The Effect of Sodium Alginate-Coated Nano-Zinc Oxide on the Growth Performance, Serum Indexes and Fecal Microbial Structure of Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Products
2.2. Animals, Dietary Treatments and Experimental Design
2.3. Growth Performance and Diarrhea Rate
2.4. Sample Collection
2.5. Serum Indexes
2.6. Zn Concentrations in Serum and Fecal Samples
2.7. SCFAs Concentrations in Feces
2.8. Microbial Analysis Based on 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
3.1. Growth Performance and Diarrhea Rate
3.2. Biochemical Indices in Serum
3.3. Zn Concentrations in Serum and Feces
3.4. Fecal SCFAs Concentrations
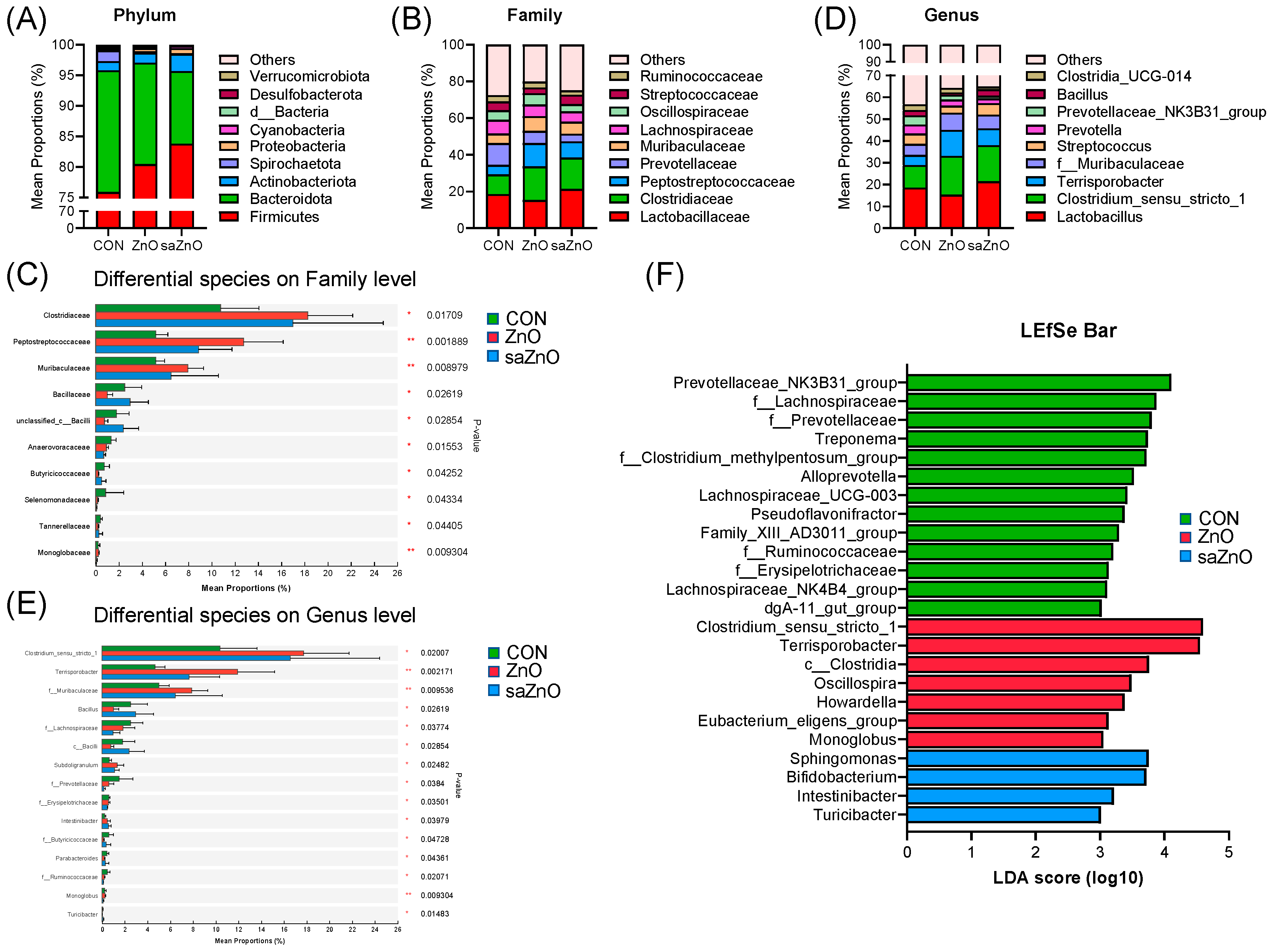
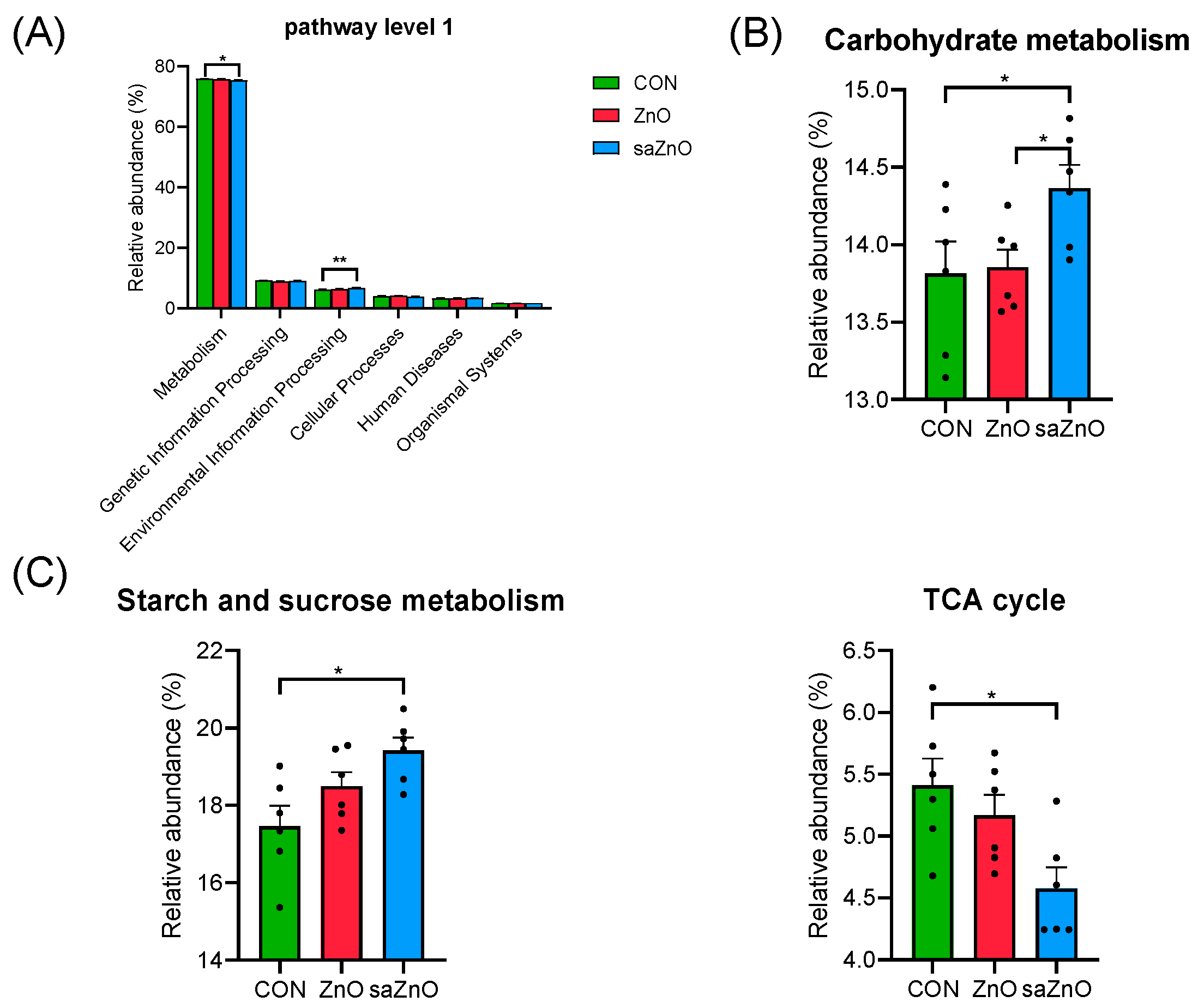
3.5. Microbiota Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutherland, M.A.; Backus, B.L.; McGlone, J.J. Effects of Transport at Weaning on the Behavior, Physiology and Performance of Pigs. Animals 2014, 4, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Gong, T.; Jiang, Z.; Lu, Z.; Wang, Y. The Role of Probiotics in Alleviating Postweaning Diarrhea in Piglets From the Perspective of Intestinal Barriers. Front. Cell Infect. Microbiol. 2022, 12, 883107. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, B.C.; Hayes, M.D.; Condotta, I.; Leonard, S.M. Impact of housing environment and management on pre-/post-weaning piglet productivity. J. Anim. Sci. 2022, 100, skac142. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Yu, C.; Zhang, H.; Song, D.; Jiang, D.; Du, H.; Wang, Y. Cathelicidin-BF suppresses intestinal inflammation by inhibiting the nuclear factor-kappaB signaling pathway and enhancing the phagocytosis of immune cells via STAT-1 in weanling piglets. Int. Immunopharmacol. 2015, 28, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Song, M.; Liu, Y.; Ji, P. Enterotoxigenic Escherichia coli infection of weaned pigs: Intestinal challenges and nutritional intervention to enhance disease resistance. Front. Immunol. 2022, 13, 885253. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, N.; Qi, Z.; Han, M.; Ma, X. Coated Zinc Oxide Improves Growth Performance of Weaned Piglets via Gut Microbiota. Front. Nutr. 2022, 9, 819722. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Xie, Y.; Chen, Y.; Yi, H.; He, D. Effects of antimicrobial peptide cathelicidin-BF on diarrhea controlling, immune responses, intestinal inflammation and intestinal barrier function in piglets with postweaning diarrhea. Int. Immunopharmacol. 2020, 85, 106658. [Google Scholar] [CrossRef]
- Li, J. Current status and prospects for in-feed antibiotics in the different stages of pork production—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 1667–1673. [Google Scholar] [CrossRef]
- Pamer, E.G. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 2016, 352, 535–538. [Google Scholar] [CrossRef]
- Szuba-Trznadel, A.; Rzasa, A.; Hikawczuk, T.; Fuchs, B. Effect of Zinc Source and Level on Growth Performance and Zinc Status of Weaned Piglets. Animals 2021, 11, 2030. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Li, Z.; Gong, T.; Wang, F.; Jin, M.; Wang, Y.; Lu, Z. An alternative ZnO with large specific surface area: Preparation, physicochemical characterization and effects on growth performance, diarrhea, zinc metabolism and gut barrier function of weaning piglets. Sci. Total Environ. 2023, 882, 163558. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.; Yin, Y.; Zhang, S.; Li, X.; Sun, Q.; Wan, D. Effects of Zinc Oxide/Zeolite on Intestinal Morphology, Intestinal Microflora, and Diarrhea Rates in Weaned Piglets. Biol. Trace Elem. Res. 2021, 199, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ying, J.; Zou, P.; Zhou, Y.; Wang, B.; Yu, D.; Li, W.; Zhan, X. Effects of Dietary Supplementation of Humic Acid Sodium and Zinc Oxide on Growth Performance, Immune Status and Antioxidant Capacity of Weaned Piglets. Animals 2020, 10, 2104. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Geng, N.; Li, Y.; Li, X.; Yu, J.; Gao, S.; Wang, H.; Pan, H.; Yang, Q.; Zhuge, Y.; et al. Treatment of cadmium and zinc-contaminated water systems using modified biochar: Contaminant uptake, adsorption ability, and mechanism. Bioresour. Technol. 2022, 363, 127817. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, J.; Mahfuz, S.; Piao, X. Effects of Hydrolysable Tannins as Zinc Oxide Substitutes on Antioxidant Status, Immune Function, Intestinal Morphology, and Digestive Enzyme Activities in Weaned Piglets. Animals 2020, 10, 757. [Google Scholar] [CrossRef]
- Kim, T.; Kim, M.; Lee, J.; Moturi, J.; Ha, S.; Tajudeen, H.; Mun, J.; Hosseindoust, A.; Chae, B. Supplementation of nano-zinc in lower doses as an alternative to pharmacological doses of ZnO in weanling pigs. J. Anim. Sci. Technol. 2022, 64, 70–83. [Google Scholar] [CrossRef]
- Liu, H.; Bai, M.; Xu, K.; Zhou, J.; Zhang, X.; Yu, R.; Huang, R.; Yin, Y. Effects of different concentrations of coated nano zinc oxide material on fecal bacterial composition and intestinal barrier in weaned piglets. J. Sci. Food Agric. 2021, 101, 735–745. [Google Scholar] [CrossRef]
- Bai, M.M.; Liu, H.N.; Xu, K.; Wen, C.Y.; Yu, R.; Deng, J.P.; Yin, Y.L. Use of coated nano zinc oxide as an additive to improve the zinc excretion and intestinal morphology of growing pigs1. J. Anim. Sci. 2019, 97, 1772–1783. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, G.; Yang, Z.; Zhao, J. Effects of Tetrabasic Zinc Chloride on Growth Performance, Nutrient Digestibility and Fecal Microbial Community in Weaned Piglets. Front. Vet. Sci. 2022, 9, 905242. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Dai, Z.; Cao, G.; Cui, Z.; Zhang, R.; Xu, Y.; Wu, Y.; Yang, C. Protective effects of Bacillus licheniformis on growth performance, gut barrier functions, immunity and serum metabolome in lipopolysaccharide-challenged weaned piglets. Front. Immunol. 2023, 14, 1140564. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, Y.L.; Zhou, Z.A.; Wu, F.; Wang, H.F.; Zong, X. Response of sediment microbial communities to different levels of PAC contamination and exposure time. Sci. Total Environ. 2023, 861, 160683. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.J.; Lee, C.Y.; Kim, M.H.; Jung, D.Y.; Han, J.H.; Jang, I.; Song, Y.M.; Park, B.C. Effects of dietary supplementation of a lipid-coated zinc oxide product on the fecal consistency, growth, and morphology of the intestinal mucosa of weanling pigs. J. Anim. Sci. Technol. 2017, 59, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hou, G.; Hu, P.; Feng, D.; Wang, J.; Zhu, W. Nano chitosan-zinc complex improves the growth performance and antioxidant capacity of the small intestine in weaned piglets. Br. J. Nutr. 2021, 126, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Park, Y.J.; Cho, J.H.; Song, M.H.; Gu, B.H.; Yun, W.; Lee, J.H.; An, J.S.; Kim, Y.J.; Lee, J.S.; et al. Changes in Diarrhea Score, Nutrient Digestibility, Zinc Utilization, Intestinal Immune Profiles, and Fecal Microbiome in Weaned Piglets by Different Forms of Zinc. Animals 2021, 11, 1356. [Google Scholar] [CrossRef]
- Holodova, M.; Cobanova, K.; Sefcikova, Z.; Barszcz, M.; Tuśnio, A.; Taciak, M.; Gresakova, L. Dietary Zinc and Fibre Source can Influence the Mineral and Antioxidant Status of Piglets. Animals 2019, 9, 497. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef]
- Cao, S.T.; Wang, C.C.; Wu, H.; Zhang, Q.H.; Jiao, L.F.; Hu, C.H. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1073–1083. [Google Scholar] [CrossRef]
- Song, D.; Cheng, Y.; Li, X.; Wang, F.; Lu, Z.; Xiao, X.; Wang, Y. Biogenic Nanoselenium Particles Effectively Attenuate Oxidative Stress-Induced Intestinal Epithelial Barrier Injury by Activating the Nrf2 Antioxidant Pathway. ACS Appl. Mater. Interfaces 2017, 9, 14724–14740. [Google Scholar] [CrossRef]
- Xiao, X.; Cheng, Y.; Song, D.; Li, X.; Hu, Y.; Lu, Z.; Wang, F.; Wang, Y. Selenium-enriched Bacillus paralicheniformis SR14 attenuates H2O2-induced oxidative damage in porcine jejunum epithelial cells via the MAPK pathway. Appl. Microbiol. Biotechnol. 2019, 103, 6231–6243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, N.; Peng, S.; Zhang, Y.; Wang, H.; Huang, S.; Zhu, M.; Ma, Y. Effects of Dietary Valine Chelated Zinc Supplementation on Growth Performance, Antioxidant Capacity, Immunity, and Intestine Health in Weaned Piglets. Biol. Trace Elem. Res. 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, T.; Hammarström, L.; Zhao, Y. The Immunoglobulins: New Insights, Implications, and Applications. Annu. Rev. Anim. Biosci. 2020, 8, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Deng, D.; Chen, S.; Li, C.; Luo, J.; Romeo, A.; Li, T.; Tang, X.; Fang, R. The Effects of Dietary Porous Zinc Oxide Supplementation on Growth Performance, Inflammatory Cytokines and Tight Junction’s Gene Expression in Early-Weaned Piglets. J. Nutr. Sci. Vitaminol. 2020, 66, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Zou, J.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, W.; Mai, K.; Xu, W.; Zhong, X. Effects of dietary zinc on gene expression of antioxidant enzymes and heat shock proteins in hepatopancreas of abalone Haliotis discus hannai. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef]
- McDonald, J.A.K.; Mullish, B.H.; Pechlivanis, A.; Liu, Z.; Brignardello, J.; Kao, D.; Holmes, E.; Li, J.V.; Clarke, T.B.; Thursz, M.R.; et al. Inhibiting Growth of Clostridioides difficile by Restoring Valerate, Produced by the Intestinal Microbiota. Gastroenterology 2018, 155, 1495–1507.e1415. [Google Scholar] [CrossRef]
- Li, Y.; Dong, J.; Xiao, H.; Zhang, S.; Wang, B.; Cui, M.; Fan, S. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes 2020, 11, 789–806. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Prykhodko, O.; Fåk Hållenius, F.; Nyman, M. Monovalerin and trivalerin increase brain acetic acid, decrease liver succinic acid, and alter gut microbiota in rats fed high-fat diets. Eur. J. Nutr. 2019, 58, 1545–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Akhtar, M.; Chen, Y.; Ma, Z.; Liang, Y.; Shi, D.; Cheng, R.; Cui, L.; Hu, Y.; Nafady, A.A.; et al. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome 2022, 10, 107. [Google Scholar] [CrossRef]
- Xiao, X.; Cheng, Y.; Fu, J.; Lu, Z.; Wang, F.; Jin, M.; Zong, X.; Wang, Y. Gut Immunity and Microbiota Dysbiosis Are Associated with Altered Bile Acid Metabolism in LPS-Challenged Piglets. Oxid. Med. Cell Longev. 2021, 2021, 6634821. [Google Scholar] [CrossRef] [PubMed]
- Dahmer, P.L.; Harrison, O.L.; Jones, C.K. Effects of formic acid and glycerol monolaurate on weanling pig growth performance, fecal consistency, fecal microbiota, and serum immunity. Transl. Anim. Sci. 2022, 6, txac145. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Wang, P. Regulatory Effects Mediated by Enteromorpha prolifera Polysaccharide and Its Zn(II) Complex on Hypoglycemic Activity in High-Sugar High-Fat Diet-Fed Mice. Foods 2023, 12, 2854. [Google Scholar] [CrossRef] [PubMed]
- Forssten, S.D.; Sindelar, C.W.; Ouwehand, A.C. Probiotics from an industrial perspective. Anaerobe 2011, 17, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Huang, K.; Wen, X.; Gao, J.; Cui, B.; Yao, K.; Zhan, X.; Hu, S.; Wu, Q.; Xiao, H.; et al. Dietary supplementation with potassium-magnesium sulfate modulates the antioxidant capacity, immunity, and gut microbiota in weaned piglets. Front. Microbiol. 2022, 13, 961989. [Google Scholar] [CrossRef]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef]
- Yang, B.; Yue, Y.; Chen, Y.; Ding, M.; Li, B.; Wang, L.; Wang, Q.; Stanton, C.; Ross, R.P.; Zhao, J.; et al. Lactobacillus plantarum CCFM1143 Alleviates Chronic Diarrhea via Inflammation Regulation and Gut Microbiota Modulation: A Double-Blind, Randomized, Placebo-Controlled Study. Front. Immunol. 2021, 12, 746585. [Google Scholar] [CrossRef]
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.D.; Delzenne, N.M.; Muccioli, G.; Clément, K.; Cani, P.D. From correlation to causality: The case of Subdoligranulum. Gut Microbes 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Liu, J.; Yu, X.; Ma, F.; Wo, Y.; Jin, Y.; Hashem, N.M.; Sun, P. Early supplementation with zinc proteinate does not change rectal microbiota but increases growth performance by improving antioxidant capacity and plasma zinc concentration in preweaned dairy calves. Front. Vet. Sci. 2023, 10, 1236635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Q.; Wang, X.; Song, J.; Lambo, M.T.; Huang, J.; He, P.; Li, Y.; Zhang, Y. Replacing alfalfa hay with industrial hemp ethanol extraction byproduct and Chinese wildrye hay: Effects on lactation performance, plasma metabolites, and bacterial communities in Holstein cows. Front. Vet. Sci. 2023, 10, 1061219. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Maia, M.R.G.; Pinna, C.; Biagi, G.; Matos, E.; Segundo, M.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Effects of Zinc Source and Enzyme Addition on the Fecal Microbiota of Dogs. Front. Microbiol. 2021, 12, 688392. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Li, P.; Yan, S.; Liu, Y.; Gao, M.; Lv, H.; Lv, Z.; Guo, Y. Effects of Dietary Astragalus Polysaccharide Supplementation on the Th17/Treg Balance and the Gut Microbiota of Broiler Chickens Challenged With Necrotic Enteritis. Front. Immunol. 2022, 13, 781934. [Google Scholar] [CrossRef]
- Drey, E.; Kok, C.R.; Hutkins, R. Role of Bifidobacterium pseudocatenulatum in Degradation and Consumption of Xylan-Derived Carbohydrates. Appl. Environ. Microbiol. 2022, 88, e0129922. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, N.; Zhao, H.; Yuan, H.; Xia, D.; Lei, H. The Microbiome-Metabolome Response in the Colon of Piglets Under the Status of Weaning Stress. Front. Microbiol. 2020, 11, 2055. [Google Scholar] [CrossRef]

| Ingredients | Content, % | Nutrient Level | Content |
|---|---|---|---|
| Corn | 55.00 | DE, MJ/Kg | 14.17 |
| Wheat midding | 3.50 | CP, % | 20.35 |
| Phospholipid | 2.00 | Lys, % | 1.34 |
| Whey powder | 5.00 | Met + Cys, % | 0.77 |
| Extruded soybean | 7.30 | Thr, % | 0.80 |
| Soybean meal | 18.50 | Ca, % | 0.95 |
| Fish meal | 5.00 | Total P, % | 0.65 |
| Dicalcium phosphate | 1.00 | Available P, % | 0.48 |
| Limestone | 1.10 | ||
| NaCl | 0.10 | ||
| L-Lysine HCl | 0.35 | ||
| DL-methionine | 0.15 | ||
| Vitamin-mineral premix 1 | 1.00 | ||
| Total | 100 |
| Item 1 | Treatments 2 | p Value | ||
|---|---|---|---|---|
| CON | ZnO | saZnO | ||
| BW, kg | ||||
| d 1 | 7.96 ± 0.05 | 7.97 ± 0.06 | 7.91 ± 0.44 | 0.687 |
| d 14 | 9.94 ± 0.87 b | 10.83 ± 0.11 a | 10.75 ± 0.20 a | 0.001 |
| d 28 | 14.08 ± 0.20 b | 15.28 ± 0.24 a | 15.34 ± 0.33 a | 0.006 |
| ADG, g | ||||
| d 1 to 14 | 141.43 ± 3.67 b | 204.29 ± 4.40 a | 202.86 ± 11.40 a | 0.000 |
| d 15 to 28 | 295.71 ± 8.25 | 317.86 ± 10.03 | 327.86 ± 11.15 | 0.095 |
| d 1 to 28 | 218.57 ± 5.85 b | 261.07 ± 6.87 a | 265.35 ± 10.70 a | 0.002 |
| ADFI, g | ||||
| d 1 to 14 | 329.65 ± 13.40 | 350.48 ± 10.16 | 355.77 ± 21.40 | 0.480 |
| d 15 to 28 | 650.60 ± 11.77 | 682.89 ± 14.39 | 686.25 ± 24.60 | 0.322 |
| d 1 to 28 | 490.13 ± 10.89 | 516.69 ± 10.81 | 521.01 ± 21.81 | 0.334 |
| F/G | ||||
| d 1 to 14 | 2.33 ± 0.06 a | 1.71 ± 0.02 b | 1.75 ± 0.01 b | 0.000 |
| d 15 to 28 | 2.21 ± 0.04 a | 2.15 ± 0.02 ab | 2.09 ± 0.02 b | 0.050 |
| d 1 to 28 | 2.25 ± 0.03 a | 1.98 ± 0.02 b | 1.96 ± 0.01 b | 0.000 |
| Diarrhea rate, % | ||||
| d 1 to 14 | 33.30 | 6.30 | 8.30 | |
| d 15 to 28 | 27.10 | 4.20 | 4.20 | |
| Item | Treatments | p Value | ||
|---|---|---|---|---|
| CON | ZnO | saZnO | ||
| T-AOC (U/mL) | 22.02 ± 1.24 b | 26.93 ± 0.34 a | 28.49 ± 0.97 a | 0.001 |
| GPx (μmol/L) | 204.22 ± 9.26 | 218.51 ± 23.72 | 255.14 ± 28.71 | 0.276 |
| SOD (U/mL) | 50.80 ± 1.31 b | 63.13 ± 3.29 ab | 66.63 ± 5.88 a | 0.031 |
| CAT (mmol/L) | 260.44 ± 32.71 ab | 337.03 ± 24.43 a | 221.41 ± 28.41 b | 0.036 |
| MDA (μmol/L) | 3.90 ± 0.05 a | 3.68 ± 0.06 b | 3.79 ± 0.06 ab | 0.046 |
| IgM (g/L) | 0.59 ± 0.04 b | 0.64 ± 0.02 ab | 0.83 ± 0.09 a | 0.025 |
| IgG (g/L) | 4.21 ± 0.26 | 4.39 ± 0.34 | 4.79 ± 0.25 | 0.376 |
| Cu/Zn-SOD (μg/L) | 2.40 ± 0.05 b | 2.49 ± 0.06 b | 2.87 ± 0.03 a | 0.000 |
| ALP (U/L) | 235.67 ± 16.36 b | 276.33 ± 16.18 a | 295.00 ± 27.27 a | 0.004 |
| Item | Treatments | p Value | ||
|---|---|---|---|---|
| CON | ZnO | saZnO | ||
| Serum Zn (mg/L) | 1.48 ± 0.23 b | 3.04 ± 0.43 a | 1.66 ± 0.19 b | 0.004 |
| Fecal Zn (mg/kg) | 235.91 ± 23.15 c | 2396.09 ± 113.43 a | 755.05 ± 58.90 b | 0.000 |
| Item | Treatments | p Value | ||
|---|---|---|---|---|
| CON | ZnO | saZnO | ||
| Acetic acid | 6359.47 ± 546.15 | 5570.28 ± 239.69 | 6680.13 ± 456.41 | 0.210 |
| Propionic acid | 3687.62 ± 319.12 | 3231.75 ± 216.74 | 3639.36 ± 171.85 | 0.373 |
| Butyric acid | 4388.08 ± 577.29 | 3716.04 ± 153.13 | 6417.06 ± 1345.34 | 0.097 |
| Isobutyric acid | 361.27 ± 59.04 | 355.99 ± 29.10 | 348.65 ± 34.90 | 0.979 |
| Valeric acid | 632.65 ± 76.63 b | 790.34 ± 65.17 ab | 942.53 ± 85.88 a | 0.038 |
| Isovaleric acid | 727.16 ± 117.14 | 689.97 ± 61.14 | 696.93 ± 65.27 | 0.948 |
| Total SCFAs | 16,156.24 ± 1332.94 | 14,354.38 ± 617.45 | 18,724.66 ± 1842.74 | 0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Guo, K.; Liu, J.; Liu, Y.; Yang, C.; Xu, Y.; Deng, B. The Effect of Sodium Alginate-Coated Nano-Zinc Oxide on the Growth Performance, Serum Indexes and Fecal Microbial Structure of Weaned Piglets. Animals 2024, 14, 146. https://doi.org/10.3390/ani14010146
Xiao X, Guo K, Liu J, Liu Y, Yang C, Xu Y, Deng B. The Effect of Sodium Alginate-Coated Nano-Zinc Oxide on the Growth Performance, Serum Indexes and Fecal Microbial Structure of Weaned Piglets. Animals. 2024; 14(1):146. https://doi.org/10.3390/ani14010146
Chicago/Turabian StyleXiao, Xiao, Kai Guo, Jinsong Liu, Yulan Liu, Caimei Yang, Yinglei Xu, and Bo Deng. 2024. "The Effect of Sodium Alginate-Coated Nano-Zinc Oxide on the Growth Performance, Serum Indexes and Fecal Microbial Structure of Weaned Piglets" Animals 14, no. 1: 146. https://doi.org/10.3390/ani14010146
APA StyleXiao, X., Guo, K., Liu, J., Liu, Y., Yang, C., Xu, Y., & Deng, B. (2024). The Effect of Sodium Alginate-Coated Nano-Zinc Oxide on the Growth Performance, Serum Indexes and Fecal Microbial Structure of Weaned Piglets. Animals, 14(1), 146. https://doi.org/10.3390/ani14010146






