Interaction of Flupyradifurone and Deltamethrin, Two Pesticides Commonly Used for Plant Pest Control, in Honeybees
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honeybee Groups
2.2. Pesticide Concentration and Administration
2.3. Solution Intake and Honeybee Behavior
2.4. Statistical Analysis
3. Results
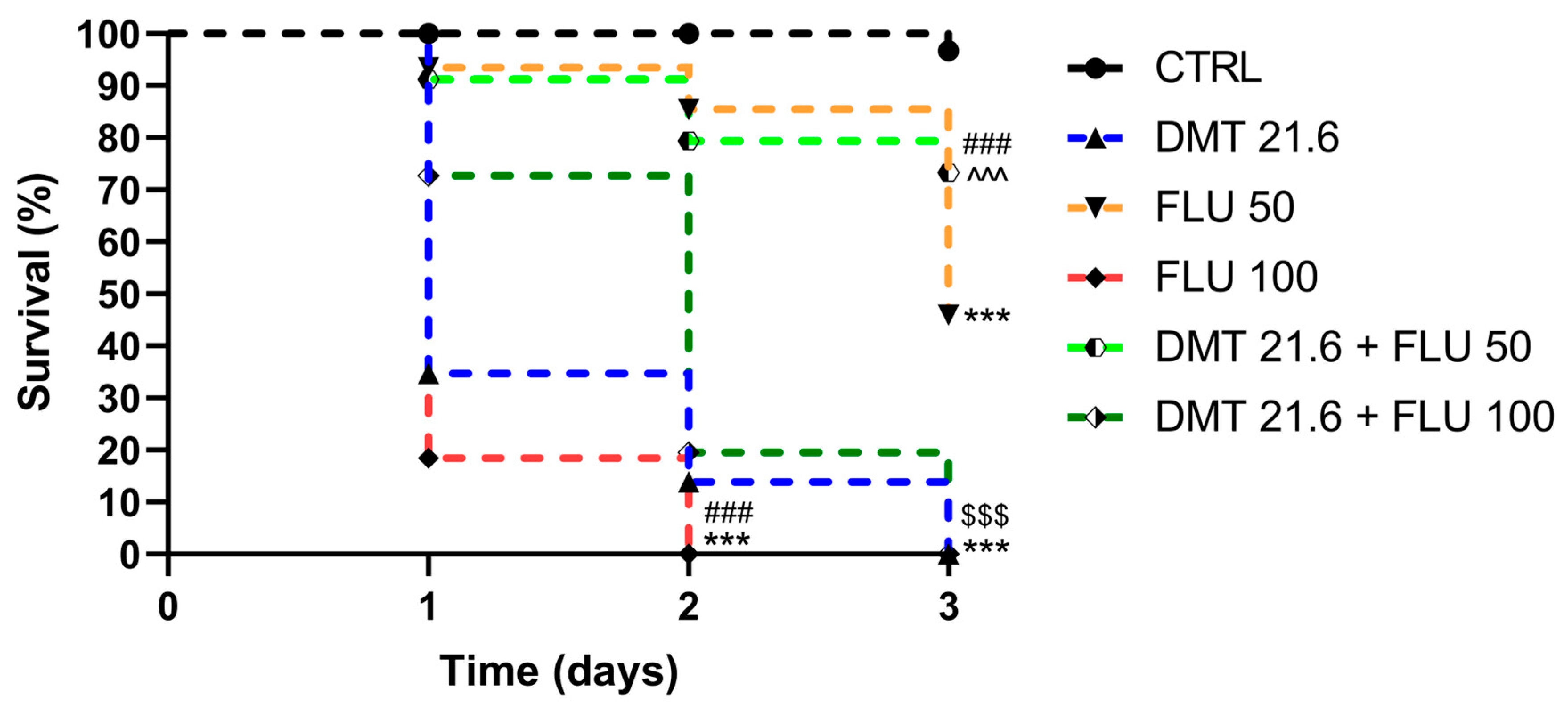
3.1. Survival Probability
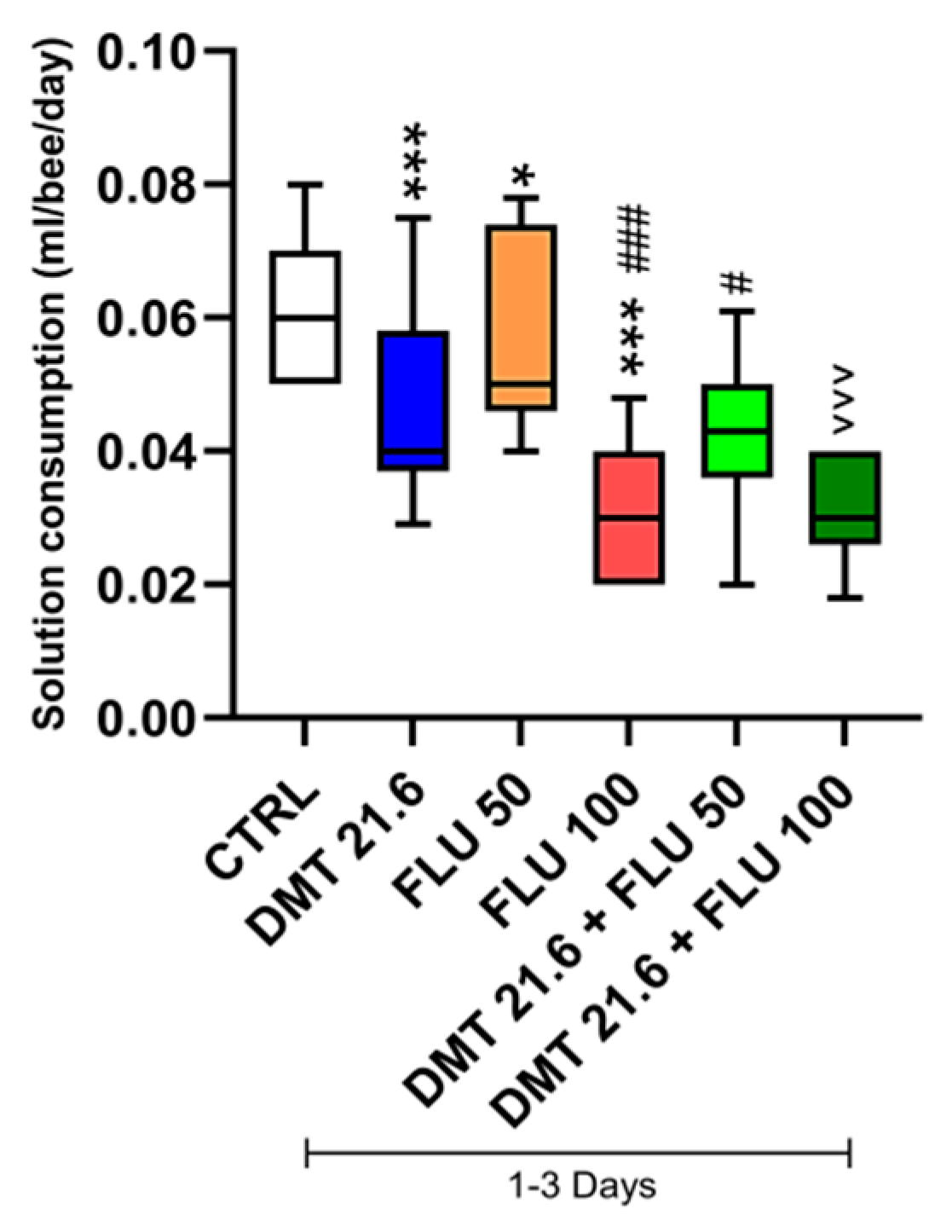
3.2. Solution Intake and Consumption
3.3. Observed Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, J.A. The Pesticide Manual: A World Compendium; BCPC: Aldershot, Hampshire, 2018. [Google Scholar]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Georghiou, G.P. Pest Resistance to Pesticides; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 1468444662. [Google Scholar]
- Bhattacharyya, A.; Barik, S.R.; Ganguly, P. New pesticide molecules, formulation technology and uses: Present status and future challenges. J. Plant Prot. Sci. 2009, 1, 9–15. [Google Scholar]
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A brief profile of a new butenolide insecticide. Pest Manag. Sci. 2015, 71, 850–862. [Google Scholar] [CrossRef]
- Cerezo-Galvez, S.; Marienhagen, C.; Weckwert, H.; Thielert, W. Active Ingredient Combinations Having Insecticidal/Acaricidal Properties. U.S. Patent 11,540,517, 3 January 2023. [Google Scholar]
- Funk, C.; Schneider, T.B.; Fischer, R.; Fischer, R.; Hengelberg, H.; Anderschwolf, W.; Tillet, G.; Kraus, A. Active Agent Combinations with Insecticidal and Acaricidal Properties. U.S. Patent CA2428101A1, 7 May 2023. [Google Scholar]
- Gewehr, M. Synergistic Compositions Comprising Pyraclostrobin and an Insecticidal Compound. U.S. Patent WO2013156331A1, 24 October 2013. [Google Scholar]
- Peng, D.; Li, S.; Chen, C.; Zhou, M. Combined application of Bacillus subtilis NJ-18 with fungicides for control of sharp eyespot of wheat. Biol. Control 2014, 70, 28–34. [Google Scholar] [CrossRef]
- Ji, X.; Li, J.; Meng, Z.; Zhang, S.; Dong, B.; Qiao, K. Synergistic effect of combined application of a new fungicide fluopimomide with a biocontrol agent Bacillus methylotrophicus TA-1 for management of gray mold in tomato. Plant Dis. 2019, 103, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Le Mauff, A.; Cartereau, A.; Plantard, O.; Taillebois, E.; Thany, S.H. Effect of the combination of DEET and flupyradifurone on the tick Ixodes ricinus: Repellency bioassay and pharmacological characterization using microtransplantation of synganglion membranes. Ticks Tick. Borne. Dis. 2023, 14, 102079. [Google Scholar] [CrossRef]
- Carleton, J. Environmental Fate and Ecological Risk Assessment for Foliar, Soil Drench, and Seed Treatment Uses of the New Insecticide Flupyradifurone; United States Environmental Protection Agency: Washington, DC, USA, 2014.
- Tosi, S.; Nieh, J.C. Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivanto®), on honeybees. Proc. R. Soc. B 2019, 286, 20190433. [Google Scholar] [CrossRef]
- Siviter, H.; Muth, F. Do novel insecticides pose a threat to beneficial insects? Proc. R. Soc. B 2020, 287, 20201265. [Google Scholar] [CrossRef]
- Tan, K.; Wang, C.; Dong, S.; Li, X.; Nieh, J.C. The pesticide flupyradifurone impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci. Rep. 2017, 7, 17772. [Google Scholar] [CrossRef]
- O’Brien, R.D. Insecticides: Action and Metabolism; Academic Press, Technology & Engineering: Cambridge, MA, USA, 2014. [Google Scholar]
- Tozzi, A. A brief history of the development of piperonyl butoxide as an insecticide synergist. In Piperonyl Butoxide; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1–5. [Google Scholar]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Lima, J.G.; Santos, J.P.; Cruz, C.D. Resistance to DDT and pyrethroids in Brazilian populations of Sitophilus zeamais Motsch (Coleoptera: Curculionidae). J. Stored Prod. Res. 1995, 31, 145–150. [Google Scholar] [CrossRef]
- Russell, R.J.; Claudianos, C.; Campbell, P.M.; Horne, I.; Sutherland, T.D.; Oakeshott, J.G. Two major classes of target site insensitivity mutations confer resistance to organophosphate and carbamate insecticides. Pestic. Biochem. Physiol. 2004, 79, 84–93. [Google Scholar] [CrossRef]
- Katsuda, Y. Progress and future of pyrethroids. Pyrethroids Chrysanth. Mod. Ind. Insectic. 2012, 79, 84–93. [Google Scholar]
- Tang, W.; Wang, D.I.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; El-Ghoneimy, A. Synergistic protective effects of ceftriaxone and ascorbic acid against subacute deltamethrin-induced nephrotoxicity in rats. Ren. Fail. 2015, 37, 297–304. [Google Scholar] [CrossRef]
- Dai, P.; Wang, Q.; Sun, J.; Liu, F.; Wang, X.; Wu, Y.; Zhou, T. Effects of sublethal concentrations of bifenthrin and deltamethrin on fecundity, growth, and development of the honeybee Apis mellifera ligustica. Environ. Toxicol. Chem. Int. J. 2010, 29, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Devillers, J.; Cluzeau, S.; Charreton, M.; Pham-Delègue, M.-H. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Saf. 2004, 57, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Li, Z.; Huang, Q.; Zhang, X.W.; Ke, L.; Yan, W.Y.; Zhang, L.Z.; Zeng, Z.J. Deltamethrin impairs honeybees (Apis mellifera) dancing communication. Arch. Environ. Contam. Toxicol. 2020, 78, 117–123. [Google Scholar] [CrossRef]
- Vandame, R.; Belzunces, L.P. Joint actions of deltamethrin and azole fungicides on honey bee thermoregulation. Neurosci. Lett. 1998, 251, 57–60. [Google Scholar] [CrossRef]
- Medrzycki, P.; Giffard, H.; Aupinel, P.; Belzunces, L.P.; Chauzat, M.-P.; Classen, C.; Colin, M.E.; Dupont, T.; Girolami, V.; Johnson, R. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 2013, 52, 1–60. [Google Scholar] [CrossRef]
- Forsgren, E.; Budge, G.E.; Charrière, J.-D.; Hornitzky, M.A.Z. Standard methods for European foulbrood research. J. Apic. Res. 2013, 52, 1–14. [Google Scholar] [CrossRef]
- De Graaf, D.C.; Alippi, A.M.; Antúnez, K.; Aronstein, K.A.; Budge, G.; De Koker, D.; De Smet, L.; Dingman, D.W.; Evans, J.D.; Foster, L.J. Standard methods for American foulbrood research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Williams, G.R.; Alaux, C.; Costa, C.; Csaki, T.; Doublet, V.; Eisenhardt, D.; Fries, I.; Kuhn, R.; McMahon, D.P.; Medrzycki, P. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Ruga, S.; Caminiti, R.; Nucera, S.; Bulotta, R.M.; Naccari, C.; Britti, D.; Mollace, V.; Palma, E. Protective Role of Bergamot Polyphenolic Fraction (BPF) against Deltamethrin Toxicity in Honeybees (Apis mellifera). Animals 2023, 13, 3764. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Lupia, C.; Castagna, F.; Ruga, S.; Nucera, S.; Caminiti, R.; Bulotta, R.M.; Naccari, C.; Carresi, C.; Musolino, V. Bergamot Polyphenolic Fraction for the Control of Flupyradifurone-Induced Poisoning in Honeybees. Animals 2024, 14, 608. [Google Scholar] [CrossRef]
- Gao, J.; Guo, Y.; Chen, J.; Diao, Q.-Y.; Wang, Q.; Dai, P.-L.; Zhang, L.; Li, W.-M.; Wu, Y.-Y. Acute oral toxicity, apoptosis, and immune response in nurse bees (Apis mellifera) induced by flupyradifurone. Front. Physiol. 2023, 14, 1150340. [Google Scholar] [CrossRef]
- OECD/OCDE. OECD/OCDE. OECD Guideline 245 for the Testing of Chemicals. Honey Bee (Apis mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding). In OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- Nogué, S.; Long, P.R.; Eycott, A.E.; de Nascimento, L.; Fernández-Palacios, J.M.; Petrokofsky, G.; Vandvik, V.; Willis, K.J. Pollination service delivery for European crops: Challenges and opportunities. Ecol. Econ. 2016, 128, 1–7. [Google Scholar] [CrossRef]
- Pettis, J.S.; Delaplane, K.S. Coordinated responses to honey bee decline in the USA. Apidologie 2010, 41, 256–263. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Carreck, N.; Neumann, P. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. Towards an integrated environmental risk assessment of multiple stressors on bees: Review of research projects in Europe, knowledge gaps and recommendations. EFSA J. 2014, 12, 3594. [Google Scholar]
- Penev, D. European bee week: What the EU should do for bees and biodiversity. Euractiv 2016, 2. [Google Scholar]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Z.; He, Q.; Liu, Q.; Li, X.; Yu, L.; Cao, H. The effect of neonicotinoid insecticide and fungicide on sugar responsiveness and orientation behavior of honey bee (Apis mellifera) in semi-field conditions. Insects 2018, 9, 130. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Brandt, A.; Colli, M.; Fourrier, J.; Giffard, H.; Hernández-López, J.; Malagnini, V.; Williams, G.R.; Simon-Delso, N. Long-term field-realistic exposure to a next-generation pesticide, flupyradifurone, impairs honey bee behaviour and survival. Commun. Biol. 2021, 4, 805. [Google Scholar] [CrossRef]
- Van der Steen, J.J.M. The foraging honey bee. BBKA News Br. Bee J. 2015, 2015, 43–46. [Google Scholar]
- Das, S.K. Scope and relevance of using pesticide mixtures in crop protection: A critical review. Int. J. Environ. Sci. Toxicol. 2014, 2, 119–123. [Google Scholar]
- Wang, Y.; Zhu, Y.C.; Li, W. Interaction patterns and combined toxic effects of acetamiprid in combination with seven pesticides on honey bee (Apis mellifera L.). Ecotoxicol. Environ. Saf. 2020, 190, 110100. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Protection Products and their Residues (PPR). Scientific Opinion on the science behind the development of a risk assessment of Plant Protection Products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 2012, 10, 2668. [Google Scholar] [CrossRef]
- Johnson, R.M. Honey bee toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [PubMed]
- OECD. 214: Honeybees, acute contact toxicity test. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 1998; Volume 2, pp. 2–4. [Google Scholar]
- European and Mediterranean Plant Protection Organization. Efficacy evaluation of plant protection products. PP 1/170 Side-effects on honeybees. EPPO Bull. 2010, 40, 313. [Google Scholar] [CrossRef]
- Laufer, J.; Roche, M.; Pelhate, M.; Elliott, M.; Janes, N.F.; Sattelles, D.B. Pyrethroid insecticides: Actions of deltamethrin and related compounds on insect axonal sodium channels. J. Insect Physiol. 1984, 30, 341–349. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Gutbrod, O.; Beck, M.E.; Matthiesen, S.; Haas, M.; Velten, R. Flupyradifurone (SivantoTM) and its novel butenolide pharmacophore: Structural considerations. Pestic. Biochem. Physiol. 2015, 121, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]

| Groups | Survival Rate (%) | ||
|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |
| CTRL | 100 | 100 | 96.6 |
| DMT 21.6 | 34.6 | 13.8 | 0 |
| FLU 50 | 93.4 | 85.4 | 45.9 |
| FLU 100 | 18.4 | 0 | / |
| DMT 21.6 + FLU 50 | 91.1 | 79.4 | 73.3 |
| DMT 21.6 + FLU 100 | 72.7 | 19.5 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bava, R.; Lupia, C.; Castagna, F.; Ruga, S.; Nucera, S.; Carresi, C.; Caminiti, R.; Bulotta, R.M.; Naccari, C.; Britti, D.; et al. Interaction of Flupyradifurone and Deltamethrin, Two Pesticides Commonly Used for Plant Pest Control, in Honeybees. Animals 2024, 14, 851. https://doi.org/10.3390/ani14060851
Bava R, Lupia C, Castagna F, Ruga S, Nucera S, Carresi C, Caminiti R, Bulotta RM, Naccari C, Britti D, et al. Interaction of Flupyradifurone and Deltamethrin, Two Pesticides Commonly Used for Plant Pest Control, in Honeybees. Animals. 2024; 14(6):851. https://doi.org/10.3390/ani14060851
Chicago/Turabian StyleBava, Roberto, Carmine Lupia, Fabio Castagna, Stefano Ruga, Saverio Nucera, Cristina Carresi, Rosamaria Caminiti, Rosa Maria Bulotta, Clara Naccari, Domenico Britti, and et al. 2024. "Interaction of Flupyradifurone and Deltamethrin, Two Pesticides Commonly Used for Plant Pest Control, in Honeybees" Animals 14, no. 6: 851. https://doi.org/10.3390/ani14060851
APA StyleBava, R., Lupia, C., Castagna, F., Ruga, S., Nucera, S., Carresi, C., Caminiti, R., Bulotta, R. M., Naccari, C., Britti, D., & Palma, E. (2024). Interaction of Flupyradifurone and Deltamethrin, Two Pesticides Commonly Used for Plant Pest Control, in Honeybees. Animals, 14(6), 851. https://doi.org/10.3390/ani14060851









