1. Introduction
Fishmeal (FM) is an important dietary ingredient and has many advantages due to its high protein content, balanced amino acid composition, good palatability, and high digestibility [
1]. However, due to its decreased production worldwide, the price of FM has risen sharply in recent years, which has affected the sustainable and rapid development of modern aquaculture [
2]. Therefore, as a research hotspot, the search for suitable FM substitutes has focused on animal and plant protein resources in recent years [
1,
3,
4]. Compared with animal protein resources with higher market prices, many plant protein resources are more widely available, cheaper, and have high nutritional value, with high contents of protein, lipids, and vitamins as nonfood protein resources [
2]. A number of research studies have been carried out on FM substitution with different plant protein resources, including cottonseed meal [
5], fermented soybean meal [
6], and peanut meal [
7]. These studies have shown that appropriate levels of plant protein resources replacing FM in the feed did not affect normal growth in aquatic animals such as golden pompano (
Trachinotusovatus) [
5], largemouth bass (
Micropterus salmoides) [
6], and hybrid grouper (
Epinephelus fuscoguttatus♀ ×
Epinephelus lanceolatus♂) [
7].
As an important plant protein with a wide range of sources and a low price, cottonseed meal is the main by-product after cottonseed processing. It is rich in protein, and it is an ideal plant protein source [
8]. Meanwhile, cottonseed meal contains antinutritional factors such as free gossypol and tannin, which can adversely influence the health and growth performance of animals [
9,
10,
11]. In order to reduce these adverse factors, a low-temperature and two-step solvent-extraction process has been developed and used to produce high-quality degossypolled cottonseed protein (DCP) that removes free gossypol, aflatoxins, and water-soluble antinutritional factors and protects the quality of crude protein and amino acids [
12,
13]. Processing is mainly used to reduce the content of water-soluble non-starch polysaccharides, oligo-oligosaccharides, and antinutritional factors (cotton-phenol and tannins), etc. [
14]. Recently, many studies have been conducted on the application of cottonseed and its products in aquatic feed, such as rainbow trout (
Oncorhynchus mykiss) [
12], juvenile golden pompano (
Trachinotus ovatus) [
15], pearl gentian grouper (
Epinephelus fuscoguttatus♀ × ♂
Epinephelus lanceolatu♂) [
16], and Japanese seabass (
Lateolabrax japonicas) [
17]. However, these studies were mainly focused on growth, body composition, hematological indices, antioxidant capacity, and innate immunity; there was little comprehensive information that detailed the impacts of FM’s replacement with DCP in the freshwater carnivorous fish species comprising the traditional “Four Domesticated Fish” in China.
Black carp (
Mylopharyngodon piceus), a cyprinid fish, is one of four major fish species and is a popular fish in China [
18]. According to the China Fishery Statistical Yearbook, the production of black carp reached about 0.80 million tons in 2023, an increase of 6.96% compared with the previous year. Recent studies primarily concentrated on the requirements of carbohydrates [
19], selenium yeast [
20], leucine [
21], different vitamins [
22,
23], and protein replacement [
24]. Previously, our team investigated the effect of replacing FM with blood meal on black carp, a study that ensured the methodological continuity of this experiment [
24]. Currently, little research has examined replacing FM with DCP in black carp feeding. Based on these ideas, this experiment looked at growth performance, serum biochemistry, antioxidant capability, inflammatory responses, and muscle quality and analyzed them from the perspective of genes. It aimed to determine the appropriate DCP replacement proportion for FM and provide theoretical support for the formulation of low-FM feed for black carp.
2. Materials and Methods
2.1. Experiment with Fish Diets
FM, DCP, soybean meal, and corn protein meal were used as protein sources; soy lecithin, rapeseed oil, and fish oil were used as lipid sources; and tapioca flour was used as a carbohydrate source. Next, 0%, 10%, 20%, 30%, 40%, and 50% substitution amounts for FM’s replacement with DCP (DCP0, DCP10, DCP20, DCP30, DCP40, and DCP50), respectively, were configured into six kinds of nitrogen-equivalent and energy-equivalent sinking experiment feeds. DCP was commercially sourced and used the low-temperature and two-step solvent-extraction process, which removed free gossypol, aflatoxins, and water-soluble antinutritional factors. The free gossypol content in DCP was about 150 mg/kg, according to the manufacturer’s detection results. Raw materials underwent sequential processing consisting of crushing, sieving through a 60-mesh screen, precise weighing, and homogeneous mixing. The components with small quantities were mixed using step-by-step expansion processing to produce pellet feeds 2 mm in diameter, which were dried at 35℃ in a hot-air-circulating oven and stored for spare use. The formulations of test feeds for each experimental group and the nutrient compositions are shown in
Table 1 and
Table 2.
2.2. Experimental Procedure
This experiment was in line with the national guidelines regarding the feeding and use of vertebrate animals. It was approved by the Ethical Committee of Huzhou University, located in Huzhou, China. The approval ID is HUZJ-DW-2023-139, and the date of approval was 6 March 2023.
The black carp used in this experiment were purchased from local farmers in Huzhou, Zhejiang, and were placed into a holding tank for a fortnight for domestication and fed with basic diets. After this, 540 black carp—uniform individuals, healthy and disease-free, and with an average body weight of 6.80 ± 0.50 g—were picked and randomly placed in 18 indoor water tanks (500 L). Six groups were set up in this experiment, and three replicates were set up in each group, with 30 fish in each replicate. In order to reduce potential tank effects, all tanks were maintained under identical environmental conditions throughout the experiment. The daily feeding was timed at 08:30 and 17:30. The daily feeding rate was about 3% of the body weight, which was adjusted appropriately according to the feeding situation of the fish and the growth of the fish species. The residual bait was removed after 1 h of baiting, and the daily water exchange was about 1/4–1/3 of the water tanks. During the experimental period, the water temperature was 27.0 ± 0.5 °C, the oxygen was continuously filled for 24 h with the dissolved oxygen above 5.8 mg/L, and the culture cycle was 60 days. The mortality rate was checked and recorded daily to calculate the survival rate.
2.3. Sample Collection and Measurement of Growth Performance
At the end of the feeding experiment, all fish in each tank were collected and anesthetized using 300 mg/L of sodium trialkyl methyl-anesthesia-sulfonate (Sigma, St. Louis, MO, USA). The total weight, total length, and quantity of each fish were recorded to evaluate growth performance. Then, using the method described by Wu et al. [
21], serum, blood, liver, intestinal, and muscle samples were collected. Fifteen fish were randomly picked from each tank, then blood was collected from the tail vein of these fifteen fish and stored at −80 °C. After collecting and processing, these 15 fish were rapidly dissected on ice, and the visceral mass was dissected and weighed to record their weights. After rapid separation from the intestines, the livers of these 15 fish in each tank were weighed, respectively. The livers, intestines, and muscles were rapidly frozen in liquid nitrogen and then transferred to a −80 °C refrigerator for storage. After sampling, three fish in each tank were selected and stored at −20 °C for the next detection of composition.
2.4. Measurement of Fish and Feed Composition
The moisture of the test feeds was determined by drying at 105 °C, while the moisture of the fish body and dorsal muscle was determined by freeze-drying (Alpha2-4 LSC Basic, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany); crude protein, crude lipid, and ash amounts were determined using Dumas automatic rapid nitrogen fixation (Rapid N exceed, Elementar Analysensysteme GmbH, Frankfurt, Germany), Soxhlet extraction, and Marfo oven burn (550 °C), respectively.
2.5. Measurement of Serum Biochemical Parameters
High-density lipoprotein cholesterol (HDL-C), albumin (ALB), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), total bile acids (TBAs), and the activities of alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), glucose (GLU), total cholesterol (TC), and glutamic oxaloacetic acid (AST) were measured with the help of Ningbo Prebis Biotec Co. (Ningbo, China) and detected using kits from Ningbo Prebon Biotechnology Co. (Ningbo, China) Insulin (INS). Insulin growth factor (IGF-1 and IGF-2) and 5-hydroxytryptamine (5-HT) were detected using kits from Shanghai Hengyuan Biotechnology Co. (Shanghai, China). All the analyses contained at least 3 replicates.
2.6. Measurement of Physiological and Biochemical Indexes
Lipase (LPS), amylase (α-AMS), trypsin (TRY), and chymotrypsin (CYT) were determined with the help of Jiancheng Biotech (Nanjing, China). The procedure was performed according to the provided instructions. Total antioxidant capacity (T-AOC), glutathione-S-transferase (GST), glutathione peroxidase (GPx), glutathione reductase (GR), catalase (CAT), hydrogen peroxide (H2O2), superoxide dismutase (T-SOD), reduced glutathione (GSH), and malondialdehyde (MDA) levels in the liver and intestine were measured using kits purchased from Shanghai Hengyuan Biotech (Shanghai, China). Immunoglobulin M (IgM), lysozyme (LZM), and alkaline phosphatase (ALP) kits were purchased from Jiancheng Biotech (Nanjing, China). All the analyses contained at least 3 replicates.
2.7. Measurement of Relative Gene Expression
We used Trizol reagent (Invitrogen, Carlsbad, CA, USA) to extract total RNA in liver tissue. The RNA concentration and integrity were evaluated via spectrophotometry and electrophoresis, following protocols described by Song et al. [
25]. The experiment employed specific primers targeting molecules involved in digestion, antioxidant systems, immune responses, lipid metabolism, and their respective signaling pathways (
Table 3). All primers were synthesized by Biosune Co. (Shanghai, China). β-actin served as the internal reference gene. Quantitative PCR (qPCR) analyses were conducted according to methodologies established by Wu et al. [
25] and Zhang et al. [
20]. All the analyses contained at least 3 replicates.
2.8. Data Processing and Statistical Analysis
The experimental data were expressed as the mean ± standard deviation (mean ± SD). Prior to statistical analysis, the homogeneity of variances was assessed using Levene’s test. The collected data were analyzed using one-way ANOVA in SPSS 26.0. When significant differences were found, multiple comparisons were performed using Tukey’s post hoc test, with a significance level of p < 0.05. Tukey’s test was chosen because it is well-suited for comparing all pairwise group differences while controlling for Type I error.
4. Discussion
Growth performance is a crucial indicator for evaluating the effectiveness of substituting FM with plant protein sources [
26]. In the present study, although WG and SGR increased in the DCP30 and DC40 groups compared with the DCP0 group, there were no significant variations in WG and SGR among these six DCP groups; similar results were also shown in juvenile golden pompano [
10], rainbow trout [
12], and hybrid grouper [
7]. Generally, there are tight relationships among normal growth, metabolic ability, and digestive enzyme activities in animals and fish species [
27,
28]. Greater digestive enzyme activity could improve growth performance by promoting the utilization of exogenous nutrients in fish [
27,
28]. In our results, higher TRY and LPS activities were also shown in the DCP30 and DCP40 groups compared with the DCP0 group, which is consistent with another study on South American white shrimp [
29]. Combined with these findings, this study indicated that a suitable FM substitution level with DCP could maintain or increase normal growth by modulating digestive enzyme activities in black carp. However, previous studies also found that a higher ratio of low-gossypol cottonseed meal could impair growth in silver sillago [
30] and juvenile black seabass [
31]. Meanwhile, higher contents of cottonseed meal could decrease FI values in Ussuri catfish
Pseudobagrus ussuriensis [
29]; similar results were also presented in our study, which indicated that higher contents of free gossypol could impair growth via lowering animal feed intake in black carp. In addition, higher VSI, HSI, and ISI were shown in DCP40 and DCP50 groups; similar results were observed in channel catfish [
32]. A number of studies found that a higher HSI value is always linked with greater hepatic lipid content and weakened lipid metabolic function in cultured fish species [
20,
22,
23]. Combined with greater hepatic TAG contents in the DCP40 and DCP50 groups, it is suggested that higher dietary DCP could impair lipid metabolic function by heightening HSI values in black carp. Moreover, a higher FCR value was shown in the DCP50 group; similar results were also demonstrated in grouper largemouth bass [
33,
34], golden pompano [
10], and red swamp crayfish [
35], which may be due to the excessive FM replacement with DCP. This could affect the hardness and palatability of feed and influence the utilization of feed in fish [
36,
37].
In this experiment, the replacement of FM with DCP did not affect the moisture content of the dorsal muscle of black carp, which is consistent with results in rainbow trout [
12]. Previous studies observed that the crude protein of whole fish and dorsal muscle decreased with increasing DCP replacement levels in juvenile largemouth bass [
33] and silver sillago [
30]; similar results were also shown in our results, which indicated that the DCP diet was less palatable and consumed at a much lower rate than the other diets [
30]. Previous studies reported no changes in the amino acid profiles in the fish muscle [
31,
38]; similar results were also shown in our study, which suggested that greater replacement of FM (50%) with DCP did not influence the amino acid profiles in the muscle of black carp. However, other studies also found that greater FM replacement with a cottonseed protein concentrate (CPC) could reduce the total and essential contents in the muscle of largemouth bass [
34] and Amur sturgeon [
39]. This diversity may be due to the imbalance of amino acid composition and utilization abilities mediated by the higher dietary DCP content in these fish species.
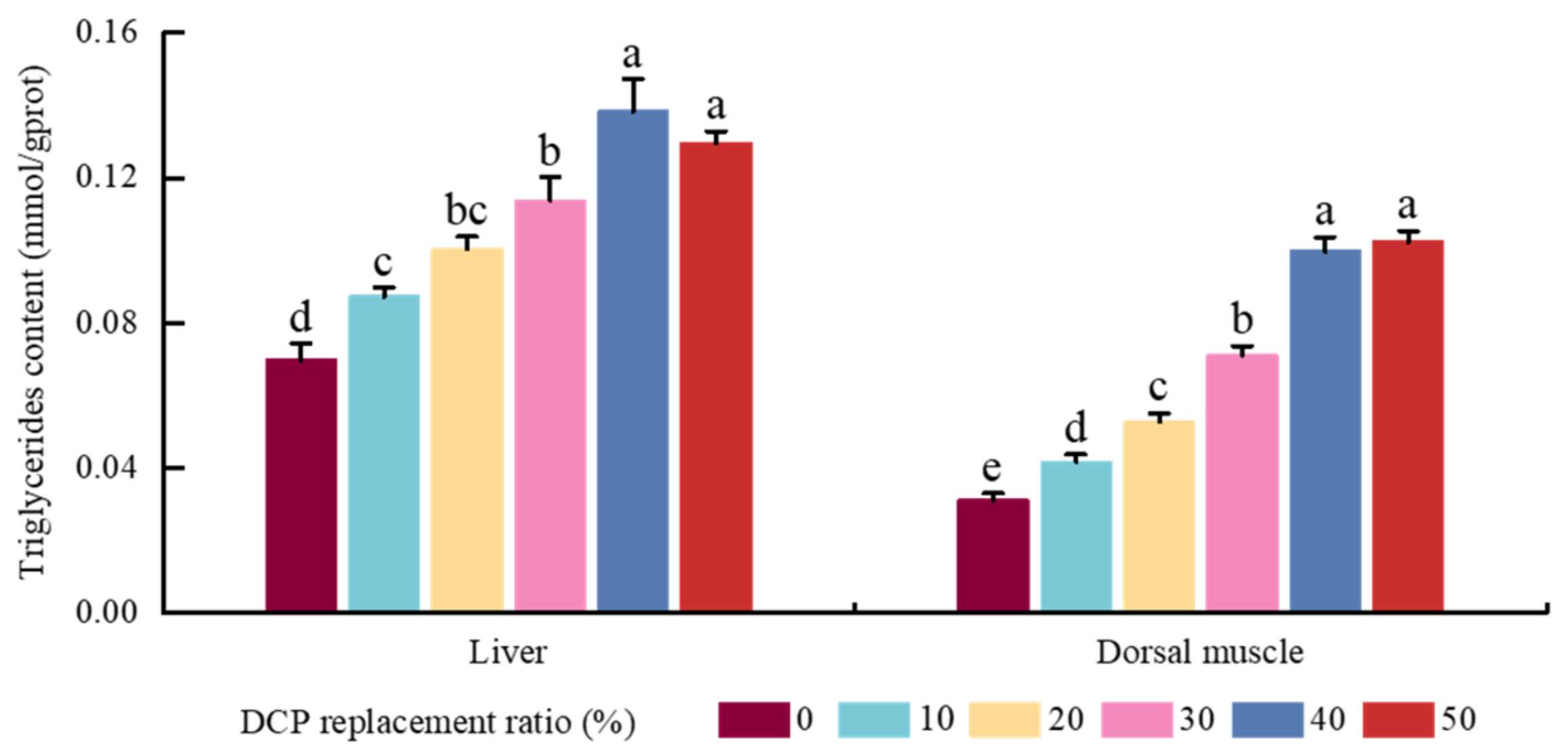
In addition, the content of crude lipid increased, which is similar to the results found in black sea bass [
31]. A previous study also reported that there were higher carbohydrate contents in cottonseed meal as a typical plant-derived protein resource (19.30 ± 0.1%) [
40], and higher contents of carbohydrates could induce lipid deposition or synthesis in fish [
41]. Combined with the results found for body lipid contents, TAG contents, and HSI values in our results, this indicated that lipid deposition and/or synthesis were induced by higher contents of carbohydrates with increasing dietary DCP replacement levels in black carp. In addition, a former study also found that the significant increase in crude lipid contents may be due to impaired liver function or lower utilization of lipids as an energy source in diets with higher contents of cottonseed meal [
42]. However, other studies also found reduced growth and lower body lipid levels in cultured fish fed diets with higher cottonseed meal levels [
34,
43]; this may be due to the differences in fish species and their utilization abilities corresponding to experimental feed formulations. Although there were no changes in ash contents in the muscle samples, ash contents were reduced in the whole fish body of the DCP40 and DCP50 groups, while there were no differences in whole fish body ash contents in rainbow trout [
12], silver sillago [
30], and pearl gentian groupers [
44], which may be due to the different experimental feed ingredients, culturing conditions, and fish species [
45].
Serum parameters of fish are critical indicators to effectively respond to the metabolic ability and health status in cultured fish species [
46,
47]. Variations in AST and ALT activities could reflect hepatic damage in fish and correlate with metabolic ability [
48,
49]. In this experiment, the levels of serum AST and ALT were decreased in the DCP30 group, which was consistent with the results in largemouth bass [
33] and large yellow croaker [
45], indicating that 30% FM replacement with DCP did not induce typical hepatic damage in black carp. Notably, higher AST and ALT levels were observed in DCP0 and DCP10 groups, which may be attributed to metabolic adaptation during transamination and their use for protein synthesis in fish associated with FM and DCP utilization [
13]. Regarding increased AST and ALT activities in the DCP50 group, this might be linked to higher levels of residual gossypol present in the DCP inducing metabolic stress at excessive DCP replacement levels [
50]. As a metabolite marker, changes in serum GLU could act as a stress marker for evaluating the effects of dietary protein resource replacement [
51]. Our results showed that serum GLU contents were heightened with increased levels of DCP replacement, aligning with findings in pearl gentian groupers [
44]. Meanwhile, higher activity of α-AMS was demonstrated in the liver and intestine of fish fed diets with higher DCP levels compared with the DCP0 group, suggesting that higher contents of GLU could be generated through the digestion of starch or polysaccharides in DCP and then be transported into the serum [
52]. These findings indicate that higher contents of GLU could be induced by higher contents of carbohydrate included in dietary DCP in black carp. Although INS could lower GLU levels, serum INS contents were markedly reduced in these higher levels of DCP replacement, which indicated that higher levels of DCP could impair glucose metabolism by lowering INS secretion, although this regulatory mechanism needed to be further studied in black carp.
Moreover, as key serum hormones, higher levels of IGFs always play critical roles in improving protein synthesis in animals [
53]. Further, variations in serum BUN are usually related to nitrogen utilization and impact the protein-synthesizing ability [
25,
54]. Combined with protein contents in the fish body and muscle and contents of IGF-1/2 and BUN in the serum of DCP40 and DCP50 groups, this indicated that high DCP might weaken dietary nitrogen utilization and reduce protein synthesis by lowering IGF-1/2 contents in black carp. Previous studies reported that 5-HT could act as an inhibitory neuromodulator and decrease food intake in fish [
55,
56]. In this study, serum 5-HT levels were noticeably heightened in the DCP40 and DCP50 groups in comparison with the DCP0, DCP10, and DCP20 groups. Along with lower FI values in the DCP40 and DCP50 groups, this indicates that FI could be inhibited by greater 5-HT amounts induced by increased dietary DCP, although its regulatory mechanism needs to be well-studied in the future. As the key transporter for lipid molecules out of hepatic cells, LDL-C with higher activities or levels usually indicates cellular damage to hepatic tissue [
57,
58]. Furthermore, higher TBA contents could be linked to lipid metabolic dysfunction induced by higher starch contents in fish [
57]. These increased LDL-C, TG, TC, and TBA amounts in the DCP40 group suggest that high DCP levels could impact lipid metabolism in black carp.
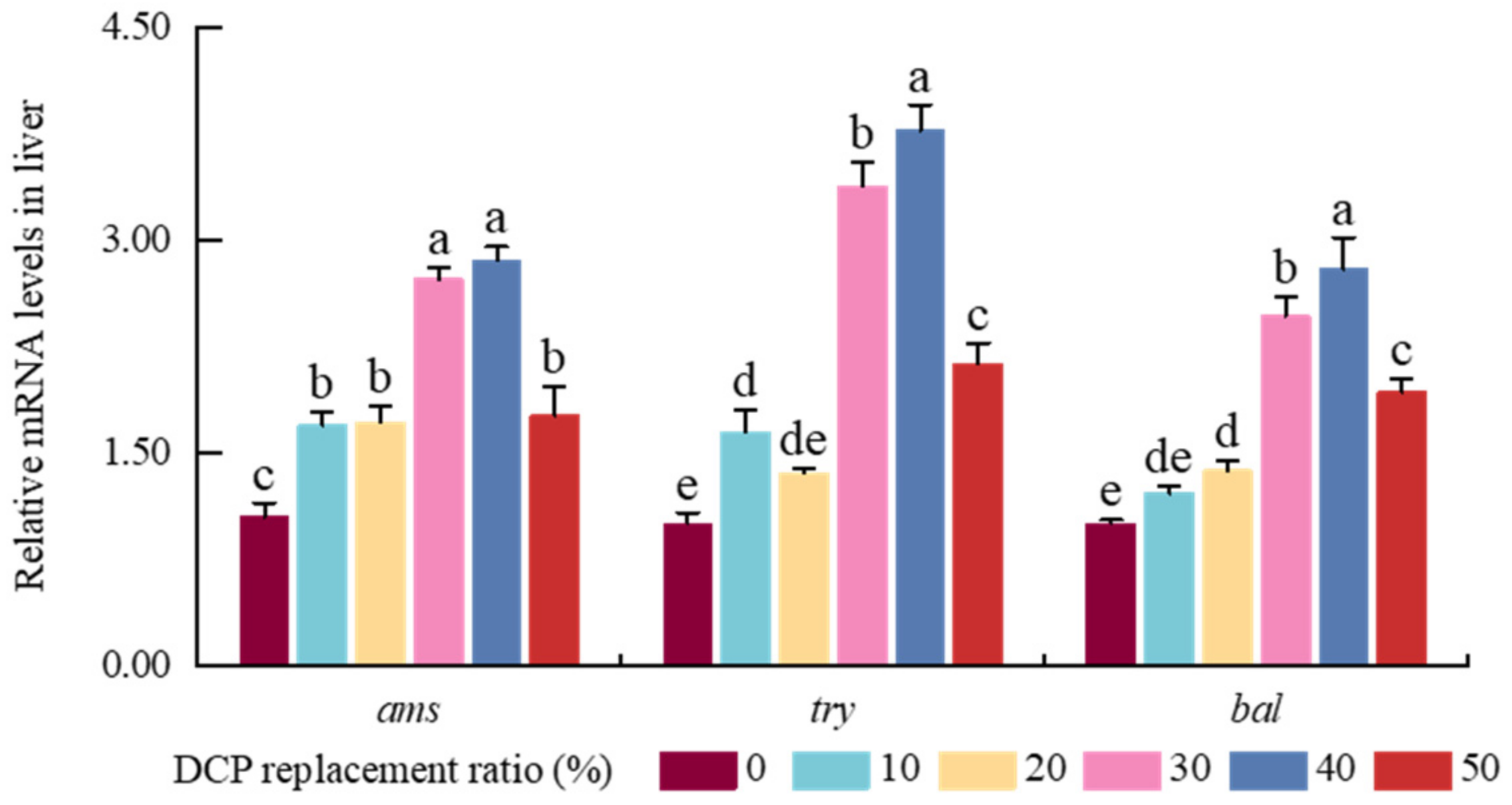
In general, FAS can be used to generate palmitate with cytoplasmic Ac-CoA and Mal-CoA molecules in hepatic cells [
59,
60]. Palmitate can be used to synthesize corresponding saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs), catalyzed by SCD, ELOVLs, FADS, etc. [
61]. In line with these previous findings in largemouth bass [
60], higher levels of
acc,
fas,
fads,
scd, and
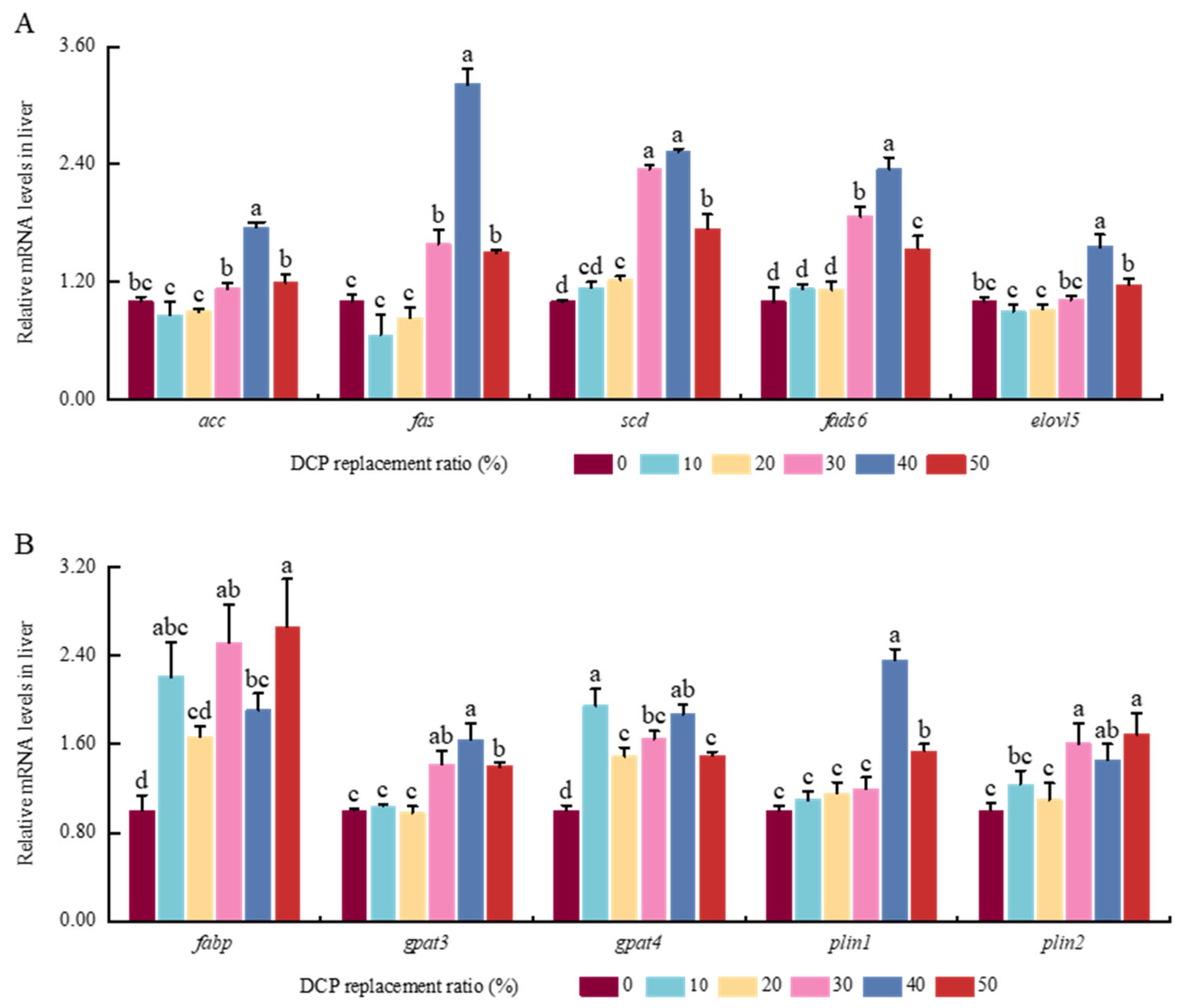
elovl5 were also shown in the liver of black carp fed higher levels of dietary DCP, which suggests that higher DCP intake could cause fatty acid synthesis by increasing the transcription levels of these genes in the hepatic cells of black carp. FABPs could assist in the transportation of fatty acids to the endoplasmic reticulum for the next generation of TAG within hepatic cells [
62]. As rate-limiting enzymes, GPAT3 and GPAT4 play key roles in synthesizing TAG [
63]. Meanwhile, as two key coated proteins, Plin1 and Plin2, are tightly linked with the biogenesis and storage of lipid droplets in hepatic cells [
64]. Many studies have found that higher expression amounts of Plins could be related to so-called metabolic dysfunction or disorders in animals [
65,
66,
67]. This study also found there were higher levels of
fabp6,
gpat3,
gpat4,
plin1, and
plin2 in black carp fed higher dietary DCP, which is in line with previous results in largemouth bass [
60] and Atlantic salmon [
68]. Along with higher TG contents and higher mRNA expression levels of these functional genes, this demonstrates that higher contents of DCP could enhance TAG synthesis in the liver of black carp. Previous research has reported that TAG stored in lipid droplets could be hydrolyzed into FAs and glycerol by adipose triglyceride lipase (ATGL) and lipoprotein lipase (LPL) in animals [
69]. Combined with lower
atgl and
lpl expression levels in this study, this suggests that higher contents of DCP could inhibit TAG hydrolysis and exacerbate lipid deposition in black carp.
Many studies have found that redox homeostasis always plays a key role in maintaining normal growth and improving immunity in animals [
70,
71]. These antioxidant enzymes (SOD, CAT, and GPx) can protect cells from oxidative stress. SOD catalyzes the dismutation of superoxide anion radical to O
2 and H
2O
2, CAT catalyzes the decomposition of H
2O
2 into water and oxygen, and GPx reduces lipid hydroperoxides to their corresponding alcohols and free hydrogen peroxide to water, thus limiting oxidative damage [
72,
73]. GST can generate disulfide bonds between GSH molecules, while GR can decompose these disulfide bonds in glutathione disulfide (GSSG), and glutamate–cysteine ligase (GCL) can catalyze the generation of GSH [
74,
75]. In general, the cellular redox balance is tightly related with variations in these antioxidant enzymes and molecules (SOD, CAT, GPx, GR, GCL, GSH, etc.) [
76]. In this experiment, suitable contents of DCP heightened the amounts of T-SOD, T-AOC, GSH, and
gcl, while higher contents of DCP reduced the
gpx expression levels in the liver of fish. Meanwhile, DCP50 also elevated the amounts of GST, H
2O
2, and MDA in fish liver, which is in agreement with previous results in largemouth bass [
33], large yellow croaker [
45], and ussuri catfish [
13]. Combined with the above results, this indicated that suitable contents of dietary DCP could improve antioxidant capacity, protect cells from ROS-induced damage, and maintain redox balance in black carp [
77]. Many studies have proved that the Nrf2/Keap1 signal pathway can regulate redox homeostasis and the expression levels of these antioxidant enzymes in animals [
78,
79]. Our study found there were higher expression levels of
nrf2 and lower amounts of
keap1 in adequate DCP test groups, which is consistent with previous results found for grass carp [
80], common carp [
81], and largemouth bass [
82]. Combined with these findings, suitable DCP (20%) could promote antioxidant capacities by regulating the Nrf2/Keap1 pathway in black carp.
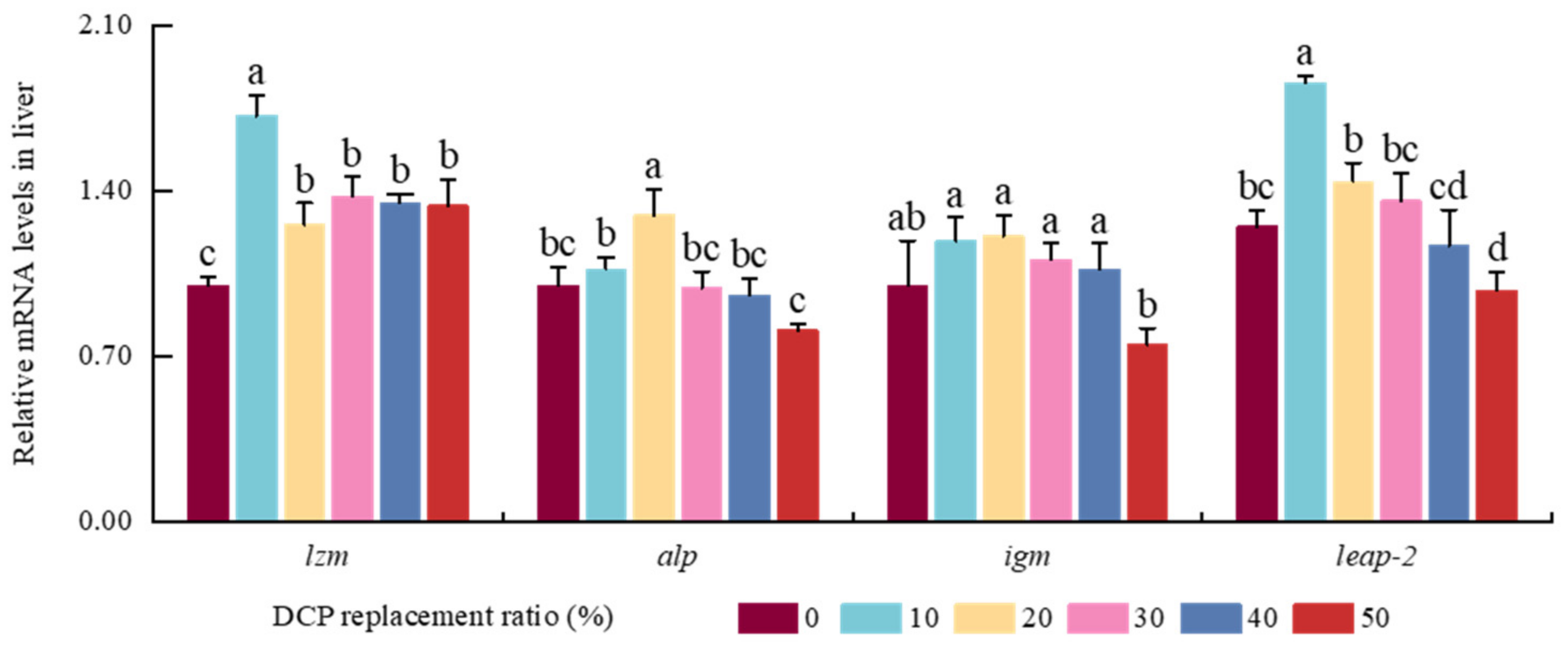
The detection of immune indices is very important for assessing animal health status and immune regulatory roles mediated by various exogenous nutrients [
20,
54]. In general, animal immune function could be heightened by increasing the contents of different immune defense molecules, including LZM, ALP, IgM, and LEAP-2 [
83,
84,
85]. LZM and LEAP-2 can act as crucial immune defense effector molecules by destroying the surface structure of these invading pathogens in fish [
86]. In the present study, LZM activities and expression levels were both heightened with an adequate amount of DCP in the liver of black carp, which was in line with former results in South American white shrimp [
29] and golden pompano [
87]. Meanwhile, higher levels of
leap-2 were also observed in fish fed lower DCP diets compared with higher DCP diets; similar results were also shown in fish fed adequate dietary nutrients [
23,
88]. Thus, higher LZM levels could be induced by adequate dietary DCP and used to improve the immunity of black carp. As a typical phosphohydrolase and diagnostic parameter, ALP can mediate crucial immune defense functions by triggering activities of immune cells and can be used to evaluate health status in fish and other animals [
89,
90]. Our results also showed there were higher ALP activities and transcription levels in the DCP20 group, which is similar to the result found for grass carp [
80]. As a major humoral immune protein produced by B cells, IgM can recognize target epitopes of foreign pathogens and help construct their complex, which is rendered to macrophages and phagocytes to destroy these pathogens in animals [
84]. Higher levels of IgM are usually closely related to better humoral immunity in fish fed suitable contents of dietary nutrients [
91]. These findings align with previous results found for hybrid grouper [
7], where higher levels of IgM were also shown in the liver and intestine of fish fed suitable amounts of DCP. This suggests that adequate dietary DCP could promote humoral immunity, enhance health status, and protect hosts by increasing IgM secretion in black carp. However, lower levels of IgM were also present in fish fed excessive DCP, similar to many cultured fish fed higher contents of DCP or cottonseed meal, respectively [
80,
92]. These findings suggest that excessive dietary DCP might weaken humoral immunity and health status by reducing the secretion of these defense molecules in black carp.