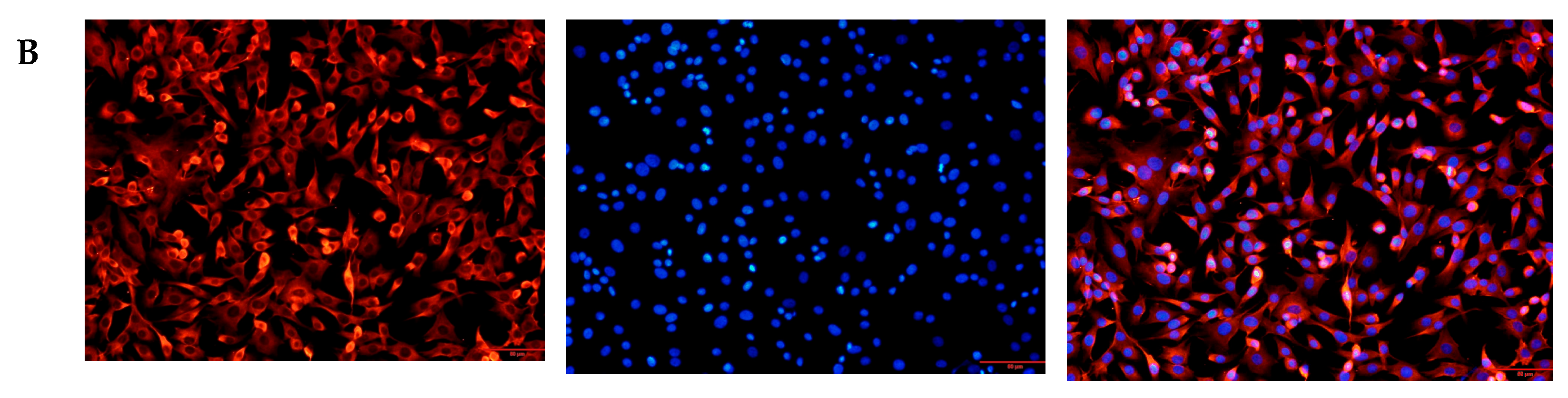
3.1. Cultivation and Identification of Goat Fibroblast Cells
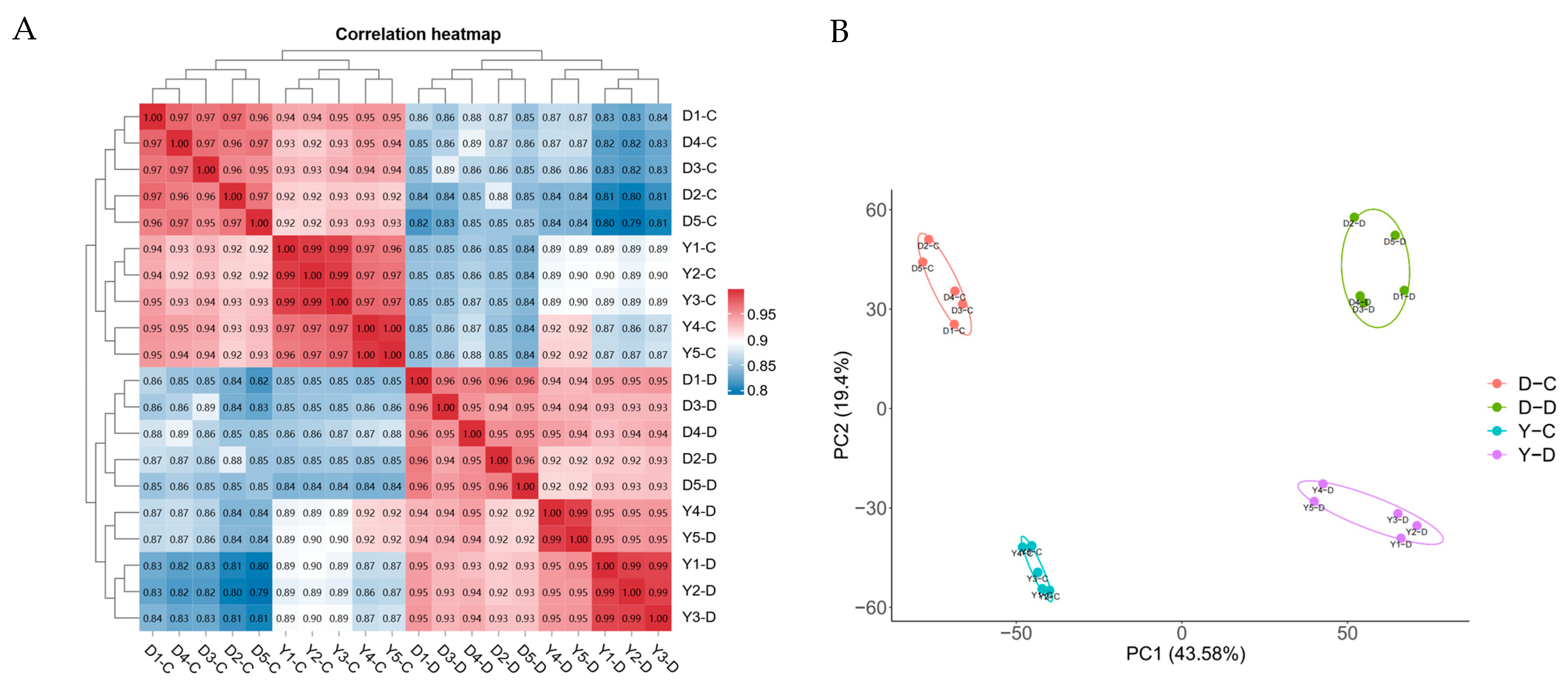
3.2. Summary of mRNA Data
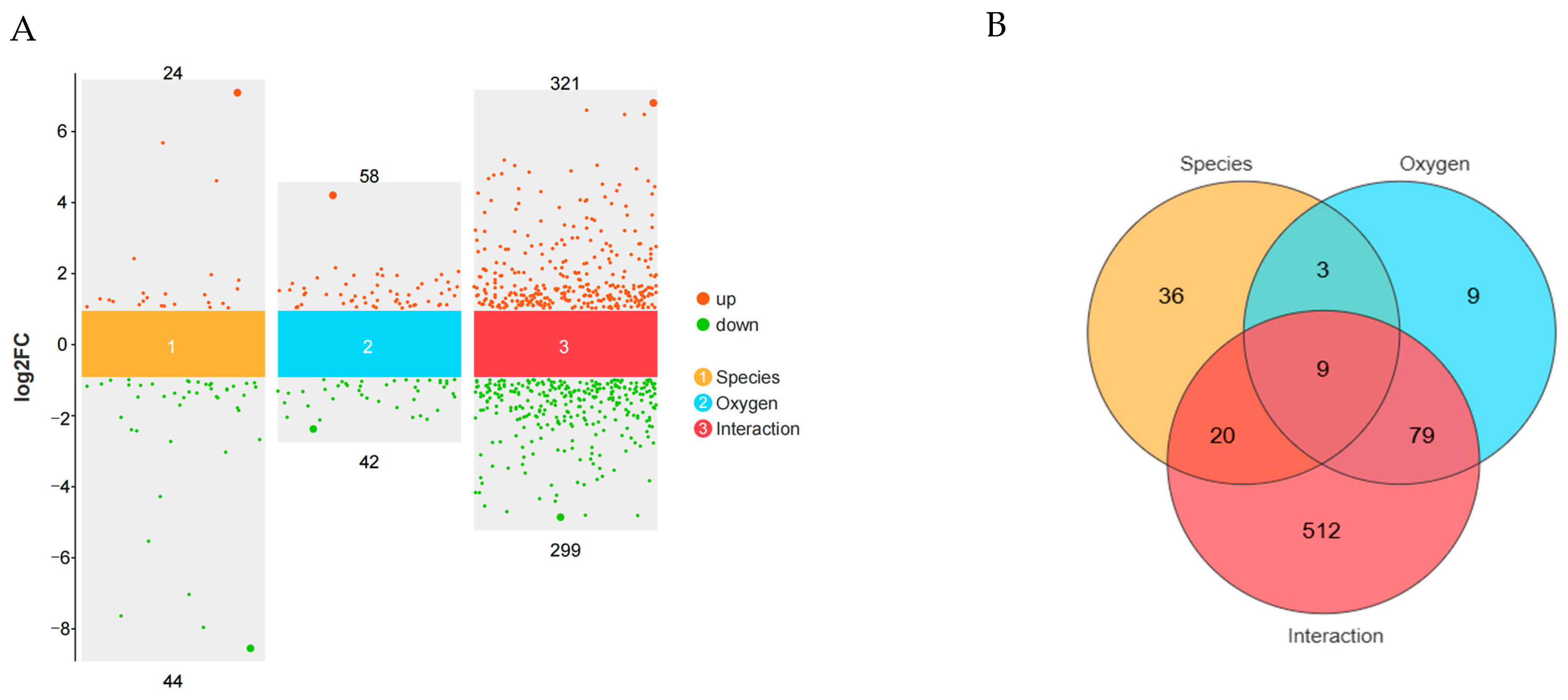
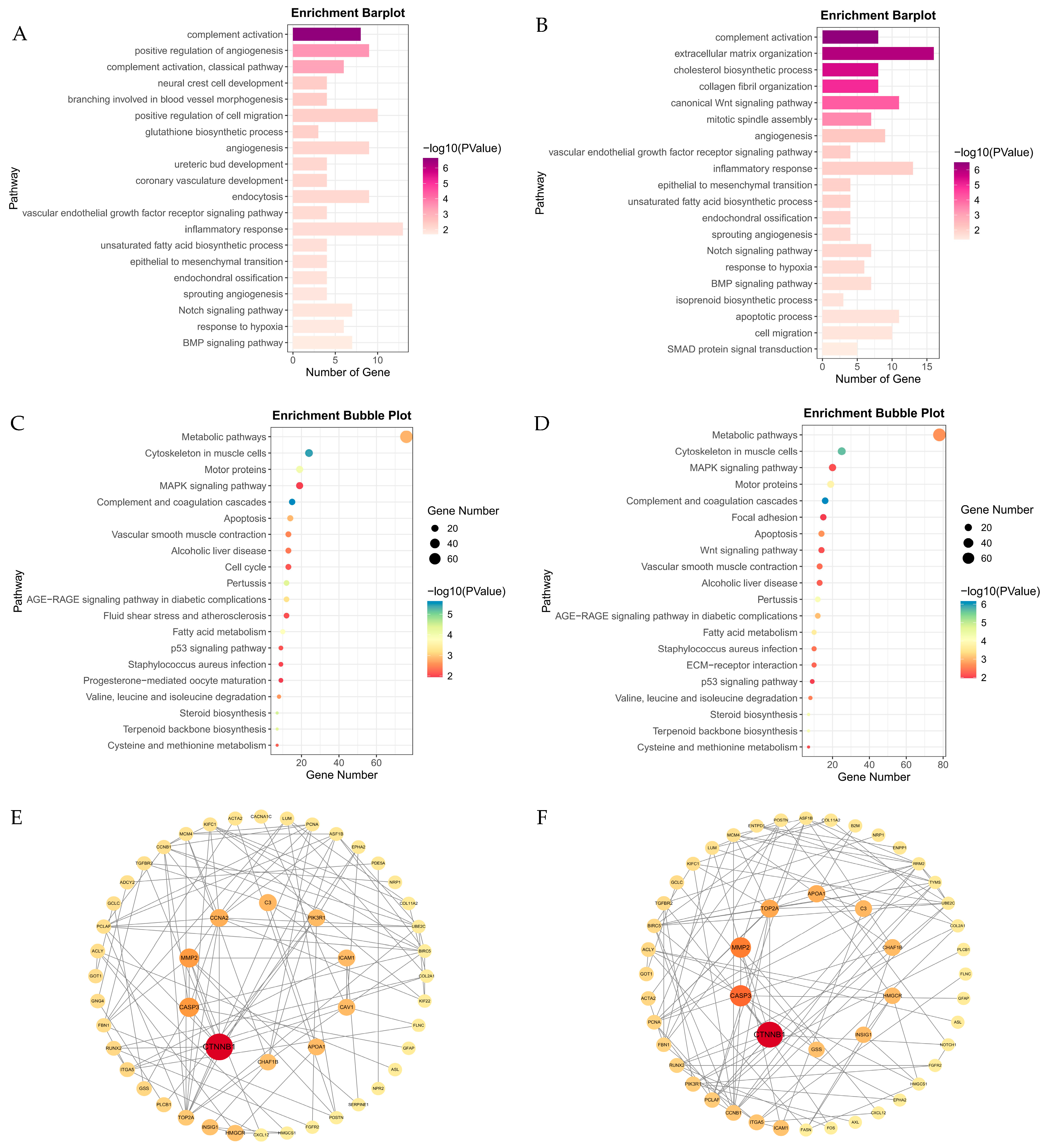
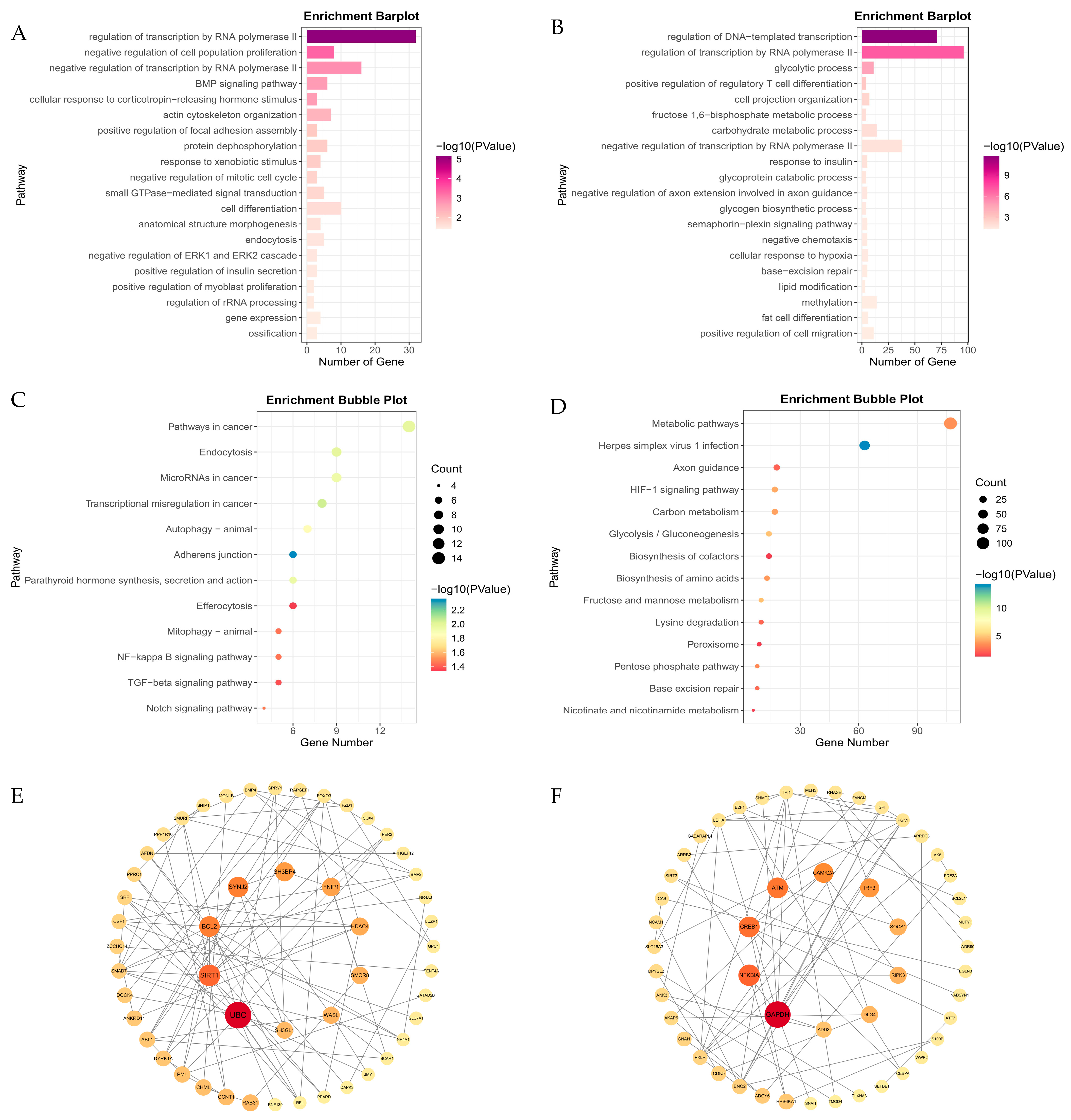
3.3. Analysis of Differentially Expressed Genes (DEGs) and Functional Enrichment
3.4. Key Transcription Genes During Hypoxia Stress Identified by Machine Learning
3.5. Key Transcription Genes During Hypoxia Stress Identified by WGCNA
3.6. RT-qPCR Validation of RNA-Seq Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Storz, J.F. Hemoglobin-oxygen affinity in high-altitude vertebrates: Is there evidence for an adaptive trend? J. Exp. Biol. 2016, 219, 3190–3203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Suo, L.; Wu, Y.; Chen, T.; Tulafu, H.; Lu, Q.; Liu, W.; Sammad, A.; Wu, C.; Fu, X. Stress adaptation in Tibetan cashmere goats is governed by inherent metabolic differences and manifested through variable cashmere phenotypes. Genomics 2024, 116, 110801. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, Y.; Chen, B.; Cai, Y.; Guo, J.; Leonard, A.S.; Kalds, P.; Zhou, S.; Zhang, J.; Zhou, P.; et al. Markhor-derived Introgression of a Genomic Region Encompassing PAPSS2 Confers High-altitude Adaptability in Tibetan Goats. Mol. Biol. Evol. 2022, 39, msac253. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tao, H.; Li, P.; Li, L.; Zhong, T.; Wang, L.; Ma, J.; Chen, X.; Song, T.; Zhang, H. Whole-genome sequencing reveals selection signatures associated with important traits in six goat breeds. Sci. Rep. 2018, 8, 10405. [Google Scholar] [CrossRef]
- Kumar, C.; Song, S.; Jiang, L.; He, X.; Zhao, Q.; Pu, Y.; Malhi, K.K.; Kamboh, A.A.; Ma, Y. Sequence Characterization of DSG3 Gene to Know Its Role in High-Altitude Hypoxia Adaptation in the Chinese Cashmere Goat. Front. Genet. 2018, 9, 553. [Google Scholar] [CrossRef]
- Jin, M.; Lu, J.; Fei, X.; Lu, Z.; Quan, K.; Liu, Y.; Chu, M.; Di, R.; Wei, C.; Wang, H. Selection Signatures Analysis Reveals Genes Associated with High-Altitude Adaptation in Tibetan Goats from Nagqu, Tibet. Animals 2020, 10, 1599. [Google Scholar] [CrossRef]
- Song, S.; Yao, N.; Yang, M.; Liu, X.; Dong, K.; Zhao, Q.; Pu, Y.; He, X.; Guan, W.; Yang, N.; et al. Exome sequencing reveals genetic differentiation due to high-altitude adaptation in the Tibetan cashmere goat (Capra hircus). BMC Genom. 2016, 17, 122. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Z.; Li, Y.; Chen, S.; Chen, W.; Ding, H.; Tan, Z.; Ma, Z.; Qiao, Z. Expression and distribution of HIF-1alpha, HIF-2alpha, VEGF, VEGFR-2 and HIMF in the kidneys of Tibetan sheep, plain sheep and goat. Folia Morphol. 2020, 79, 748–755. [Google Scholar] [CrossRef]
- Mordhorst, B.R.; Murphy, S.L.; Schauflinger, M.; Rojas Salazar, S.; Ji, T.; Behura, S.K.; Wells, K.D.; Green, J.A.; Prather, R.S. Porcine Fetal-Derived Fibroblasts Alter Gene Expression and Mitochondria to Compensate for Hypoxic Stress During Culture. Cell Reprogram 2018, 20, 225–235. [Google Scholar] [CrossRef]
- Ye, H.; Zheng, Y.; Ma, W.; Ke, D.; Jin, X.; Liu, S.; Wang, D. Hypoxia down-regulates secretion of MMP-2, MMP-9 in porcine pulmonary artery endothelial and smooth muscle cells and the role of HIF-1. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005, 25, 382–384. [Google Scholar]
- Tiwari, M.; Sodhi, M.; Sharma, M.; Sharma, V.; Mukesh, M. Hypoxia related genes modulate in similar fashion in skin fibroblast cells of yak (Bos grunniens) adapted to high altitude and native cows (Bos indicus) adapted to tropical climate during hypoxia stress. Int. J. Biometeorol. 2024, 68, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Yang, C.; Delcher, C.; Shenkman, E.; Ranka, S. Machine learning approaches for predicting high cost high need patient expenditures in health care. Biomed. Eng. Online 2018, 17, 131. [Google Scholar] [CrossRef]
- Daneshvar, A.; Golalizadeh, M. Quantile regression shrinkage and selection via the Lqsso. J. Biopharm. Stat. 2024, 34, 297–322. [Google Scholar] [CrossRef]
- Ellis, K.; Kerr, J.; Godbole, S.; Lanckriet, G.; Wing, D.; Marshall, S. A random forest classifier for the prediction of energy expenditure and type of physical activity from wrist and hip accelerometers. Physiol. Meas. 2014, 35, 2191–2203. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Cheviron, Z.A.; Brumfield, R.T. Genomic insights into adaptation to high-altitude environments. Heredity 2012, 108, 354–361. [Google Scholar] [CrossRef]
- Storz, J.F.; Scott, G.R.; Cheviron, Z.A. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 2010, 213, 4125–4136. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xing, Y.; Zhang, J.; He, M.; Dong, J.; Chen, S.; Wu, H.; Huang, H.Y.; Chou, C.H.; Bai, L.; et al. MED1 Regulates BMP/TGF-beta in Endothelium: Implication for Pulmonary Hypertension. Circ. Res. 2022, 131, 828–841. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, G.; Ma, X.; Xiao, J.; Yu, G.; Yang, C.; Xu, N.; Zhang, B.; Zhou, J.; Ye, Z.; et al. Attenuation of TGFBR2 expression and tumour progression in prostate cancer involve diverse hypoxia-regulated pathways. J. Exp. Clin. Cancer Res. 2018, 37, 89. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Huang, L.; Fang, C.; Yao, L.; Liu, F.; Jin, T. ROS-mediated ITGB5 promotes tongue squamous cell carcinoma metastasis through epithelial mesenchymal transition and cell adhesion signal pathway. J. Cancer Res. Clin. Oncol. 2024, 150, 398. [Google Scholar] [CrossRef]
- Leifheit-Nestler, M.; Conrad, G.; Heida, N.M.; Limbourg, A.; Limbourg, F.P.; Seidler, T.; Schroeter, M.R.; Hasenfuss, G.; Konstantinides, S.; Schafer, K. Overexpression of integrin beta 5 enhances the paracrine properties of circulating angiogenic cells via Src kinase-mediated activation of STAT3. Arter. Thromb. Vasc. Biol. 2010, 30, 1398–1406. [Google Scholar] [CrossRef]
- Tang, K.; Toyozumi, T.; Murakami, K.; Sakata, H.; Kano, M.; Endo, S.; Matsumoto, Y.; Suito, H.; Takahashi, M.; Sekino, N.; et al. HIF-1alpha stimulates the progression of oesophageal squamous cell carcinoma by activating the Wnt/beta-catenin signalling pathway. Br. J. Cancer 2022, 127, 474–487. [Google Scholar] [CrossRef]
- Hu, J.; Hameed, M.R.; Agaram, N.P.; Whiting, K.A.; Qin, L.X.; Villano, A.M.; O’Connor, R.B.; Rozenberg, J.M.; Cohen, S.; Prendergast, K.; et al. PDGFRbeta Signaling Cooperates with beta-Catenin to Modulate c-Abl and Biologic Behavior of Desmoid-Type Fibromatosis. Clin. Cancer Res. 2024, 30, 450–461. [Google Scholar] [CrossRef]
- Zhang, J.; Long, K.; Wang, J.; Zhang, J.; Jin, L.; Tang, Q.; Li, X.; Ma, J.; Li, M.; Jiang, A. Yak miR-2285o-3p attenuates hypoxia-induced apoptosis by targeting caspase-3. Anim. Genet. 2022, 53, 49–57. [Google Scholar] [CrossRef]
- Park, C.H.; Park, J.Y.; Cho, W.G. Chemical Hypoxia Induces Pyroptosis in Neuronal Cells by Caspase-Dependent Gasdermin Activation. Int. J. Mol. Sci. 2024, 25, 2185. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wei, R.Q.; Zhang, W.M.; Shi, C.Y.; Yang, R.; Jin, M.; Piao, C. Crocin’s role in modulating MMP2/TIMP1 and mitigating hypoxia-induced pulmonary hypertension in mice. Sci. Rep. 2024, 14, 12716. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and adipocyte physiology: Implications for adipose tissue dysfunction in obesity. Annu. Rev. Nutr. 2014, 34, 207–236. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Qian, Y.; Zhang, H.; Guo, S.; Sunagawa, M.; Hisamitsu, T.; Liu, Y. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-kappaB/HIF-1alpha pathway. Mol. Immunol. 2013, 53, 227–236. [Google Scholar] [CrossRef]
- Cai, J.; Pardali, E.; Sanchez-Duffhues, G.; ten Dijke, P. BMP signaling in vascular diseases. FEBS Lett. 2012, 586, 1993–2002. [Google Scholar] [CrossRef]
- Rezzola, S.; Di Somma, M.; Corsini, M.; Leali, D.; Ravelli, C.; Polli, V.A.B.; Grillo, E.; Presta, M.; Mitola, S. VEGFR2 activation mediates the pro-angiogenic activity of BMP4. Angiogenesis 2019, 22, 521–533. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.; Liang, Y.; Li, R.; Zhang, L.; Chen, S.; Yang, K.; Ding, H.; Tan, X.; Zhang, Q.; et al. Study of Transcriptomic Analysis of Yak (Bos grunniens) and Cattle (Bos taurus) Pulmonary Artery Smooth Muscle Cells Under Oxygen Concentration Gradients and Differences in Their Lung Histology and Expression of Pyruvate Dehydrogenase Kinase 1-Related Factors. Animals 2023, 13, 3450. [Google Scholar] [CrossRef]
- Li, C.; Chen, B.; Langda, S.; Pu, P.; Zhu, X.; Zhou, S.; Kalds, P.; Zhang, K.; Bhati, M.; Leonard, A.; et al. Multi-omic Analyses Shed Light on The Genetic Control of High-Altitude Adaptation in Sheep. Genom. Proteom. Bioinform. 2024, 22, qzae030. [Google Scholar] [CrossRef]
- Zhong, C.; Li, S.; Li, J.; Li, F.; Ran, M.; Qiu, L.; Li, D.; Zhu, Q.; Wang, Y.; Yin, H.; et al. Polymorphisms in the Egl nine homolog 3 (EGLN3) and Peroxisome proliferator activated receptor-alpha (PPARalpha) genes and their correlation with hypoxia adaptation in Tibetan chickens. PLoS ONE 2018, 13, e0194156. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Gu, Y.; Zhou, X.; Jin, L.; Guan, J.; Liu, R.; Li, J.; Long, K.; Tian, S.; Che, T.; et al. Comparative transcriptomics of 5 high-altitude vertebrates and their low-altitude relatives. Gigascience 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Lin, F. Molecular regulation and function of FoxO3 in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Murphy, K.; Murphy, Z.; Getman, M.; Rahman, N.; Nakamura, Y.; Blanc, L.; Gallagher, P.G.; Palis, J.; Mohandas, N.; et al. HEXIM1 is an essential transcription regulator during human erythropoiesis. Blood 2023, 142, 2198–2215. [Google Scholar] [CrossRef] [PubMed]
- Piqueras, L.; Reynolds, A.R.; Hodivala-Dilke, K.M.; Alfranca, A.; Redondo, J.M.; Hatae, T.; Tanabe, T.; Warner, T.D.; Bishop-Bailey, D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arter. Thromb. Vasc. Biol. 2007, 27, 63–69. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Smith, L.A.; Lu, T.; Joyner, M.J.; Katusic, Z.S. Activation of peroxisome proliferator-activated receptor-delta enhances regenerative capacity of human endothelial progenitor cells by stimulating biosynthesis of tetrahydrobiopterin. Hypertension 2011, 58, 287–294. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, X.; Lee, S.; Hong, H.; Cao, X.; Lau, C.W.; Liu, B.; Chawla, A.; Ma, R.C.W.; Huang, Y.; et al. Endothelial PPARdelta facilitates the post-ischemic vascular repair through interaction with HIF1alpha. Theranostics 2022, 12, 1855–1869. [Google Scholar] [CrossRef]
- Bouletreau, P.J.; Warren, S.M.; Spector, J.A.; Peled, Z.M.; Gerrets, R.P.; Greenwald, J.A.; Longaker, M.T. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: Implications for fracture healing. Plast. Reconstr. Surg. 2002, 109, 2384–2397. [Google Scholar] [CrossRef]
- Li, S.; Cai, X.; Guo, J.; Li, X.; Li, W.; Liu, Y.; Qi, M. Cell communication and relevant signaling pathways in osteogenesis-angiogenesis coupling. Bone Res. 2025, 13, 45. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Y.; Jiang, X.; Peng, M.; Liu, Y.; Mo, Y.; Ren, D.; Hua, Y.; Yu, B.; Zhou, Y.; et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol. Cancer 2021, 20, 7. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, X.; Wen, L.; Wang, J.; Li, Z.; Zhang, Y.; Cheng, J.; Kan, R.; Zhang, W.; Shen, Y.; et al. USP9X integrates TGF-beta and hypoxia signalings to promote ovarian cancer chemoresistance via HIF-2alpha-maintained stemness. Cell Death Dis. 2025, 16, 312. [Google Scholar] [CrossRef]
- Fong, G.H.; Takeda, K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 2008, 15, 635–641. [Google Scholar] [CrossRef]
- Wong, B.W.; Kuchnio, A.; Bruning, U.; Carmeliet, P. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem. Sci. 2013, 38, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.D.; Ding, X.D.; Wang, S.; Wojcik, J.M.; Zhang, Y.; Tokarska, M.; Li, Y.; Wang, M.S.; Faruque, O.; Nielsen, R.; et al. Pervasive introgression facilitated domestication and adaptation in the Bos species complex. Nat. Ecol. Evol. 2018, 2, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wang, F.; Cheng, H.; Han, J.; Dang, R.; Xia, X.; Wang, H.; Zhong, J.; Lenstra, J.A.; Zhang, H.; et al. Recent selection and introgression facilitated high-altitude adaptation in cattle. Sci. Bull. 2024, 69, 3415–3424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, C.; Yang, T.; Sha, Y.; Cai, Y.; Wang, X.; Yang, Q.; Liu, C.; Wang, B.; Zhao, S. Vascular characteristics and expression of hypoxia genes in Tibetan pigs’ hearts. Vet. Med. Sci. 2022, 8, 177–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Gou, W.; Zhang, Y.; Zhang, H.; Wu, C. Insights into hypoxic adaptation in Tibetan chicken embryos from comparative proteomics. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 31, 100602. [Google Scholar] [CrossRef]
- Kim, J.H. Commentary on “Remission in pediatric Graves’ disease treated with antithyroid drug and the risk factors associated with relapse”. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 245–246. [Google Scholar] [CrossRef]
- Li, C.; Lv, J.; Wumaier, G.; Zhao, Y.; Dong, L.; Zeng, Y.; Zhu, N.; Zhang, X.; Wang, J.; Xia, J.; et al. NDRG1 promotes endothelial dysfunction and hypoxia-induced pulmonary hypertension by targeting TAF15. Precis. Clin. Med. 2023, 6, pbad024. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef]
- Simonson, T.S.; Yang, Y.; Huff, C.D.; Yun, H.; Qin, G.; Witherspoon, D.J.; Bai, Z.; Lorenzo, F.R.; Xing, J.; Jorde, L.B.; et al. Genetic evidence for high-altitude adaptation in Tibet. Science 2010, 329, 72–75. [Google Scholar] [CrossRef]
- He, H.; Zhang, H.; Pan, Y.; Zhang, T.; Yang, S.; Liu, M.; Robert, N.; Wang, J.; Zhao, T.; Zhao, L.; et al. Low oxygen concentration improves yak oocyte maturation and inhibits apoptosis through HIF-1 and VEGF. Reprod. Domest. Anim. 2022, 57, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qiangba, Y.; Shang, P.; Lu, Y.; Yang, Y.; Wang, Z.; Zhang, H. Gene expression of vascular endothelial growth factor A and hypoxic adaptation in Tibetan pig. J. Anim. Sci. Biotechnol. 2016, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, B.; An, Z.; Wang, Z.; Li, Y.; Wei, L.; Wei, D. Evolutionary analysis of TSP-1 gene in Plateau zokor (MyospalaxBaileyi) and its expression pattern under hypoxia. Cell Mol. Biol. 2019, 65, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 347–353. [Google Scholar] [CrossRef]
- Wang, M.S.; Li, Y.; Peng, M.S.; Zhong, L.; Wang, Z.J.; Li, Q.Y.; Tu, X.L.; Dong, Y.; Zhu, C.L.; Wang, L.; et al. Genomic Analyses Reveal Potential Independent Adaptation to High Altitude in Tibetan Chickens. Mol. Biol. Evol. 2015, 32, 1880–1889. [Google Scholar] [CrossRef]
- Wei, C.; Wang, H.; Liu, G.; Zhao, F.; Kijas, J.W.; Ma, Y.; Lu, J.; Zhang, L.; Cao, J.; Wu, M.; et al. Genome-wide analysis reveals adaptation to high altitudes in Tibetan sheep. Sci. Rep. 2016, 6, 26770. [Google Scholar] [CrossRef]
- Finley, L.W.; Haigis, M.C. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Res. Rev. 2009, 8, 173–188. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, Q.; He, Y.; Yang, L.; Zhang, X.; Shi, P.; Yang, L.; Liu, Z.; Zhang, F.; Liu, F.; et al. The Transcriptomic Landscape of Yaks Reveals Molecular Pathways for High Altitude Adaptation. Genome Biol. Evol. 2019, 11, 72–85. [Google Scholar] [CrossRef]
- Bao, G.; Li, S.; Zhao, F.; Wang, J.; Liu, X.; Hu, J.; Shi, B.; Wen, Y.; Zhao, L.; Luo, Y. Comprehensive Transcriptome Analysis Reveals the Role of lncRNA in Fatty Acid Metabolism in the Longissimus Thoracis Muscle of Tibetan Sheep at Different Ages. Front. Nutr. 2022, 9, 847077. [Google Scholar] [CrossRef]



| Coefficient | Comparison | Interpretation |
|---|---|---|
| Intercept | (D-D + D-C + Y-D + Y-C)/4 | Grand mean |
| Strain1 | (D-D + D-C − Y-D − Y-C)/4 | Species effect (Breed effect) |
| Treatment1 | (D-D + D-C + Y-D + Y-C)/4 | Environment effect (Oxygen effect) |
| Strain1:Treatment1 | (D-D − D-C − Y-D + Y-C)/4 | Interaction effect |
| Gene | Forward Primer (5’ → 3’) | Reverse Primer (5’ → 3’) |
|---|---|---|
| CTNNB1 | GGTGTGGGTAATAGAAC | AGAAAAACAGAAAAGGT |
| CASP3 | CACCTCTAAATCTAACC | AGTCTCAACTACCCAAC |
| MMP2 | ATGCCATCCCTGATAACC | CTTCCGAACTTCACGCTC |
| TGFBR2 | GTTTGTTTTCTCCTTAT | ATTCACATTCCTATTTT |
| ITGB5 | CCACCTGCTGCCTCTCA | ACTCGTTGGCCTCGTTC |
| RSPO1 | CACACCCTCTCTGTCCCC | GCCTCTGTCTCTTCCCCT |
| β-actin | AGATGTGGATCAGCAAGCAG | CCAATCTCATCTCGTTTTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Zhu, L.; Basang, Z.; Zhao, Y.; Li, S.; Kong, X.; Gou, X. Transcriptomic Profiling of Hypoxia-Adaptive Responses in Tibetan Goat Fibroblasts. Animals 2025, 15, 1407. https://doi.org/10.3390/ani15101407
Tang L, Zhu L, Basang Z, Zhao Y, Li S, Kong X, Gou X. Transcriptomic Profiling of Hypoxia-Adaptive Responses in Tibetan Goat Fibroblasts. Animals. 2025; 15(10):1407. https://doi.org/10.3390/ani15101407
Chicago/Turabian StyleTang, Lin, Li Zhu, Zhuzha Basang, Yunong Zhao, Shanshan Li, Xiaoyan Kong, and Xiao Gou. 2025. "Transcriptomic Profiling of Hypoxia-Adaptive Responses in Tibetan Goat Fibroblasts" Animals 15, no. 10: 1407. https://doi.org/10.3390/ani15101407
APA StyleTang, L., Zhu, L., Basang, Z., Zhao, Y., Li, S., Kong, X., & Gou, X. (2025). Transcriptomic Profiling of Hypoxia-Adaptive Responses in Tibetan Goat Fibroblasts. Animals, 15(10), 1407. https://doi.org/10.3390/ani15101407





