Histiocytic Sarcoma in a Captive Hybrid Orangutan (Pongo sp.): Morphological and Immunohistochemical Features
Abstract
Simple Summary
Abstract
1. Introduction
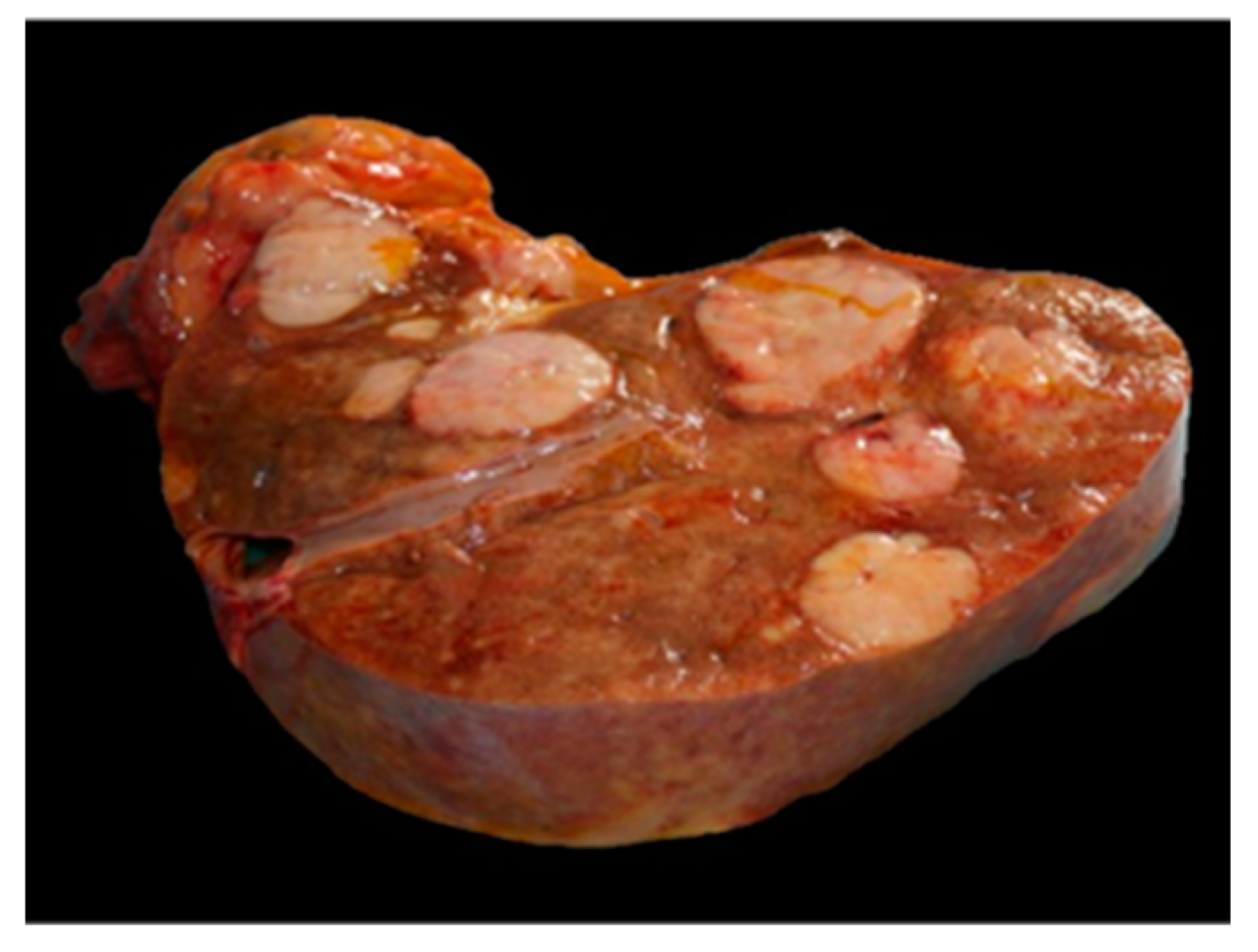
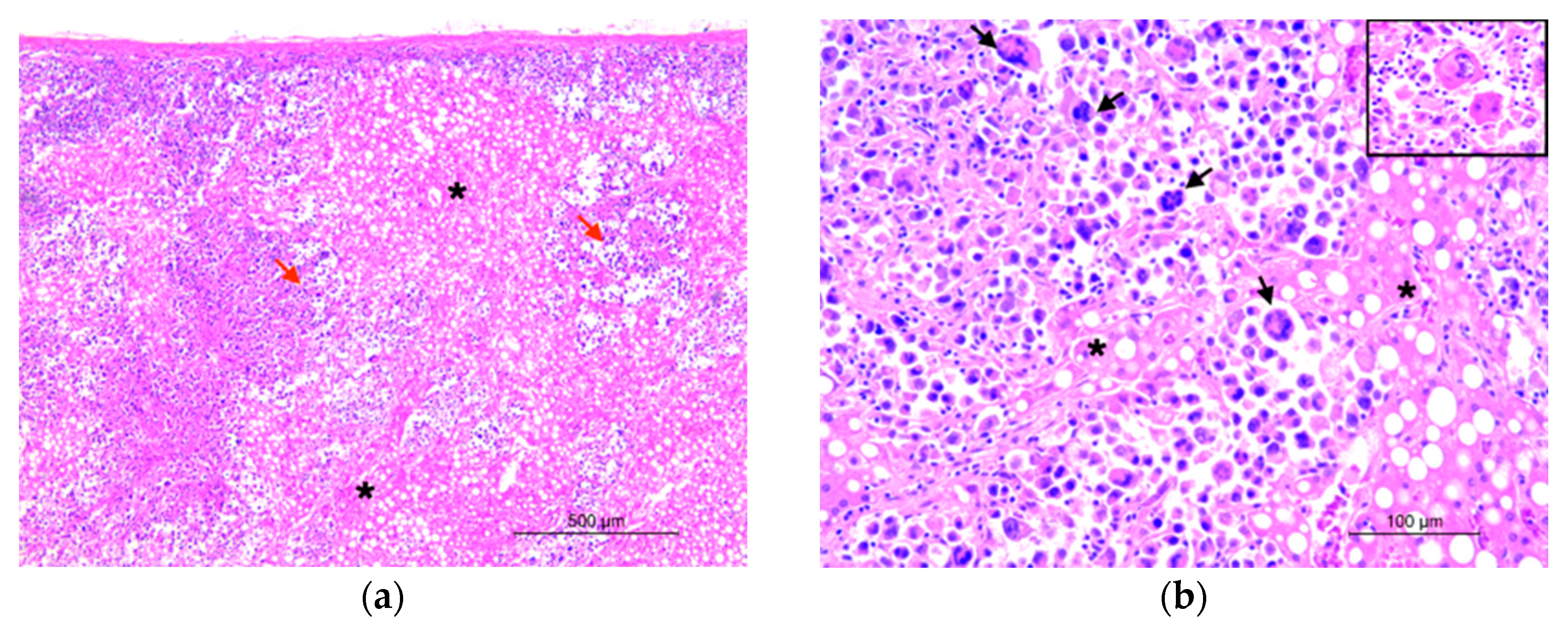
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erich, S.A.; Dobson, J.M.; Teske, E. Comparison of the Clinical Characteristics of Histiocytic Sarcoma in Bernese Mountain Dogs and Flat-Coated Retrievers. Vet. Sci. 2022, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Setoguchi-Mukai, A.; Nakagawa, T.; Uetsuka, K.; Nakayama, H.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Disseminated histiocytic sarcoma with excessive hemophagocytosis in a cat. J. Vet. Med. Sci. 2009, 71, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Son, N.V.; Chambers, J.K.; Dung, L.T.; Kishimoto, T.E.; Nishimura, M.; Kita, C.; Takada, Y.; Miwa, Y.; Nakayama, H.; Uchida, K. Histological and Immunohistochemical Features of Normal Histiocytes and Langerhans Cells, and Histiocytic Sarcomas in Four-Toed Hedgehogs (Atelerix albiventris). J. Comp. Pathol. 2020, 178, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Boonsri, K.; Dechkajorn, S.; Photichai, K.; Srivorakul, S.; Boonsriroj, H.; Thongtharb, A.; Pringproa, K. Disseminated histiocytic sarcoma in Asian palm civet (Paradoxurus hermaphroditus). J. Vet. Med. Sci. 2021, 83, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, D.S.; Stacy, N.I.; Kinsel, M.J.; de Wit, M. Disseminated Histiocytic Sarcoma in Two Free-Living Florida Manatees (Trichechus manatus latirostris). J. Wildl. Dis. 2022, 58, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Nakamura, S. Histiocytic sarcoma: An updated literature review based on the 2008 WHO classification. J. Clin. Exp. Hematop. 2013, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Soshin, T.; Adachi, K.; Suzuki, S.; Kanisawa, K. Histiocytic Sarcoma in a Cynomolgus Macaque (Macaca fascicularis) Fed with a High-Fat Diet. J. Toxicol. Pathol. 2008, 21, 69–72. [Google Scholar] [CrossRef][Green Version]
- Remick, A.K.; Van Wettere, A.J.; Williams, C.V. Neoplasia in prosimians: Case series from a captive prosimian population and literature review. Vet. Path. 2009, 46, 746–772. [Google Scholar] [CrossRef] [PubMed]
- Canuti, M.; Williams, C.V.; Gadi, S.R.; Jebbink, M.F.; Oude Munnink, B.B.; Jazaeri Farsani, S.M.; Cullen, J.M.; van der Hoek, L. Persistent viremia by a novel parvovirus in a slow loris (Nycticebus coucang) with diffuse histiocytic sarcoma. Front. Microbiol. 2014, 5, 655. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, A.; Díaz-Delgado, J.; Balamayooran, G.; Anguiano, M.; Groch, K.; Krol, L. Leukemic histiocytic sarcoma in a captive common squirrel monkey (Saimiri sciureus) with Saimiriine Gammaherpesvirus 2 (Rhadinovirus), Saimiri sciureus lymphocryptovirus 2 (Lymphocryptovirus) and Squirrel monkey retrovirus (β-Retrovirus) coinfection. J. Med. Primatol. 2020, 49, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Yamamoto, Y.; Tamaru, M.; Shimoda, H.; Sakai, Y.; Morimoto, M. Disseminated histiocytic sarcoma in a squirrel monkey (Saimiri sciureus). J. Med. Primatol. 2023, 52, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.F. Histiocytic Diseases. Vet. Clin. North. Am. Small Anim. Pract. 2023, 53, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Ablashi, D.V.; Pearson, G.R. Animal models: Herpesvirus saimiri, a nonhuman primate model for herpesvirus-associated neoplasia of man. Cancer Res. 1974, 34, 1232–1236. [Google Scholar] [PubMed]
- Qayyum, S.; Choi, J.K. Adult T-cell leukemia/lymphoma. Arch. Pathol. Lab. Med. 2014, 138, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.F. Canine and feline histiocytic diseases. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhang, X.; Wang, L.P.; Ziober, A.; Zhang, P.J.; Bagg, A. Ionized Calcium Binding Adaptor Molecule 1 (IBA1): A Novel, Highly Sensitive, and Specific Marker for Histiocytic, Dendritic, and Monocytic Neoplasms. Am. J. Clin. Pathol. 2021, 156, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Pierezan, F.; Mansell, J.; Ambrus, A.; Rodrigues Hoffmann, A. Immunohistochemical expression of ionized calcium binding adapter molecule 1 in cutaneous histiocytic proliferative, neoplastic and inflammatory disorders of dogs and cats. J. Comp. Pathol. 2014, 151, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Wang, L.; Xu, S.; Fang, G.; Guo, X.; Chen, Z.; Gu, Z. PBPK Modeling on Organs-on-Chips: An Overview of Recent Advancements. Front. Bioeng. Biotechnol. 2022, 10, 900481. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Gardin, J.F.; Tooze, J.A.; Cline, J.M. Organ Weights in Relation to Age and Sex in Cynomolgus Monkeys (Macaca fascicularis). Toxicol. Pathol. 2022, 50, 574–590. [Google Scholar] [CrossRef] [PubMed]
- Van Devanter, D.R.; Warrener, P.; Bennet, L.; Schultz, E.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and Analysis of Diverse Herpesviral Species by Consensus Primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Skala, S.L.; Lucas, D.R.; Dewar, R. Histiocytic Sarcoma: Review, Discussion of Transformation From B-Cell Lymphoma, and Differential Diagnosis. Arch. Pathol. Lab. Med. 2018, 142, 1322–1329. [Google Scholar] [CrossRef] [PubMed]

| Antigen b | Antibody c | Clone | Dilution | Source | Result |
|---|---|---|---|---|---|
| Iba-1 | rabbit pAb | N/A | 1:300 | FUJIFILM Wako, Osaka, Japan | + |
| HLA-DR | mouse mAb | TAL 1B5 | 1:100 | Novus Biologicals, Centennial, CO, USA | + |
| CD204 | mouse mAb | SRA-E5 | 1:500 | TransGenic Inc., Kumamoto, Japan | - |
| CD163 | mouse mAb | AM-3K | 1:75 | TransGenic Inc., Kumamoto, Japan | - |
| CD3 | rabbit pAb | N/A | 1:200 | Dako, Glostrup, Denmark | - |
| CD79a | mouse mAb | HM57 | 1:100 | Santa Cruz, Dallas, TX, USA | - |
| Ki67 * | mouse mAb | MIB-1 | 1:100 | Dako, Glostrup, Denmark | + |
| Vimentin | mouse mAb | V9 | 1:100 | Dako, Glostrup, Denmark | + |
| pan-Cytokeratin | mouse mAb | AE1/AE3 | 1:100 | Santa Cruz, Dallas, TX, USA | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galietta, V.; Fonti, N.; Cocumelli, C.; Raso, C.; Di Cerbo, P.; Parisi, F.; Bovi, E.; Parmigiani, R.; Pietrella, G.; Cersini, A.; et al. Histiocytic Sarcoma in a Captive Hybrid Orangutan (Pongo sp.): Morphological and Immunohistochemical Features. Animals 2024, 14, 852. https://doi.org/10.3390/ani14060852
Galietta V, Fonti N, Cocumelli C, Raso C, Di Cerbo P, Parisi F, Bovi E, Parmigiani R, Pietrella G, Cersini A, et al. Histiocytic Sarcoma in a Captive Hybrid Orangutan (Pongo sp.): Morphological and Immunohistochemical Features. Animals. 2024; 14(6):852. https://doi.org/10.3390/ani14060852
Chicago/Turabian StyleGalietta, Valentina, Niccolò Fonti, Cristiano Cocumelli, Caterina Raso, Pilar Di Cerbo, Francesca Parisi, Emanuela Bovi, Raffaella Parmigiani, Gabriele Pietrella, Antonella Cersini, and et al. 2024. "Histiocytic Sarcoma in a Captive Hybrid Orangutan (Pongo sp.): Morphological and Immunohistochemical Features" Animals 14, no. 6: 852. https://doi.org/10.3390/ani14060852
APA StyleGalietta, V., Fonti, N., Cocumelli, C., Raso, C., Di Cerbo, P., Parisi, F., Bovi, E., Parmigiani, R., Pietrella, G., Cersini, A., Friedrich, K. G., & Eleni, C. (2024). Histiocytic Sarcoma in a Captive Hybrid Orangutan (Pongo sp.): Morphological and Immunohistochemical Features. Animals, 14(6), 852. https://doi.org/10.3390/ani14060852





