Maternal Supplementation with Ornithine Promotes Placental Angiogenesis and Improves Intestinal Development of Suckling Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Treatments
2.2. Data Collection and Sampling
2.3. RNA Extraction and Real-Time Quantitative RT-PCR
2.4. Hematoxylin-Eosin (H&E) and Immunofluorescence Staining
2.5. Cell Culture
2.6. ELESA Assay
2.7. Analysis of Polyamine Content in Placenta
2.8. Analysis of Free Amino Acid Content in Placenta
2.9. Small Interfering RNA (siRNA) Transfection
2.10. Tube Formation Assay
2.11. Transwell Assay
2.12. mRNA Stability Assay
2.13. Analyses of Microbial Communities
2.14. Determination of Short Chain Fatty Acids (SCFAs) in Colonic Contents
2.15. Statistical Analysis
3. Results
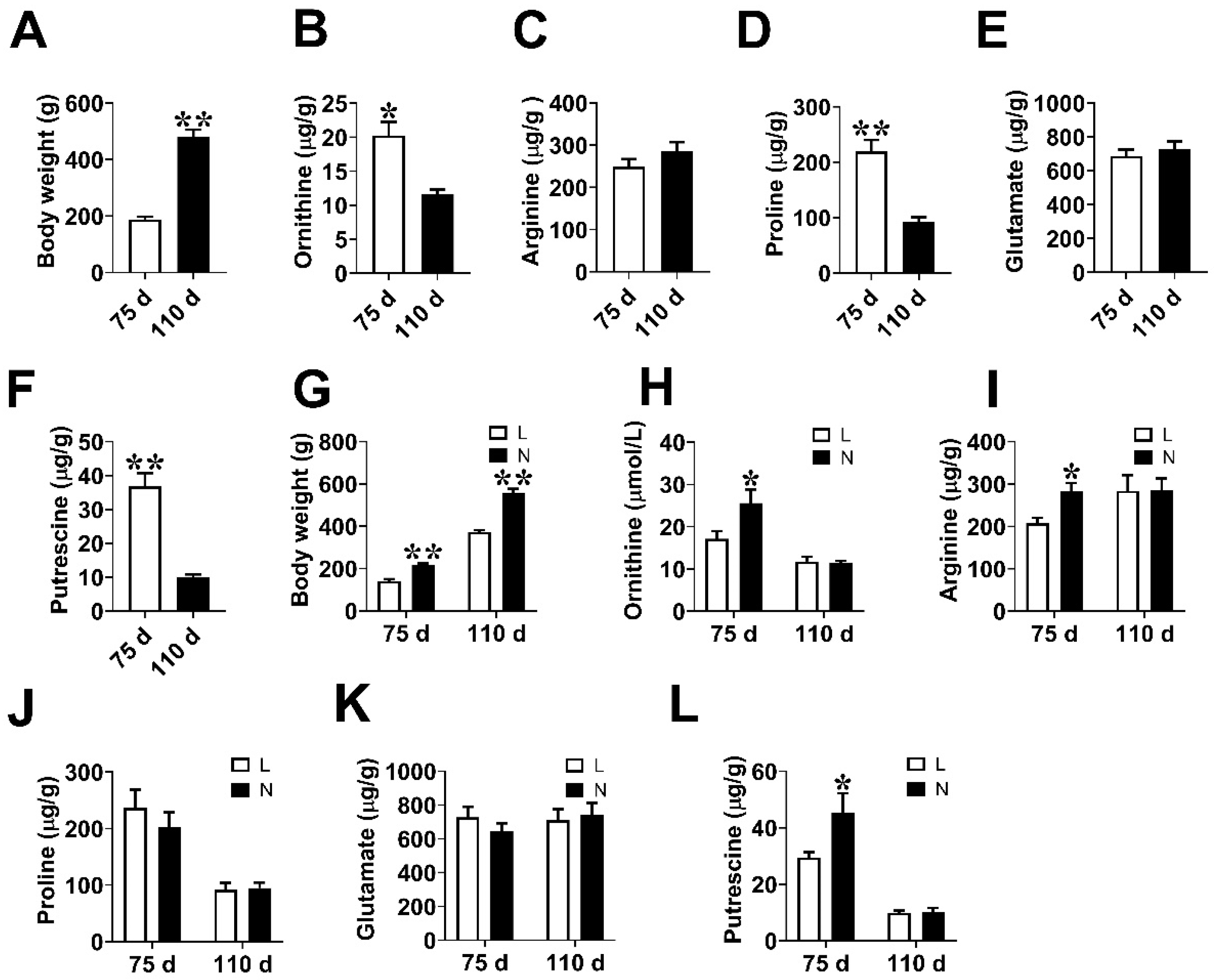
3.1. Fetal Weight and Amino Acids Contents in Placenta
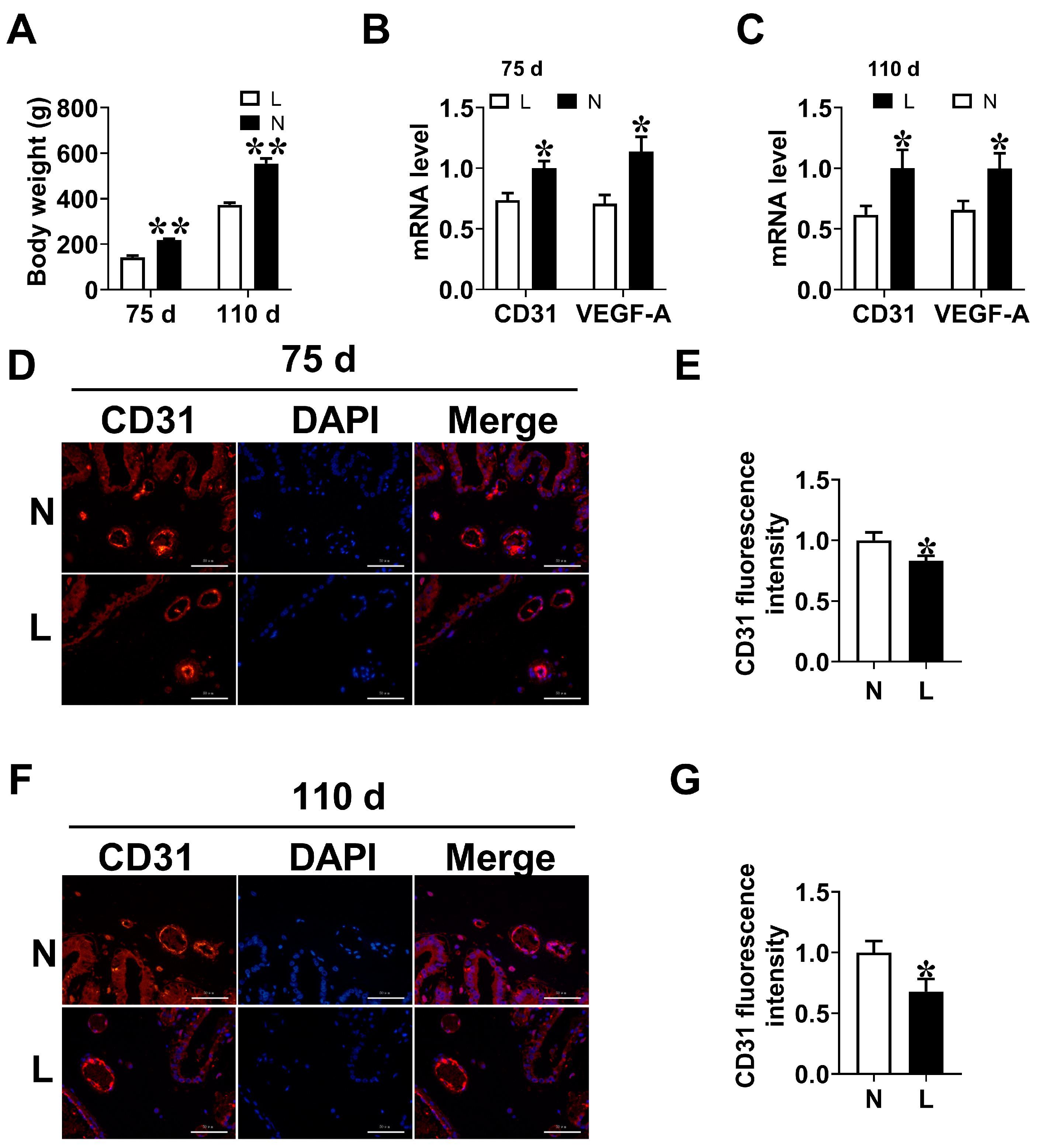
3.2. Placental Vascular Density
3.3. Reproductive Performance of Sows
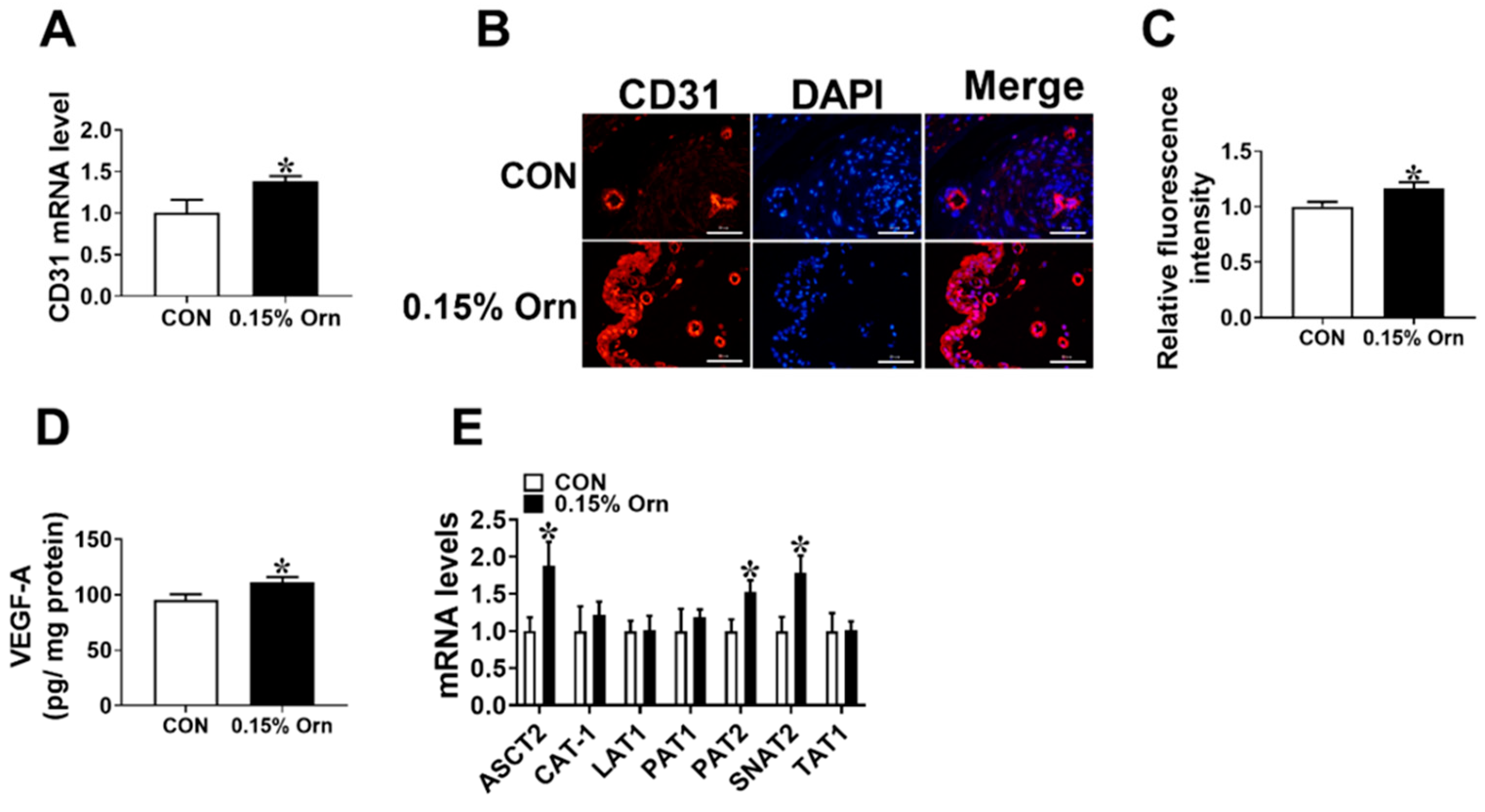
3.4. Maternal Orn Supplementation Promotes Angiogenesis in Placental Vascular Density
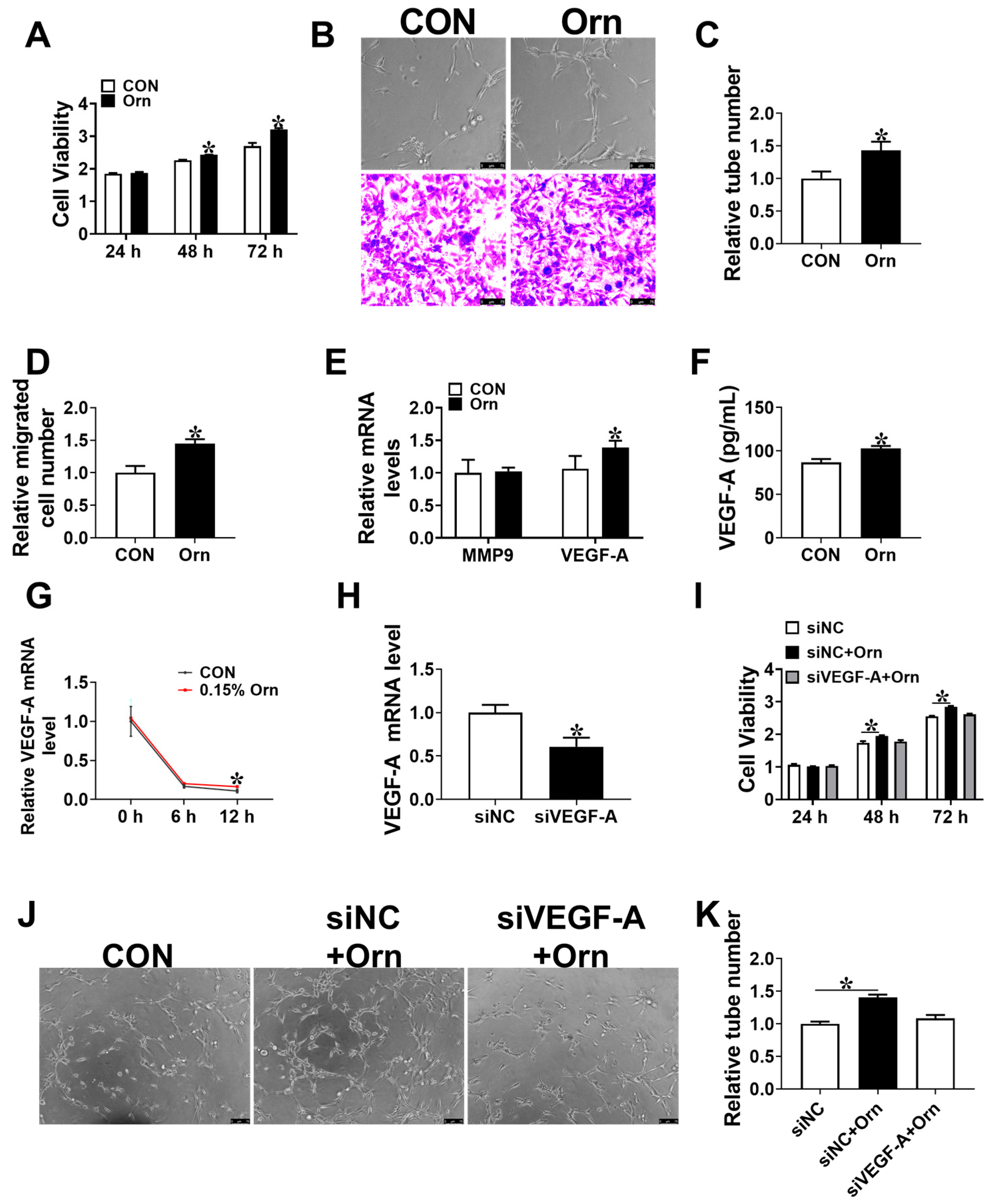
3.5. VEGF-A Mediates Angiogenesis of Orn Controlled
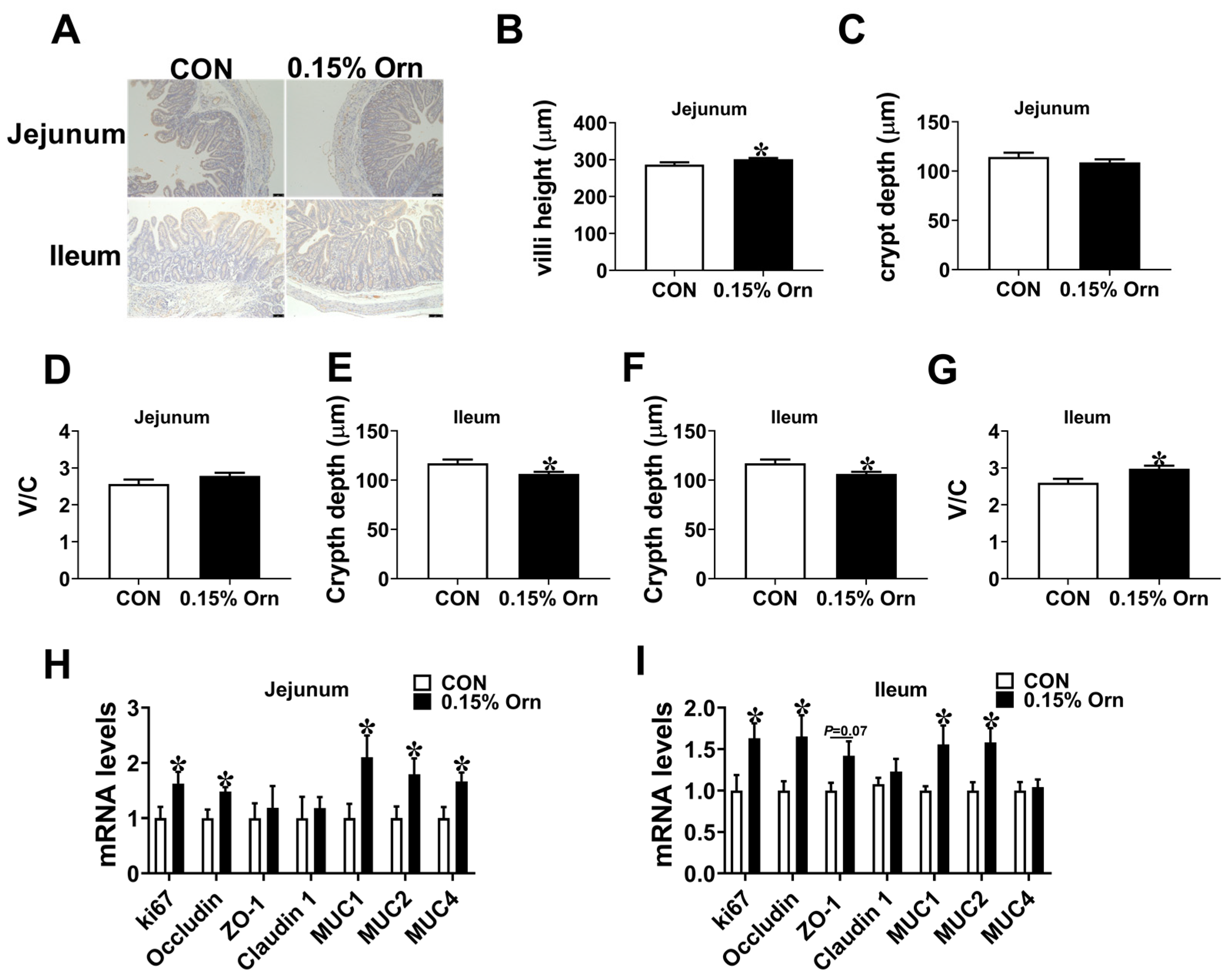
3.6. Intestinal Development of Suckling Piglets
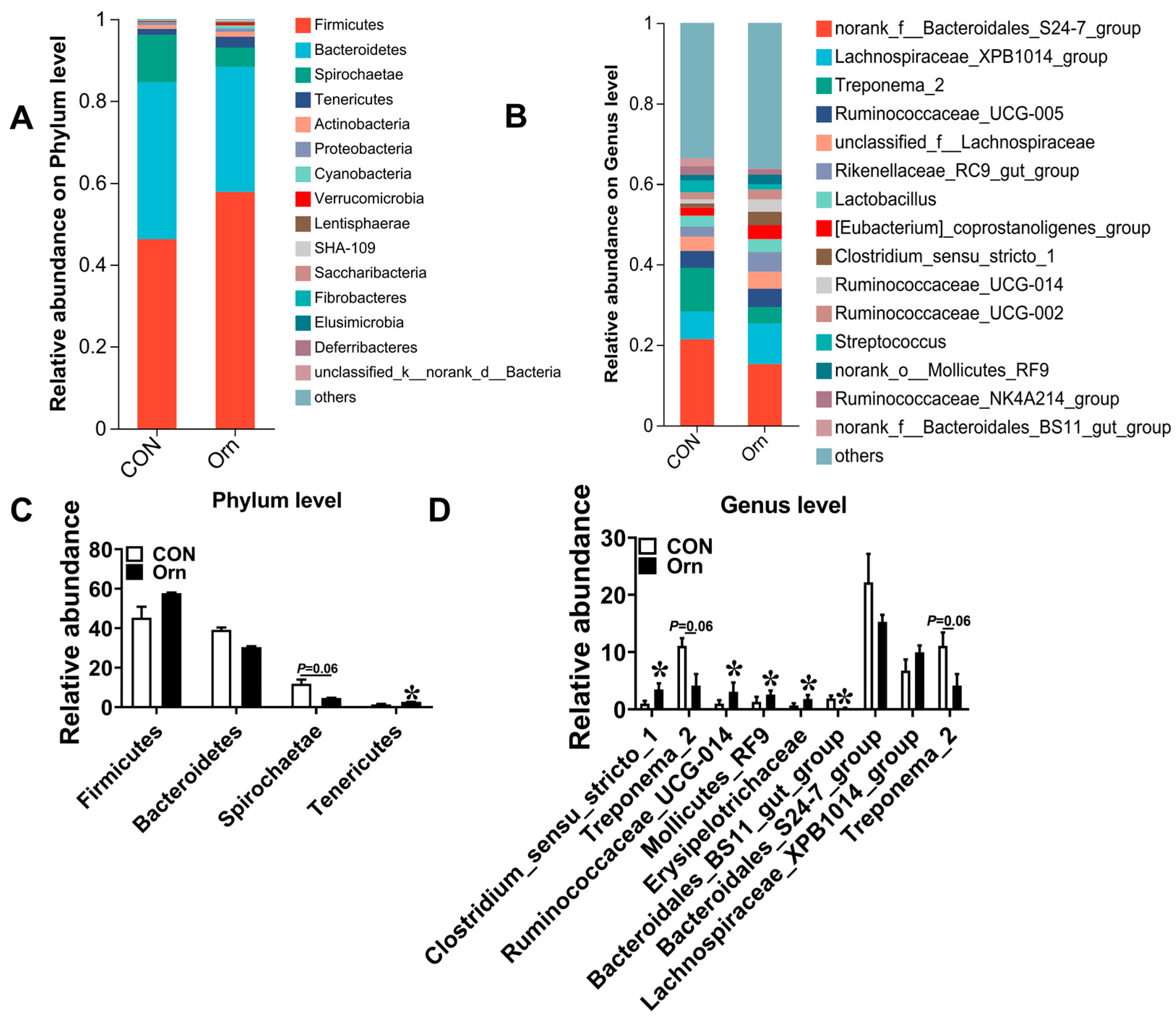
3.7. Colonic Bacterial Community Structure
3.8. SCFAs in Colonic Contents of Suckling Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armengaud, J.B.; Yzydorczyk, C.; Siddeek, B.; Peyter, A.C.; Simeoni, U. Intrauterine growth restriction: Clinical consequences on health and disease at adulthood. Reprod. Toxicol. 2021, 99, 168–176. [Google Scholar] [CrossRef]
- Yang, L.; Feng, L.; Huang, L.; Li, X.; Qiu, W.; Yang, K.; Qiu, J.; Li, H. Maternal Factors for Intrauterine Growth Retardation: Systematic Review and Meta-Analysis of Observational Studies. Reprod. Sci. 2023, 30, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tan, C.; Xin, Z.; Huang, S.; Ma, J.; Zhang, M.; Shu, G.; Luo, H.; Deng, B.; Jiang, Q.; et al. UPLC-Orbitrap-MS/MS Combined with Biochemical Analysis to Determine the Growth and Development of Mothers and Fetuses in Different Gestation Periods on Tibetan Sow Model. Front. Nutr. 2022, 9, 836938. [Google Scholar] [CrossRef] [PubMed]
- Zur, R.L.; Kingdom, J.C.; Parks, W.T.; Hobson, S.R. The Placental Basis of Fetal Growth Restriction. Obstet. Gynecol. Clin. N. Am. 2020, 47, 81–98. [Google Scholar] [CrossRef]
- Crispi, F.; Hernandez-Andrade, E.; Pelsers, M.M.A.L.; Plasencia, W.; Benavides-Serralde, J.A.; Eixarch, E.; Le Noble, F.; Ahmed, A.; Glatz, J.F.C.; Nicolaides, K.H.; et al. Cardiac dysfunction and cell damage across clinical stages of severity in growth-restricted fetuses. Am. J. Obstet. Gynecol. 2008, 199, 254.e1–254.e8. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.H.; Bhalla, M.; Hyeon, S.J.; Oh, J.E.; Yoo, S.; Chae, U.; Kwon, J.; Koh, W.; Lim, J.; Park, Y.M.; et al. Astrocytic urea cycle detoxifies Aβ-derived ammonia while impairing memory in Alzheimer’s disease. Cell Metab. 2022, 34, 1104–1120.e8. [Google Scholar] [CrossRef]
- Sivashanmugam, M.; Jaidev, J.; Umashankar, V.; Sulochana, K.N. Ornithine and its role in metabolic diseases: An appraisal. Biomed. Pharmacother. 2017, 86, 185–194. [Google Scholar] [CrossRef]
- Liu, N.; Dai, Z.; Zhang, Y.; Jia, H.; Chen, J.; Sun, S.; Wu, G.; Wu, Z. Maternal L-proline supplementation during gestation alters amino acid and polyamine metabolism in the first generation female offspring of C57BL/6J mice. Amino Acids 2019, 51, 805–811. [Google Scholar] [CrossRef]
- Gonzalez-Añover, P.; Gonzalez-Bulnes, A. Maternal age modulates the effects of early-pregnancy L-proline supplementation on the birth-weight of piglets. Anim. Reprod. Sci. 2017, 181, 63–68. [Google Scholar] [CrossRef]
- Tan, C.; Huang, Z.; Xiong, W.; Ye, H.; Deng, J.; Yin, Y. A review of the amino acid metabolism in placental function response to fetal loss and low birth weight in pigs. J. Anim. Sci. Biotechnol. 2022, 13, 28. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, T.; Hori, H.; Yoshimoto, M.; Seyama, Y.; Kubota, S. Overexpression of ornithine decarboxylase enhances endothelial proliferation by suppressing endostatin expression. Blood 2002, 99, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, N.; Liu, C.; Li, T.; Wang, W.; Jiang, L.; Li, Z.; Han, D.; Tao, S.; Wang, J. Characteristics of the gut microbiota colonization, inflammatory profile, and plasma metabolome in intrauterine growth restricted piglets during the first 12 hours after birth. J. Microbiol. 2019, 57, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Liu, S.; Liu, H.; Mahfuz, S.; Piao, X. Maternal supplementation with a combination of wheat bran and sugar beet pulp during late gestation and lactation improves growth and intestinal functions in piglets. Food Funct. 2021, 12, 7329–7342. [Google Scholar] [CrossRef]
- Wang, J.; Tan, B.; Li, J.; Kong, X.; Tan, M.; Wu, G. Regulatory role of L-proline in fetal pig growth and intestinal epithelial cell proliferation. Anim. Nutr. 2020, 6, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Niechcial, A.; Schwarzfischer, M.; Wawrzyniak, M.; Atrott, K.; Laimbacher, A.; Morsy, Y.; Katkeviciute, E.; Häfliger, J.; Westermann, P.; Akdis, C.A.; et al. Spermidine Ameliorates Colitis via Induction of Anti-Inflammatory Macrophages and Prevention of Intestinal Dysbiosis. J. Crohns Colitis 2023, 17, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- GB/T 39235-2020; Nutrient Requirement of Swine. China Agriculture Press: Beijing, China, 2020.
- Wu, Z.; Nie, J.; Wu, D.; Huang, S.; Chen, J.; Liang, H.; Hao, X.; Feng, L.; Luo, H.; Tan, C. Dietary adenosine supplementation improves placental angiogenesis in IUGR piglets by up-regulating adenosine A2a receptor. Anim. Nutr. 2023, 13, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, Y.; Deng, M.; Yang, L.; Shu, G.; Jiang, Q.; Zhang, S.; Li, X.; Yin, Y.; Tan, C.; et al. Placentae for Low Birth Weight Piglets Are Vulnerable to Oxidative Stress, Mitochondrial Dysfunction, and Impaired Angiogenesis. Oxid. Med. Cell Longev. 2020, 2020, 8715412. [Google Scholar] [CrossRef]
- Zou, D.; Yang, Y.; Ji, F.; Lv, R.; Xu, T.; Hu, C. DUOX2-Induced Oxidative Stress Inhibits Intestinal Angiogenesis through MMP3 in a Low-Birth-Weight Piglet Model. Antioxidants 2023, 12, 1800. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Li, J.; Li, Y.; Huang, P.; Ding, X.; Yin, J.; He, S.; Yang, H.; Yin, Y. Dietary vitamin E affects small intestinal histomorphology, digestive enzyme activity, and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets1. J. Anim. Sci. 2019, 97, 1212–1221. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Fan, Y.; Guo, Z.; Liu, B.; Chen, L.; Tang, G.; Jiang, Y.; Li, X.; Zhang, S.; et al. High Altitude Adaptability and Meat Quality in Tibetan Pigs: A Reference for Local Pork Processing and Genetic Improvement. Animals 2019, 9, 1080. [Google Scholar] [CrossRef]
- Hu, C.; Li, F.; Duan, Y.; Yin, Y.; Kong, X. Glutamic acid supplementation reduces body fat weight in finishing pigs when provided solely or in combination with arginine and it is associated with colonic propionate and butyrate concentrations. Food Funct. 2019, 10, 4693–4704. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-f.; Ji, Y.-j.; Li, H.-w.; Zhu, Q.; Blachier, F.; Geng, M.-m.; Chen, W.; Yin, Y.-l. Colonic luminal microbiota and bacterial metabolite composition in pregnant Huanjiang mini-pigs: Effects of food composition at different times of pregnancy. Sci. Rep. 2016, 6, 37224. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, S.; Song, T.; Yin, Y.; Tan, C. Placental Angiogenesis in Mammals: A Review of the Regulatory Effects of Signaling Pathways and Functional Nutrients. Adv. Nutr. 2021, 12, 2415–2434. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, K.; Yu, C.; Wang, L.; Liang, T.; Zhu, H.; Xu, X.; Liu, Y. Xylooligosaccharide attenuates lipopolysaccharide-induced intestinal injury in piglets via suppressing inflammation and modulating cecal microbial communities. Anim. Nutr. 2021, 7, 609–620. [Google Scholar] [CrossRef]
- Elmetwally, M.A.; Li, X.; Johnson, G.A.; Burghardt, R.C.; Herring, C.M.; Kramer, A.C.; Meininger, C.J.; Bazer, F.W.; Wu, G. Dietary supplementation with l-arginine between days 14 and 25 of gestation enhances NO and polyamine syntheses and the expression of angiogenic proteins in porcine placentae. Amino Acids 2022, 54, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-R.; Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef]
- Hu, C.; Wu, Z.; Huang, Z.; Hao, X.; Wang, S.; Deng, J.; Yin, Y.; Tan, C. Nox2 impairs VEGF-A-induced angiogenesis in placenta via mitochondrial ROS-STAT3 pathway. Redox Biol. 2021, 45, 102051. [Google Scholar] [CrossRef]
- Chen, K.; Bai, L.; Lu, J.; Chen, W.; Liu, C.; Guo, E.; Qin, X.; Jiao, X.; Huang, M.; Tian, H. Human Decidual Mesenchymal Stem Cells Obtained From Early Pregnancy Improve Cardiac Revascularization Postinfarction by Activating Ornithine Metabolism. Front. Cardiovasc. Med. 2022, 9, 837780. [Google Scholar] [CrossRef]
- Shi, H.P.; Fishel, R.S.; Efron, D.T.; Williams, J.Z.; Fishel, M.H.; Barbul, A. Effect of supplemental ornithine on wound healing. J. Surg. Res. 2002, 106, 299–302. [Google Scholar] [CrossRef]
- Herrero del Valle, A.; Seip, B.; Cervera-Marzal, I.; Sacheau, G.; Seefeldt, A.C.; Innis, C.A. Ornithine capture by a translating ribosome controls bacterial polyamine synthesis. Nat. Microbiol. 2020, 5, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Gombedza, F.C.; Bandyopadhyay, B.C. l-ornithine activates Ca(2+) signaling to exert its protective function on human proximal tubular cells. Cell Signal. 2020, 67, 109484. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- Ginguay, A.; Cynober, L.; Curis, E.; Nicolis, I. Ornithine Aminotransferase, an Important Glutamate-Metabolizing Enzyme at the Crossroads of Multiple Metabolic Pathways. Biology 2017, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Oberkersch, R.E.; Pontarin, G.; Astone, M.; Spizzotin, M.; Arslanbaeva, L.; Tosi, G.; Panieri, E.; Ricciardi, S.; Allega, M.F.; Brossa, A.; et al. Aspartate metabolism in endothelial cells activates the mTORC1 pathway to initiate translation during angiogenesis. Dev. Cell 2022, 57, 1241–1256.e8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bazer, F.W.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Dietary supplementation with L-citrulline improves placental angiogenesis and embryonic survival in gilts. Exp. Biol. Med. 2023, 248, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Ikeda, K.; Yamazaki, E.; Katayama, A.; Urata, R.; Matoba, S. Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice. Sci. Rep. 2023, 13, 8338. [Google Scholar] [CrossRef]
- Hu, C.; Ji, F.; Lv, R.; Zhou, H.; Hou, G.; Xu, T. Putrescine promotes MMP9-induced angiogenesis in skeletal muscle through hydrogen peroxide/METTL3 pathway. Free Radic. Biol. Med. 2024, 212, 433–447. [Google Scholar] [CrossRef]
- Kadife, E.; Harper, A.; De Alwis, N.; Chien, K.; Hannan, N.; Brownfoot, F.C. SLC38A4 Amino Acid Transporter Expression Is Significantly Lower in Early Preterm Intrauterine Growth Restriction Complicated Placentas. Int. J. Mol. Sci. 2022, 24, 403. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Umapathy, A.; Chamley, L.W.; James, J.L. Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis 2020, 23, 105–117. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, D.; Wu, Y.; Sun, L.; Chen, J.; Chen, J.; Huang, X.; Yang, J.; Li, S. YTHDF1 Protects Auditory Hair Cells from Cisplatin-Induced Damage by Activating Autophagy via the Promotion of ATG14 Translation. Mol. Neurobiol. 2022, 59, 7134–7151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, J.; Li, J.; Wang, Z.; Chen, Z.; Lv, Z.; Ge, L.; Xie, G.; Deng, G.; Rui, Y.; et al. N6-Methyladenosine Promotes Translation of VEGFA to Accelerate Angiogenesis in Lung Cancer. Cancer Res. 2023, 83, 2208–2225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, W.; Mao, Z.; Mou, D.; Huang, L.; Yang, M.; Ding, D.; Yan, H.; Fang, Z.; Che, L.; et al. Maternal VD(3) supplementation during gestation improves intestinal health and microbial composition of weaning piglets. Food Funct. 2022, 13, 6830–6842. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Pi, Y.; Han, D.; Feng, C.; Zhao, J.; Chen, L.; Che, D.; Bao, H.; Xie, Z.; et al. Maternal galactooligosaccharides supplementation programmed immune defense, microbial colonization and intestinal development in piglets. Food Funct. 2021, 12, 7260–7270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, S.; Li, M.; Piao, X. Effects of maternal 25-hydroxycholecalciferol during the last week of gestation and lactation on serum parameters, intestinal morphology and microbiota in suckling piglets. Arch. Anim. Nutr. 2020, 74, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Li, Y.; Yun, H.; Zhang, T.; Huang, Y.; Zhou, J.; Yan, H.; Wei, J.; Liu, Y.; Zhang, Z.; et al. Lactobacillus maintains healthy gut mucosa by producing L-Ornithine. Commun. Biol. 2019, 2, 171. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.J.M.; Lo, Y.H.; Mah, A.T.; Kuo, C.J. The Intestinal Stem Cell Niche: Homeostasis and Adaptations. Trends Cell Biol. 2018, 28, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, Z.; Yuan, X.; Li, N.; Li, T.; Wang, J.; Levesque, C.L.; Feng, C. Transcriptome Differences Suggest Novel Mechanisms for Intrauterine Growth Restriction Mediated Dysfunction in Small Intestine of Neonatal Piglets. Front. Physiol. 2020, 11, 561. [Google Scholar] [CrossRef]
- Che, L.; Zhou, Q.; Liu, Y.; Hu, L.; Peng, X.; Wu, C.; Zhang, R.; Tang, J.; Wu, F.; Fang, Z.; et al. Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct. 2019, 10, 8149–8160. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Yang, S.; Li, Z.; Zhong, J.; Fang, R. Epidermal Growth Factor and Intestinal Barrier Function. Mediat. Inflamm. 2016, 2016, 1927348. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential Application of Lonicera japonica Extracts in Animal Production: From the Perspective of Intestinal Health. Front. Microbiol. 2021, 12, 719877. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.; Yung, K.K. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; He, C.; Zhu, Y.; Lu, N.H. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J. Gastroenterol. 2020, 26, 2187–2193. [Google Scholar] [CrossRef]
- Martínez-López, M.; Iborra, S.; Conde-Garrosa, R.; Mastrangelo, A.; Danne, C.; Mann, E.R.; Reid, D.M.; Gaboriau-Routhiau, V.; Chaparro, M.; Lorenzo, M.P.; et al. Microbiota Sensing by Mincle-Syk Axis in Dendritic Cells Regulates Interleukin-17 and -22 Production and Promotes Intestinal Barrier Integrity. Immunity 2019, 50, 446–461.e9. [Google Scholar] [CrossRef]
- Stephens, C.P.; Hampson, D.J. Intestinal spirochete infections of chickens: A review of disease associations, epidemiology and control. Anim. Health Res. Rev. 2001, 2, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liu, X.; Xu, J.; Li, F.; Wang, J.; Zhang, X.; Yang, X.; Wang, L.; Ma, S.; Li, D.; et al. Effects of Silage Diet on Meat Quality through Shaping Gut Microbiota in Finishing Pigs. Microbiol. Spectr. 2022, 11, e02416–e02422. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; Zhang, B.; Zhang, L.; Wu, K.; Yang, A.; Li, C.; Wang, Y.; Zhang, J.; Qi, D. Potential Link between Gut Microbiota and Deoxynivalenol-Induced Feed Refusal in Weaned Piglets. J. Agric. Food Chem. 2019, 67, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- González-Ortiz, G.; Olukosi, O.A.; Jurgens, G.; Apajalahti, J.; Bedford, M.R. Short-chain fatty acids and ceca microbiota profiles in broilers and turkeys in response to diets supplemented with phytase at varying concentrations, with or without xylanase. Poult. Sci. 2020, 99, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Isayama, K.; Rini, D.M.; Yamamoto, Y.; Suzuki, T. Propionate regulates tight junction barrier by increasing endothelial-cell selective adhesion molecule in human intestinal Caco-2 cells. Exp. Cell Res. 2023, 425, 113528. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]

| Items | Gestating Diet | Lactating Diet |
|---|---|---|
| Ingredient, % | ||
| Corn | 46.00 | 52.20 |
| Soybean meal | 13.00 | 20.00 |
| Barley | 12.00 | 10.00 |
| Wheat bran | 15.00 | 10.00 |
| soybean hull | 10.00 | - |
| soybean oil | - | 3.80 |
| Premix 1 | 4 | 4 |
| Total | 100.00 | 100.00 |
| Chemical composition | ||
| DE, MJ/kg | 12.33 | 14.00 |
| CP, % | 13.71 | 15.19 |
| EE, % | 2.86 | 6.48 |
| ASH | 2.75 | 2.63 |
| CF, % | 6.80 | 3.32 |
| Ca, % | 0.85 | 0.82 |
| P, % | 0.61 | 0.60 |
| Lys, % | 0.66 | 0.76 |
| Met + Cys, % | 0.54 | 0.55 |
| Trp, % | 0.16 | 0.18 |
| Thr, % | 0.53 | 0.60 |
| Genes | Primers | Sequences (5′ to 3′) |
|---|---|---|
| ASCT2 | Forward | GATTGTGGAGATGGAGGATGTGG |
| Reverse | TGCGAGTGAAGAGGAAGTAGATGA | |
| CAT1 | Forward | TGCCCATACTTCCCGTCC |
| Reverse | GGTCCAGGTTACCGTCAG | |
| CD31 | Forward | TGTGGACTTCTTCCTGATTGTC |
| Reverse | CGTTGTTGTGGCAGTTGTTGGT | |
| Claudin-1 | Forward | GATTTACTCCTACGCTGGTGAC |
| Reverse | CACAAAGATGGCTATTAGTCCC | |
| Occludin | Forward | GCACCCAGCAACGACAT |
| Reverse | CATAGACAGAATCCGAATCAC | |
| PAT1 | Forward | TGTGGACTTCTTCCTGATTGTC |
| Reverse | CGTTGTTGTGGCAGTTGTTGGT | |
| PAT2 | Forward | GGGCTACTTGCGGTTCGG |
| Reverse | GCGCTTTGACACCTGGGAG | |
| TAT1 | Forward | GCCCATTGCCTTCGAGTTAG |
| Reverse | AGCGAGGTAGAATGCCACAT | |
| LAT1 | Forward | TTTGTTATGCGGAACTGG |
| Reverse | AAAGGTGATGGCAATGAC | |
| SNAT2 | Forward | TACTTGGTTCTGCTGGTGTCC |
| Reverse | GTTGTGGGCTGTGTAAAGGTG | |
| VEGF-A | Forward | CCTCGGAGCGGAGAAAGCAT |
| Items | CON | 0.05% Orn | 0.10% Orn | 0.15% Orn | p-Value |
|---|---|---|---|---|---|
| Body weight of sows, kg | |||||
| Day 1 | 91.71 ± 6.11 | 91.88 ± 3.23 | 89.79 ± 4.91 | 91.29 ± 4.89 | 0.99 |
| Day 110 | 124.21 ± 4.19 | 124.42 ± 3.41 | 125.37 ± 4.59 | 126.92 ± 4.75 | 0.97 |
| Body weight gain | 32.50 ± 3.17 | 33.54 ± 2.76 | 35.58 ± 1.41 | 35.63 ± 2.02 | 0.66 |
| Backfat thickness of sows, mm | |||||
| Day 1 | 29.25 ± 0.93 | 29.58 ± 0.73 | 29.41 ± 1.12 | 29.08 ± 0.71 | 0.98 |
| Day 110 | 32.42 ± 0.83 | 32.58 ± 0.50 | 32.83 ± 0.79 | 32.25 ± 0.48 | 0.93 |
| Backfat thickness gain | 3.16 ± 0.42 | 2.92 ± 0.46 | 3.42 ± 0.71 | 3.16 ± 0.34 | 0.92 |
| Items | CON | 0.05% Orn | 0.10% Orn | 0.15% Orn | p-Value |
|---|---|---|---|---|---|
| Serum Orn, μmol/L | 55.81 ± 3.24 b | 58.53 ± 2.09 b | 66.28 ± 1.87 a | 68.65 ± 1.71 a | <0.01 |
| litter size | 10.50 ± 0.73 | 10.42 ± 0.45 | 10.75 ± 0.78 | 10.75 ± 0.68 | 0.98 |
| Born alive | 9.33 ± 0.69 | 9.83 ± 0.42 | 9.58 ± 0.70 | 9.75 ± 0.85 | 0.96 |
| Stillborn piglets | 1.17 ± 0.24 | 0.90 ± 0.25 | 1.17 ± 0.34 | 1.18 ± 0.50 | 0.95 |
| Birth weight, g | 590.92 ± 17.25 b | 601.42 ± 19.55 b | 654.27 ± 15.80 a | 665.27 ± 19.98 a | 0.01 |
| Lactation | |||||
| ADFI of sows, kg | 2.61 ± 0.24 | 2.56 ± 0.25 | 2.59 ± 0.39 | 2.55 ± 0.38 | 0.89 |
| Piglet weight at weaning, g | 3423.29 ± 120.89 | 3291.07 ± 87.15 | 3406.95 ± 128.53 | 3529.25 ± 69.80 | 0.47 |
| ADG of piglets, g/d | 100.61 ± 4.10 | 96.78 ± 3.68 | 97.40 ± 4.81 | 102.38 ± 2.97 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Hou, G.; Ji, F.; Zhou, H.; Lv, R.; Hu, C. Maternal Supplementation with Ornithine Promotes Placental Angiogenesis and Improves Intestinal Development of Suckling Piglets. Animals 2024, 14, 689. https://doi.org/10.3390/ani14050689
Yang Y, Hou G, Ji F, Zhou H, Lv R, Hu C. Maternal Supplementation with Ornithine Promotes Placental Angiogenesis and Improves Intestinal Development of Suckling Piglets. Animals. 2024; 14(5):689. https://doi.org/10.3390/ani14050689
Chicago/Turabian StyleYang, Yun, Guanyu Hou, Fengjie Ji, Hanlin Zhou, Renlong Lv, and Chengjun Hu. 2024. "Maternal Supplementation with Ornithine Promotes Placental Angiogenesis and Improves Intestinal Development of Suckling Piglets" Animals 14, no. 5: 689. https://doi.org/10.3390/ani14050689
APA StyleYang, Y., Hou, G., Ji, F., Zhou, H., Lv, R., & Hu, C. (2024). Maternal Supplementation with Ornithine Promotes Placental Angiogenesis and Improves Intestinal Development of Suckling Piglets. Animals, 14(5), 689. https://doi.org/10.3390/ani14050689






