Carcass and Meat Quality Traits in Female Lidia Cattle Slaughtered at Different Ages
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Carcass and Meat Quality Analyses
2.3. Sensory Analysis
2.4. Statistical Analyses
3. Results
3.1. Carcass Traits
3.2. pH and Carcass Color
3.3. 6th Rib Cut Dissection
3.4. Meat Traits
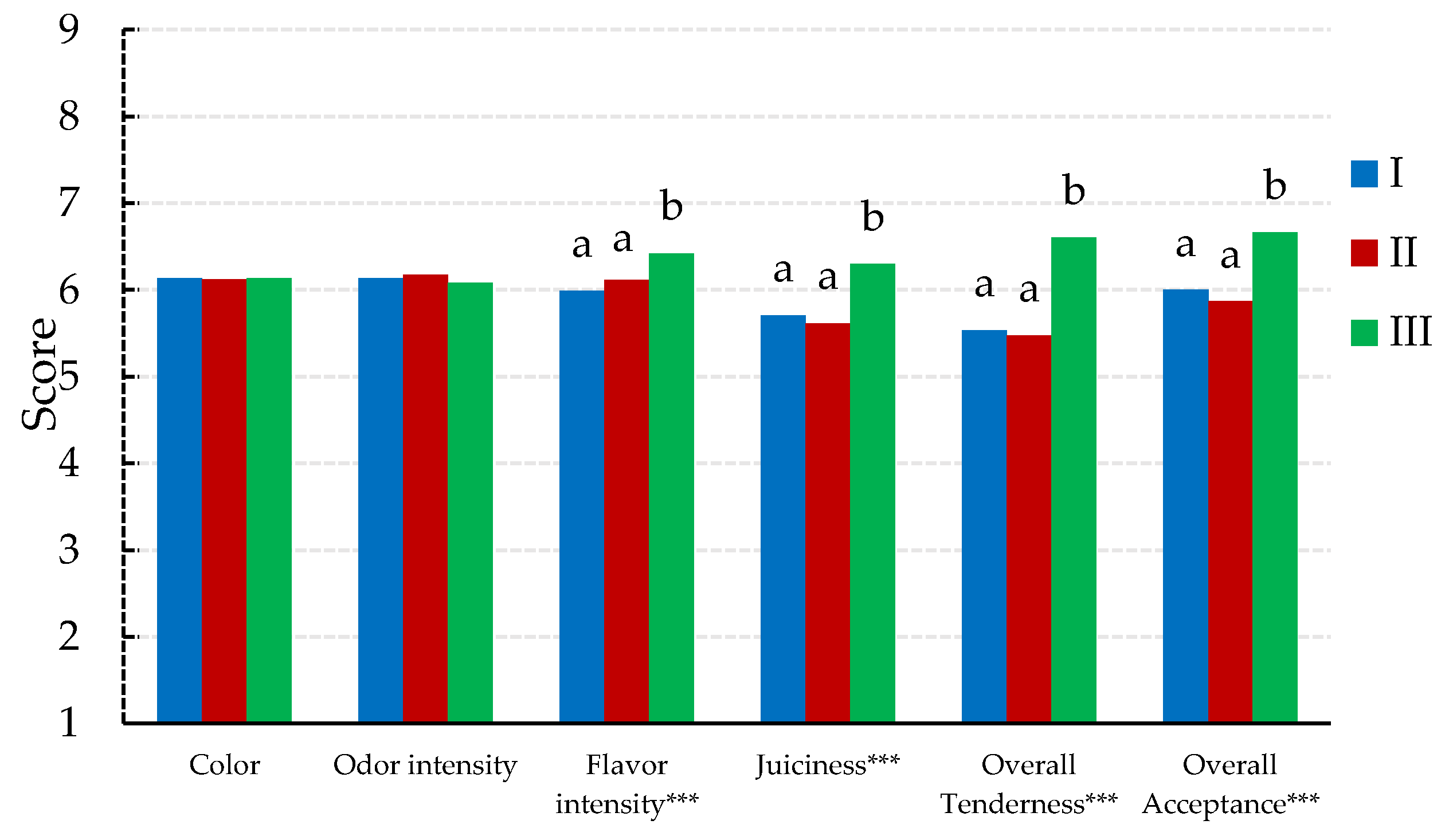
3.5. Sensory Analysis
4. Discussion
4.1. Carcass Traits
4.2. pH and Carcass Color
4.3. 6th Rib Cut Dissection
4.4. Meat Traits
4.5. Sensory Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MAPAMA Datos Censales de La Raza LIDIA. Available online: https://servicio.mapa.gob.es/arca/flujos.html?_flowId=datosCensalesRaza-flow&tipoOperacion=CONSULTA&formatoPagina=0&id=50100 (accessed on 17 January 2024).
- Cañón, J.; Tupac-Yupanqui, I.; García-Atance, M.A.; Cortés, O.; García, D.; Fernández, J.; Dunner, S. Genetic Variation within the Lidia Bovine Breed. Anim. Genet. 2008, 39, 439–445. [Google Scholar] [CrossRef]
- Pelayo, R.; Valera, M.; Molina, A.; Royo, L.J. Contribution of Lidia Cattle Breed Historical Castes to the Paternal Genetic Stock of Spain. Anim. Genet. 2015, 46, 312–315. [Google Scholar] [CrossRef]
- Lomillos, J.M.; Alonso, M.E. Morphometric Characterization of the Lidia Cattle Breed. Animals 2020, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J. El Toro de Lidia En La Biología, En La Zootecnia y En La Cultura; de Castilla y León, J., Ed.; Consejería de Agricultura y Ganadería: Valladolid, Spain, 1991. [Google Scholar]
- Almeida, J.P.F. Sistemas Extensivos de Pastoreio—Estimativa Das Emissões de Metano e Do Balanço de Carbono Na Região Alentejo. AGROTEC 2019, 32, 30–32. [Google Scholar]
- Stampa, E.; Schipmann-Schwarze, C.; Hamm, U. Consumer Perceptions, Preferences, and Behavior Regarding Pasture-Raised Livestock Products: A Review. Food Qual. Prefer. 2020, 82, 103872. [Google Scholar] [CrossRef]
- Koistinen, L.; Pouta, E.; Heikkilä, J.; Forsman-Hugg, S.; Kotro, J.; Mäkelä, J.; Niva, M. The Impact of Fat Content, Production Methods and Carbon Footprint Information on Consumer Preferences for Minced Meat. Food Qual. Prefer. 2013, 29, 126–136. [Google Scholar] [CrossRef]
- Sierra, V.; Guerrero, L.; Fernández-Suárez, V.; Martínez, A.; Castro, P.; Osoro, K.; Rodríguez-Colunga, M.J.; Coto-Montes, A.; Oliván, M. Eating Quality of Beef from Biotypes Included in the PGI “Ternera Asturiana” Showing Distinct Physicochemical Characteristics and Tenderization Pattern. Meat Sci. 2010, 86, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Resano, H.; Sanjuán, A.I. Exploring the Role of Mountain Origin and Autochthonous Breed on Urban Consumers’ Acceptability. Sustainability 2018, 10, 4423. [Google Scholar] [CrossRef]
- Perea, J.; Arias, R. Competitiveness of Spanish Local Breeds. Animals 2022, 12, 2060. [Google Scholar] [CrossRef]
- Buxadé, C. Producciones Equinas y de Ganado de Lidia. In Zootecnia; Mundiprensa: Madrid, Spain, 1996; Volume Tomo XI, 350p. [Google Scholar]
- Bernabéu, R.; Rabadán, A.; El Orche, N.E.; Díaz, M. Influence of Quality Labels on the Formation of Preferences of Lamb Meat Consumers. A Spanish Case Study. Meat Sci. 2018, 135, 129–133. [Google Scholar] [CrossRef]
- Real Decreto 75/2009, de 30 de Enero, Por El Que Se Modifica El Real Decreto 1698/2003, de 12 de Diciembre, Por El Que Se Establecen Las Disposiciones de Aplicación de Los Reglamentos Comunitarios Sobre El Sistema de Etiquetado de La Carne de Vacuno. 2009. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2009-1602 (accessed on 15 January 2024).
- Lomillos Pérez, J.M.; Alonso de la Varga, M. Características, Tratamiento y Comercialización de La Carne Procedente de La Raza de Lidia. Rev. Complut. Cienc. Vet. 2016, 10, 94–111. [Google Scholar] [CrossRef]
- Lomillos, J.M.; Alonso, M.E. Revisión de La Alimentación de La Raza de Lidia y Caracterización de Las Principales Patologías Asociadas al Cebo Del Toro En La Actualidad. Inf. Tec. Econ. Agrar. 2019, 115, 376–398. [Google Scholar] [CrossRef]
- Council Regulation 1099/2009 Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Off. J. Eur. Union 2009, L303, 1–30.
- Hickey, J.M.; Keane, M.G.; Kenny, D.A.; Cromie, A.R.; Veerkamp, R.F. Genetic Parameters for EUROP Carcass Traits within Different Groups of Cattle in Ireland1. J. Anim. Sci. 2007, 85, 314–321. [Google Scholar] [CrossRef] [PubMed]
- ISO/CIE 11664-4:2019; Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space. ISO: Geneva, Switzerland, 2019.
- Boer, H.D.; Dumont, B.L.; Pomeroy, R.W.; Weniger, J.H. Manual on E.A.A.P. Reference Methods for the Assessment of Carcass Characteristics in Cattle. Livest. Prod. Sci. 1974, 1, 151–164. [Google Scholar] [CrossRef]
- Robelin, J.; Geay, Y.; Jailler, R.; Cuylle, G. Estimation de La Composition Des Carcasses de Jeunes Bovins a Partir de La Composition d’un Morceau Monocostal Prélevé Au Niveau de La 11e Côte. I. Composition Anatomique de La Carcasse. Ann. Zootech. 1975, 24, 391–402. [Google Scholar] [CrossRef]
- Beriain, M.J.; Horcada, A.; Purroy, A.; Lizaso, G.; Chasco, J.; Mendizabal, J.A. Characteristics of Lacha and Rasa Aragonesa Lambs Slaughtered at Three Live Weights. J. Anim. Sci. 2000, 78, 3070. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference Methods for the Assessment of Physical Characteristics of Meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Moreno Díaz, M.; Domenech García, V.; Avilés Ramírez, C.; Peña Blanco, F.; Requena Domenech, F.; Martínez Marín, A. Effects of A Concentrate Rich in Agro-Industrial By-Products on Productivity Results, Carcass Characteristics and Meat Quality Traits of Finishing Heifers. Animals 2020, 10, 1311. [Google Scholar] [CrossRef]
- ISO 13299:2017; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. ISO International Organization for Standarization: Geneva, Switzerland, 2017.
- ISO 8586:2014; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO International Organization for Standarization: Geneva, Switzerland, 2014.
- Cittadini, A.; Domínguez, R.; Sarriés, M.V.; Pateiro, M.; Lorenzo, J.M. Study of Pansalt® or Laminaria Ochroleuca Seaweed Powder as Potential NaCl Replacers in Dry-Cured Foal “Cecina”. Meat Sci. 2023, 204, 109253. [Google Scholar] [CrossRef]
- Resconi, V.C.; Campo, M.M.; Font i Furnols, M.; Montossi, F.; Sañudo, C. Sensory quality of beef from different finishing diets. Meat Sci. 2010, 86, 865–869. [Google Scholar] [CrossRef] [PubMed]
- UNE-EN ISO 8589:2010; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO International Organization for Standarization: Geneva, Switzerland, 2010.
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Designs to Balance the Effect of Order of Presentation and First-Order Carry-over Effects in Hall Tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Quintana, Á.R.; Perea, J.M.; García-Béjar, B.; Jiménez, L.; Garzón, A.; Arias, R. Dominant Yeast Community in Raw Sheep’s Milk and Potential Transfers of Yeast Species in Relation to Farming Practices. Animals 2020, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; García, M.D.; Cerdeño, A.; Mantecón, A.R. Effect of Diet Composition and Slaughter Weight on Animal Performance, Carcass and Meat Quality, and Fatty Acid Composition in Veal Calves. Livest. Prod. Sci. 2005, 93, 263–275. [Google Scholar] [CrossRef]
- Piedrafita, J.; Quintanilla, R.; Sañudo, C.; Olleta, J.-L.; Campo, M.-M.; Panea, B.; Renand, G.; Turin, F.; Jabet, S.; Osoro, K.; et al. Carcass Quality of 10 Beef Cattle Breeds of the Southwest of Europe in Their Typical Production Systems. Livest. Prod. Sci. 2003, 82, 1–13. [Google Scholar] [CrossRef]
- Román-Trufero, A.; García-Prieto, V.; Martínez, A.; Osoro, K.; Celaya, R. Beef Steer Production from Two Local Breeds under Two Management Systems Differing in the Utilisation of Mountain Pastures. Ital. J. Anim. Sci. 2019, 18, 1174–1185. [Google Scholar] [CrossRef]
- Soji, Z.; Muchenje, V. Effect of Genotype and Age on Some Carcass and Meat Quality Traits of Beef Carcasses Subjected to the South African Classification System. Meat Sci. 2016, 117, 205–211. [Google Scholar] [CrossRef]
- Vallejo, M.; Gutiérrez, J.P.; Alonso, L.; Cañón, J.; Revuelta, J.R.; Goyache, F.; Cima, M. Características de Las Canales de Razas Bovinas Asturianas. II. Valoración Cuantitativa y Predicción de La Composición de Canales En La Raza Asturiana de La Montaña. Arch. Zootec. 1992, 41, 645–656. [Google Scholar]
- Indurain, G.; Carr, T.R.; Goñi, M.V.; Insausti, K.; Beriain, M.J. The relationship of carcass measurements to carcass composition and intramuscular fat in Spanish beef. Meat Sci. 2009, 82, 155–161. [Google Scholar] [CrossRef]
- Serra, X.; Gil, M.; Gispert, M.; Guerrero, L.; Oliver, M.A.; Sañudo, C.; Campo, M.M.; Panea, B.; Olleta, J.L.; Quintanilla, R.; et al. Characterisation of Young Bulls of the Bruna Dels Pirineus Cattle Breed (Selected from Old Brown Swiss) in Relation to Carcass, Meat Quality and Biochemical Traits. Meat Sci. 2004, 66, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Albertí, P.; Ripoll, G.; Goyache, F.; Lahoz, F.; Olleta, J.L.; Panea, B.; Sañudo, C. Carcass Characterisation of Seven Spanish Beef Breeds Slaughtered at Two Commercial Weights. Meat Sci. 2005, 71, 514–521. [Google Scholar] [CrossRef]
- Vieira, C.; García-Cachán, M.D.; Recio, M.D.; Domínguez, M.; Sañudo Astiz, C. Effect of Ageing Time on Beef Quality of Rustic Type and Rustic x Charolais Crossbreed Cattle Slaughtered at the Same Finishing Grade. Span. J. Agric. Res. 2006, 4, 225. [Google Scholar] [CrossRef]
- Sañudo, C.; Olleta, J.L.; Campo, M.M.; Panea, B.; Renand, G.; Turin, F.; Jabet, S.; Osoro, K.; Oliván, C.; Noval, G.; et al. Meat Quality of Ten Cattle Breeds of the Southwest of Europe; FAIR1 CT95 0702—Final Report; RECERCAT: Barcelona, Spain, 2007; Available online: https://recercat.cat/handle/2072/4685 (accessed on 20 December 2023).
- Wood, J.D.; MacFie, H.J.H.; Pomeroy, R.W.; Twinn, D.J. Carcass Composition in Four Sheep Breeds: The Importance of Type of Breed and Stage of Maturity. Anim. Sci. 1980, 30, 135–152. [Google Scholar] [CrossRef]
- Yambayamba, E.S.K.; Price, M.A.; Jones, S.D.M. Compensatory Growth of Carcass Tissues and Visceral Organs in Beef Heifers. Livest. Prod. Sci. 1996, 46, 19–32. [Google Scholar] [CrossRef]
- Díez, J.; Albertí, P.; Ripoll, G.; Lahoz, F.; Fernández, I.; Olleta, J.L.; Panea, B.; Sañudo, C.; Bahamonde, A.; Goyache, F. Using Machine Learning Procedures to Ascertain the Influence of Beef Carcass Profiles on Carcass Conformation Scores. Meat Sci. 2006, 73, 109–115. [Google Scholar] [CrossRef]
- Soulat, J.; Picard, B.; Monteils, V. Influence of Cattle Category and Slaughter Age on Charolais-Breed Carcase and Meat Traits. Ital. J. Anim. Sci. 2023, 22, 263–275. [Google Scholar] [CrossRef]
- Peña, F.; Avilés, C.; Domenech, V.; González, A.; Martínez, A.; Molina, A. Effects of Stress by Unfamiliar Sounds on Carcass and Meat Traits in Bulls from Three Continental Beef Cattle Breeds at Different Ageing Times. Meat Sci. 2014, 98, 718–725. [Google Scholar] [CrossRef]
- Lage, J.F.; Paulino, P.V.R.; Filho, S.C.V.; Souza, E.J.O.; Duarte, M.S.; Benedeti, P.D.B.; Souza, N.K.P.; Cox, R.B. Influence of Genetic Type and Level of Concentrate in the Finishing Diet on Carcass and Meat Quality Traits in Beef Heifers. Meat Sci. 2012, 90, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Horcada-Ibáñez, A.; Polvillo-Polo, O.; Lafuente-García, A.; González-Redondo, P.; Molina-Alcalá, A.; Luque-Moya, A. Beef Quality of Native Pajuna Breed Calves in Two Production Systems. Agrociencia 2016, 50, 167–182. [Google Scholar]
- Zamuz, S.; García-Torres, S.; Cabeza de Vaca, M.; Tejerina, D.; Ortiz, A.; Oliván, M.; Sierra, V.; Diñeiro, Y.; Sentandreu, M.A.; López-Pedrouso, M.; et al. Impacto Del Modo de Transporte al Matadero (Mezcla o No Mezcla Con Animales Extraños) de Terneros de Las Razas Asturiana de Los Valles y Retinta En Los Atributos Físico químicos y Organolépticos de Carne Madurada. Aceptabilidad y Preferencia de Los Consumidores. ITEA Inf. Tec. Econ. Agrar. 2021, 118, 213–218. [Google Scholar] [CrossRef]
- Blanco, M.; Ripoll, G.; Casasús, I.; Bertolín, J.R.; Joy, M. Carotenoids and Tocopherol in Plasma and Subcutaneous Fat Colour to Trace Forage-Feeding in Growing Steers. Livest. Sci. 2019, 219, 104–110. [Google Scholar] [CrossRef]
- Blanco, M.; Ripoll, G.; Delavaud, C.; Casasús, I. Performance, Carcass and Meat Quality of Young Bulls, Steers and Heifers Slaughtered at a Common Body Weight. Livest. Sci. 2020, 240, 104156. [Google Scholar] [CrossRef]
- Vieira, C.; Cerdeño, A.; Serrano, E.; Lavín, P.; Mantecón, A.R. Breed and Ageing Extent on Carcass and Meat Quality of Beef from Adult Steers (Oxen). Livest. Sci. 2007, 107, 62–69. [Google Scholar] [CrossRef]
- Varela, A.; Oliete, B.; Moreno, T.; Portela, C.; Monserrrat, L.; Carballo, J.A.; Sánchez, L. Effect of Pasture Finishing on the Meat Characteristics and Intramuscular Fatty Acid Profile of Steers of the Rubia Gallega Breed. Meat Sci. 2004, 67, 515–522. [Google Scholar] [CrossRef]
- Schmutz, M.; Weindl, P.; Carrasco, S.; Bellof, G.; Schmidt, E. Effect of Breed, Grazing System and Concentrate Supplementation on Fattening Performance, Carcass Value and Meat Quality of Steers. Arch. Anim. Breed. 2013, 56, 943–957. [Google Scholar] [CrossRef]
- Avilés, C.; Martínez, A.L.; Domenech, V.; Peña, F. Effect of Feeding System and Breed on Growth Performance, and Carcass and Meat Quality Traits in Two Continental Beef Breeds. Meat Sci. 2015, 107, 94–103. [Google Scholar] [CrossRef]
- Kempster, A.J. Fat Partition and Distribution in the Carcasses of Cattle, Sheep and Pigs: A Review. Meat Sci. 1981, 5, 83–98. [Google Scholar] [CrossRef]
- Lawrie, R.A.; Ledward, D.A. Lawrie’s Meat Science; Woodhead Publishing: Sawston, UK, 2014. [Google Scholar]
- Galli, I.; Teira, G.; Perlo, F.; Bonato, P.; Tisocco, O.; Monje, A.; Vittone, S. Animal Performance and Meat Quality in Cull Cows with Early Weaned Calves in Argentina. Meat Sci. 2008, 79, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Marenčić, D.; Ivanković, A.; Kozačinski, L.; Popović, M.; Cvrtila, Ž. The Effect of Sex and Age at Slaughter on the Physicochemical Properties of Baby-Beef Meat. Vet. Arh. 2018, 88, 101–110. [Google Scholar] [CrossRef]
- Du Plessis, I.; Hoffman, L. Effect of Slaughter Age and Breed on the Carcass Traits and Meat Quality of Beef Steers Finished on Natural Pastures in the Arid Subtropics of South Africa. S. Afr. J. Anim. Sci. 2007, 37, 143–153. [Google Scholar] [CrossRef]
- Martin Polo, J.L.; García Bellido, I.; Sánchez Rodríguez, M.E. Meat Production on Savannah-like Grasslands (dehesas) in Semi-Arid Zones of the Province of Salamanca. Span. J. Agric. Res. 2004, 2, 107. (In Spanish) [Google Scholar] [CrossRef]
- Page, J.K.; Wulf, D.M.; Schwotzer, T.R. A Survey of Beef Muscle Color and PH. J. Anim. Sci. 2001, 79, 678. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B.; Monteils, V. Associations among animal, carcass, muscle characteristics, and fresh meat color traits in Charolais cattle. Meat Sci. 2018, 140, 145–156. [Google Scholar] [CrossRef]
- De Smet, S.; Claeys, E.; Buysse, G.; Lenaerts, C.; Demeyer, D. Tenderness Measurements in Four Muscles of Belgian Blue Normal and Double-Muscled Bulls. In Proceedings of the 44th ICoMST, Barcelona, Spain, 30 August–4 September 1998; pp. 288–289. [Google Scholar]
- Duckett, S.K.; Neel, J.P.S.; Lewis, R.M.; Fontenot, J.P.; Clapham, W.M. Effects of Forage Species or Concentrate Finishing on Animal Performance, Carcass and Meat Quality1,2. J. Anim. Sci. 2013, 91, 1454–1467. [Google Scholar] [CrossRef] [PubMed]
- Albertí Lasalle, P.; Lahoz Castelló, F.; Tena Gómez, R.; Jaime Cuello, S.; Sañudo Astiz, C.; Olleta Castañer, J.L.; Campo Arribas, M.d.M.; Panea Doblado, B.; Pardos, J.J. Producción y Rendimiento Carnicero de Siete Razas Bovinas Españolas Faenadas a Distintos Pesos. Inf. Técnicas/D.G. Tecnol. Agrar. 2001, 101, 1–15. [Google Scholar]
- Conroy, S.B.; Drennan, M.J.; McGee, M.; Keane, M.G.; Kenny, D.A.; Berry, D.P. Predicting beef carcass meat, fat and bone proportions from carcass conformation and fat scores or hindquarter dissection. Animal 2009, 4, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Nogalski, Z.; Pogorzelska-Przybyłek, P.; Sobczuk-Szul, M.; Nogalska, A.; Modzelewska-Kapituła, M.; Purwin, C. Carcass characteristics and meat quality of bulls and steers slaughtered at two different ages. Ital. J. Anim. Sci. 2018, 17, 279–288. [Google Scholar] [CrossRef]
- Oliván, M.; Martínez, A.; García, P.; Noval, G.; Osoro, K. Estimation of the carcass composition of yearling bulls of “Asturiana de los Valles” breed from the dissection of a rib joint. Meat Sci. 2001, 57, 185–190. [Google Scholar] [CrossRef]
- Fruet, A.P.B.; Stefanello, F.S.; Trombetta, F.; De Souza, A.N.M.; Rosado Júnior, A.G.; Tonetto, C.J.; Flores, J.L.C.; Scheibler, R.B.; Bianchi, R.M.; Pacheco, P.S.; et al. Growth Performance and Carcass Traits of Steers Finished on Three Different Systems Including Legume–Grass Pasture and Grain Diets. Animal 2019, 13, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P.; Warner, R.D.; Clarke, F.M.; Hughes, J.M. Variations in Meat Colour Due to Factors Other than Myoglobin Chemistry; a Synthesis of Recent Findings (Invited Review). Meat Sci. 2020, 159, 107941. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, G.; Albertí, P.; Panea, B.; Failla, S.; Hocquette, J.F.; Dunner, S.; Sañudo, C.; Olleta, J.L.; Christensen, M.; Ertbjerg, P.; et al. Colour Variability of Beef in Young Bulls from Fifteen European Breeds. Int. J. Food Sci. Technol. 2018, 53, 2777–2785. [Google Scholar] [CrossRef]
- Albertí, P.; Ripoll, G.; Albertí, C.; Panea, B. Etude de La Couleur Des Différents Viande Bovine Vendus En Espagne. Viandes Prod. Carnés 2017, 33, 1–9. [Google Scholar]
- Priolo, A.; Micol, D.; Agabriel, J. Effects of Grass Feeding Systems on Ruminant Meat Colour and Flavour. A Review. Anim. Res. 2001, 50, 185–200. [Google Scholar] [CrossRef]
- Qian, S.; Li, X.; Wang, H.; Wei, X.; Mehmood, W.; Zhang, C.; Blecker, C. Contribution of Calpain to Protein Degradation, Variation in Myowater Properties and the Water-Holding Capacity of Pork during Postmortem Ageing. Food Chem. 2020, 324, 126892. [Google Scholar] [CrossRef]
- Lucherk, L.W.; O’Quinn, T.G.; Legako, J.F.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Consumer and Trained Panel Evaluation of Beef Strip Steaks of Varying Marbling and Enhancement Levels Cooked to Three Degrees of Doneness. Meat Sci. 2016, 122, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Destefanis, G.; Brugiapaglia, A.; Barge, M.T.; Dal Molin, E. Relationship between Beef Consumer Tenderness Perception and Warner–Bratzler Shear Force. Meat Sci. 2008, 78, 153–156. [Google Scholar] [CrossRef]
- Shackelford, S.D.; Koohmaraie, M.; Miller, M.F.; Crouse, J.D.; Reagan, J.O. An Evaluation of Tenderness of the Longissimus Muscle of Angus by Hereford versus Brahman Crossbred Heifers. J. Anim. Sci. 1991, 69, 171. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.F.; Carr, M.A.; Ramsey, C.B.; Crockett, K.L.; Hoover, L.C. Consumer Thresholds for Establishing the Value of Beef Tenderness. J. Anim. Sci. 2001, 79, 3062. [Google Scholar] [CrossRef]
- Giaretta, E.; Mordenti, A.L.; Canestrari, G.; Brogna, N.; Palmonari, A.; Formigoni, A. Assessment of Muscle Longissimus Thoracis et Lumborum Marbling by Image Analysis and Relationships between Meat Quality Parameters. PLoS ONE 2018, 13, e0202535. [Google Scholar] [CrossRef]
- Duarte, M.S.; Paulino, P.V.R.; Fonseca, M.A.; Diniz, L.L.; Cavali, J.; Serão, N.V.L.; Gomide, L.A.M.; Reis, S.F.; Cox, R.B. Influence of Dental Carcass Maturity on Carcass Traits and Meat Quality of Nellore Bulls. Meat Sci. 2011, 88, 441–446. [Google Scholar] [CrossRef]
- Lucero-Borja, J.; Pouzo, L.B.; de la Torre, M.S.; Langman, L.; Carduza, F.; Corva, P.M.; Santini, F.J.; Pavan, E. Slaughter Weight, Sex and Age Effects on Beef Shear Force and Tenderness. Livest. Sci. 2014, 163, 140–149. [Google Scholar] [CrossRef]
- Vasta, V.; Priolo, A. Ruminant Fat Volatiles as Affected by Diet. A Review. Meat Sci. 2006, 73, 218–228. [Google Scholar] [CrossRef]
- Gorraiz, C.; Beriain, M.J.; Chasco, J.; Insausti, K. Effect of Aging Time on Volatile Compounds, Odor, and Flavor of Cooked Beef from Pirenaica and Friesian Bulls and Heifers. J. Food Sci. 2002, 67, 916–922. [Google Scholar] [CrossRef]
- Koutsidis, G.; Elmore, J.S.; Oruna-Concha, M.J.; Campo, M.M.; Wood, J.D.; Mottram, D.S. Water-Soluble Precursors of Beef Flavour. Part II: Effect of Post-Mortem Conditioning. Meat Sci. 2008, 79, 270–277. [Google Scholar] [CrossRef]
- King, N.J.; Whyte, R. Does It Look Cooked? A Review of Factors That Influence Cooked Meat Color. J. Food Sci. 2006, 71, R31–R40. [Google Scholar] [CrossRef]
- Bello Acebrón, L.; Calvo Dopico, D. The Importance of Intrinsic and Extrinsic Cues to Expected and Experienced Quality: An Empirical Application for Beef. Food Qual. Prefer. 2000, 11, 229–238. [Google Scholar] [CrossRef]
- Fořtová, J.; del Mar Campo, M.; Valenta, J.; Needham, T.; Řehák, D.; Lebedová, N.; Bartoň, L.; Klouček, P.; Bureš, D. Preferences and Acceptance of Czech and Spanish Consumers Regarding Beef with Varying Intramuscular Fat Content. Meat Sci. 2022, 192, 108912. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Monteiro, M.J.; Voss, H.-P.; Komora, N.; Teixeira, P.; Pintado, M. The Most Important Attributes of Beef Sensory Quality and Production Variables That Can Affect It: A Review. Livest. Sci. 2021, 250, 104573. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat Flavor Precursors and Factors Influencing Flavor Precursors—A Systematic Review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D.; Meinert, L. Meat Flavour in Pork and Beef—From Animal to Meal. Meat Sci. 2017, 132, 112–117. [Google Scholar] [CrossRef]
- Hilton, G.G.; Tatum, J.D.; Williams, S.E.; Belk, K.E.; Williams, F.L.; Wise, J.W.; Smith, G.C. An Evaluation of Current and Alternative Systems for Quality Grading Carcasses of Mature Slaughter Cows. J. Anim. Sci. 1998, 76, 2094. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ellies-Oury, M.-P.; Stoyanchev, T.; Hocquette, J.-F. Consumer Perception of Beef Quality and How to Control, Improve and Predict It? Focus on Eating Quality. Foods 2022, 11, 1732. [Google Scholar] [CrossRef] [PubMed]
- Schönfeldt, H.C.; Strydom, P.E. Effect of Age and Cut on Tenderness of South African Beef. Meat Sci. 2011, 87, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ellies-Oury, M.-P.; Chriki, S.; Legrand, I.; Pogorzelski, G.; Wierzbicki, J.; Farmer, L.; Troy, D.; Polkinghorne, R.; Hocquette, J.-F. Contributions of Tenderness, Juiciness and Flavor Liking to Overall Liking of Beef in Europe. Meat Sci. 2020, 168, 108190. [Google Scholar] [CrossRef]
- Miller, R. Drivers of Consumer Liking for Beef, Pork, and Lamb: A Review. Foods 2020, 9, 428. [Google Scholar] [CrossRef]
| Variable 2 | Mean ± SE | Age at Slaughter 3 | Farm | |||
|---|---|---|---|---|---|---|
| I | II | III | p-Value | p-Value 4 | ||
| LHCW (kg) | 59.48 ± 0.93 | 53.41 ± 2.22 a | 56.77 ± 1.41 a | 68.27 ± 1.95 b | <0.001 | ns |
| CL (cm) | 114.03 ± 0.46 | 108.59 ± 1.07 a | 110.75 ± 1.09 a | 122.76 ± 1.21 b | <0.001 | ns |
| DCh (cm) | 38.80 ± 0.18 | 36.79 ± 0.43 a | 37.86 ± 0.27 b | 41.77 ± 0.38 c | <0.001 | ns |
| HL (cm) | 70.84 ± 0.78 | 67.39 ± 1.80 a | 70.14 ± 1.14 a | 74.99 ± 1.59 b | <0.01 | ns |
| HP (cm) | 78.48 ± 0.76 | 76.01 ± 1.84 a | 78.75 ± 1.16 ab | 80.67 ± 1.61 b | <0.01 | <0.01 |
| CC (kg/cm) | 1.03 ± 0.13 | 0.98 ± 2.22 a | 1.02 ± 2.22 a | 1.11 ± 2.22 b | <0.01 | ns |
| CS (1-15) | 5.50 ± 0.04 | 5.52 ± 0.09 | 5.54 ± 0.06 | 5.44 ± 0.08 | ns | <0.05 |
| FS (1-15) | 5.85 ± 0.15 | 5.86 ± 0.37 | 5.56 ± 0.23 | 6.14 ± 0.42 | ns | <0.05 |
| Variable 2 | Mean ± SE | Age at Slaughter 3 | Farm | |||
|---|---|---|---|---|---|---|
| I | II | III | p-Value 4 | p-Value 4 | ||
| pH24 | 5.66 ± 0.03 | 5.67 ± 0.06 | 5.65 ± 0.04 | 5.66 ± 0.06 | ns | <0.05 |
| Subcutaneous fat | ||||||
| L* | 59.77 ± 0.63 | 62.17 ± 1.51 | 59.82 ± 0.97 | 57.33 ± 1.34 | ns | ns |
| a* | 8.22 ± 0.48 | 5.62 ± 1.15 a | 7.54 ± 0.73 a | 11.50 ± 1.02 b | <0.001 | ns |
| b* | 23.48 ± 0.33 | 19.44 ± 1.74 a | 20.60 ± 1.12 a | 30.41 ± 1.54 b | <0.001 | <0.05 |
| Chroma | 25.06 ± 0.37 | 20.45 ± 1.97 a | 21.92 ± 1.27 a | 32.80 ± 1.74 b | <0.001 | <0.05 |
| Hue angle | 72.03 ± 0.80 | 74.64 ± 1.92 | 71.41 ± 1.23 | 70.06 ± 1.69 | ns | <0.05 |
| Rectus abdominis | ||||||
| L* | 38.46 ± 0.38 | 39.03 ± 0.89 | 38.12 ± 0.57 | 38.24 ± 0.79 | ns | <0.01 |
| a* | 15.85 ± 0.37 | 14.94 ± 0.88 a | 14.69 ± 0.56 a | 17.90 ± 0.77 b | <0.01 | ns |
| b* | 15.03 ± 0.38 | 13.58 ± 0.89 a | 15.13 ± 0.57 ab | 16.37 ± 0.79 b | <0.01 | ns |
| Chroma | 21.93 ± 0.49 | 20.09 ± 1.16 a | 21.36 ± 0.74 a | 24.35 ± 1.02 b | <0.01 | ns |
| Hue angle | 44.22 ± 0.53 | 46.42 ± 1.26 | 43.50 ± 0.81 | 42.75 ± 1.11 | ns | <0.05 |
| Variable 2 | Mean ± SE | Age at Slaughter 3 | Farm | |||
|---|---|---|---|---|---|---|
| I | II | III | p-Value 4 | p-Value 4 | ||
| Rib weight (g) | 1991.76 ± 40.50 | 1885.90 ± 96.81 a | 1930.46 ± 66.14 a | 2158.92 ± 87.76 b | <0.05 | ns |
| SF thickness (cm) | 0.20 ± 0.02 | 0.20 ± 0.04 | 0.22 ± 0.03 | 0.19 ± 0.04 | ns | ns |
| Bone + waste (g) | 483.05 ± 10.36 | 460.37 ± 24.65 | 480.79 ± 16.84 | 504.98 ± 22.34 | ns | <0.01 |
| Bone + waste (%) * | 24.81 ± 0.40 | 24.78 ± 0.95 | 25.22 ± 0.65 | 24.43 ± 0.86 | ns | <0.001 |
| Lean (g) | 1150.20 ± 27.16 | 1098.13 ± 64.98 a | 1110.85 ± 44.39 a | 1233.63 ± 58.91 b | <0.05 | ns |
| Lean (%) * | 58.64 ± 0.55 | 59.12 ± 1.25 | 58.05 ± 0.85 | 58.77 ± 1.13 | ns | <0.000 |
| LT (g) | 138.32 ± 3.57 | 136.96 ± 8.50 | 139.53 ± 5.80 | 138.46 ± 7.70 | ns | <0.05 |
| LT (%) ** | 12.02 ± 0.29 | 12.40 ± 0.40 b | 12.74 ± 0.27 b | 10.91 ± 0.36 a | <0.05 | ns |
| Fat (g) | 312.66 ± 13.71 | 270.59 ± 26.37 a | 280.56 ± 18.01 a | 360.82 ± 23.90 b | <0.05 | <0.01 |
| Fat (%) * | 14.28 ± 0.55 | 13.31 ± 1.00 a | 13.26 ± 0.68 a | 16.25 ± 0.91 b | <0.05 | <0.000 |
| SF (g) | 48.88 ± 4.16 | 48.57 ± 9.86 ab | 35.48 ± 6.73 a | 58.60 ± 8.93 b | <0.05 | <0.01 |
| SF (%) *** | 14.75 ± 0.76 | 17.14 ± 1.81 | 12.67 ± 1.24 | 14.45 ± 1.64 | ns | ns |
| IF (g) | 266.77 ± 10.96 | 222.03 ± 26.37 a | 243.07 ± 18.01 a | 299.22 ± 23.90 b | <0.05 | <0.01 |
| IF (%) *** | 85.21 ± 0.76 | 82.86 ± 1.81 | 87.24 ± 1.24 | 85.53 ± 1.64 | ns | ns |
| Variable 2 | Mean ± SE | Age at Slaughter 3 | Farm | |||
|---|---|---|---|---|---|---|
| I | II | III | p-Value 4 | p-Value 4 | ||
| pH | 5.73 ± 0.02 | 5.87 ± 0.06 b | 5.64 ± 0.04 a | 5.68 ± 0.06 a | <0.01 | <0.05 |
| L* | 25.49 ± 0.43 | 24.51 ± 1.02 | 26.13 ± 0.65 | 25.83 ± 0.90 | ns | ns |
| a* | 14.42 ± 0.33 | 14.28 ± 0.74 | 14.64 ± 0.47 | 13.90 ± 0.66 | ns | ns |
| b* | 12.86 ± 0.23 | 11.86 ± 0.54 a | 13.69 ± 0.35 b | 13.02 ± 0.48 ab | <0.05 | ns |
| Chroma | 19.63 ± 0.34 | 18.77 ± 0.77 | 20.13 ± 0.49 | 19.08 ± 0.68 | ns | ns |
| Hue angle | 42.40 ± 0.64 | 40.38 ± 1.44 | 43.18 ± 0.93 | 42.86 ± 1.28 | ns | ns |
| Thawing loss (%) | 5.34 ± 0.26 | 5.45 ± 0.58 | 4.73 ± 0.37 | 4.37 ± 0.51 | ns | ns |
| Drip loss (%) | 0.97 ± 0.02 | 0.96 ± 0.13 | 1.11 ± 0.08 | 0.85 ± 0.12 | ns | <0.01 |
| Pressure loss (%) | 8.90 ± 0.23 | 7.97 ± 0.51 | 9.21 ± 0.33 | 9.52 ± 0.45 | ns | ns |
| Cooking loss (%) | 22.16 ± 0.40 | 21.75 ± 0.97 | 23.12 ± 0.62 | 21.61 ± 0.88 | ns | ns |
| WBSF (kg/cm2) | 4.59 ± 0.16 | 4.07 ± 0.37 | 4.55 ± 0.24 | 5.14 ± 0.33 | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantarero-Aparicio, M.Á.; Angón, E.; González-Esquivel, C.; Peña, F.; Caballero-Villalobos, J.; Ryan, E.G.; Perea, J.M. Carcass and Meat Quality Traits in Female Lidia Cattle Slaughtered at Different Ages. Animals 2024, 14, 850. https://doi.org/10.3390/ani14060850
Cantarero-Aparicio MÁ, Angón E, González-Esquivel C, Peña F, Caballero-Villalobos J, Ryan EG, Perea JM. Carcass and Meat Quality Traits in Female Lidia Cattle Slaughtered at Different Ages. Animals. 2024; 14(6):850. https://doi.org/10.3390/ani14060850
Chicago/Turabian StyleCantarero-Aparicio, Miguel Ángel, Elena Angón, Carlos González-Esquivel, Francisco Peña, Javier Caballero-Villalobos, Eoin G. Ryan, and José Manuel Perea. 2024. "Carcass and Meat Quality Traits in Female Lidia Cattle Slaughtered at Different Ages" Animals 14, no. 6: 850. https://doi.org/10.3390/ani14060850
APA StyleCantarero-Aparicio, M. Á., Angón, E., González-Esquivel, C., Peña, F., Caballero-Villalobos, J., Ryan, E. G., & Perea, J. M. (2024). Carcass and Meat Quality Traits in Female Lidia Cattle Slaughtered at Different Ages. Animals, 14(6), 850. https://doi.org/10.3390/ani14060850








