Effect of IGFBP-4 during In Vitro Maturation on Developmental Competence of Bovine Cumulus Oocyte Complexes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant Bovine IGFBP-4
2.2. rbIGFBP4 Purity and Stability
2.3. rbIGFBP4 Binding Capacity
2.4. In Vitro Embryo Production
2.4.1. Recovery of Cumulus–Oocyte Complexes
2.4.2. In Vitro Maturation
2.4.3. In Vitro Fertilization
2.4.4. In Vitro Culture
2.5. mRNA Transcript Analysis
2.5.1. mRNA Isolation
2.5.2. Reverse Transcription
2.5.3. Quantitative Polymerase Chain Reaction (qPCR)
2.6. Assessment of Nuclear Maturation by Aceto-Orcein Staining
2.7. Statistical Analysis
3. Results
3.1. rbIGFBP-4 Purity and Stability
3.2. rbIGFBP4 Binding Capacity
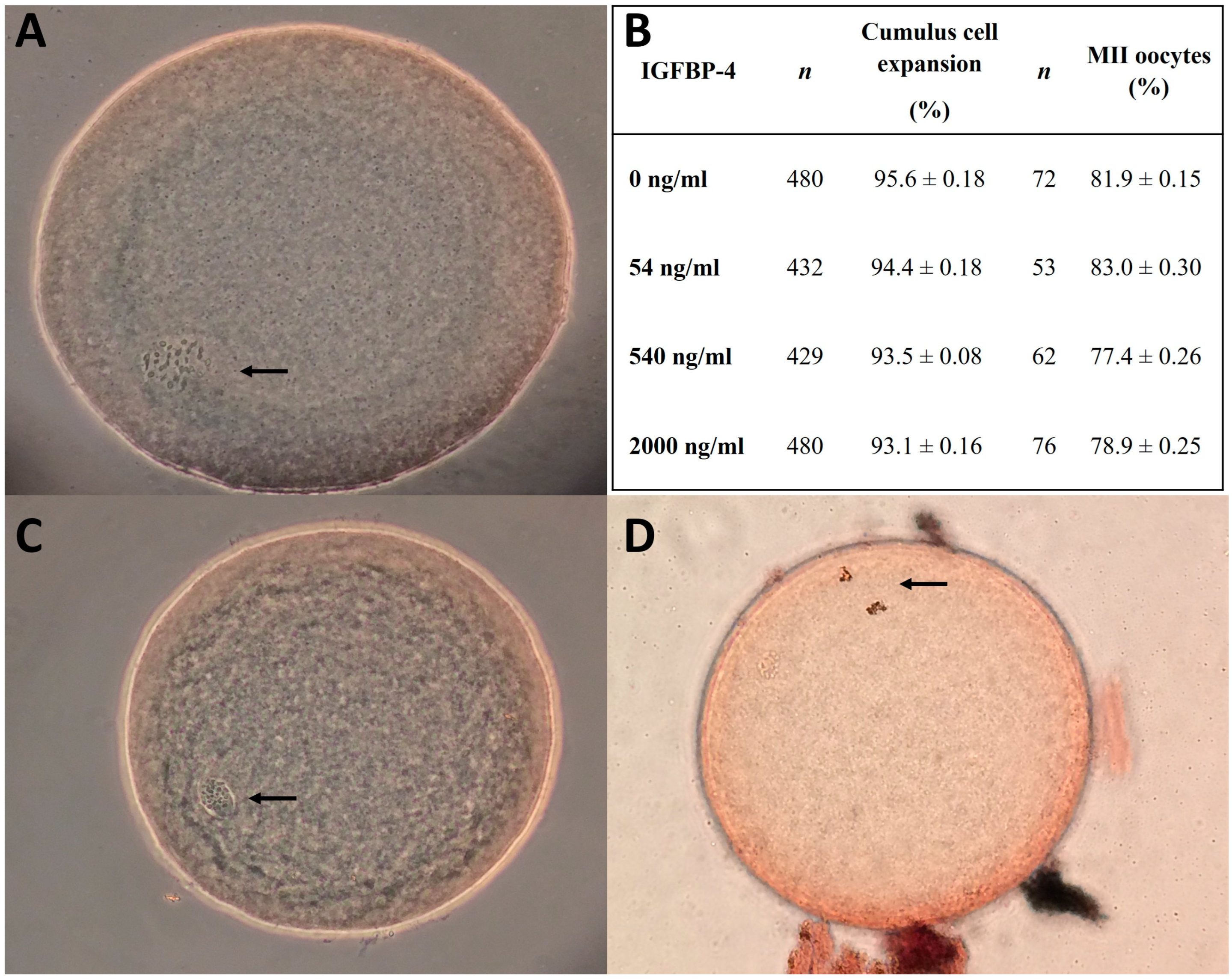
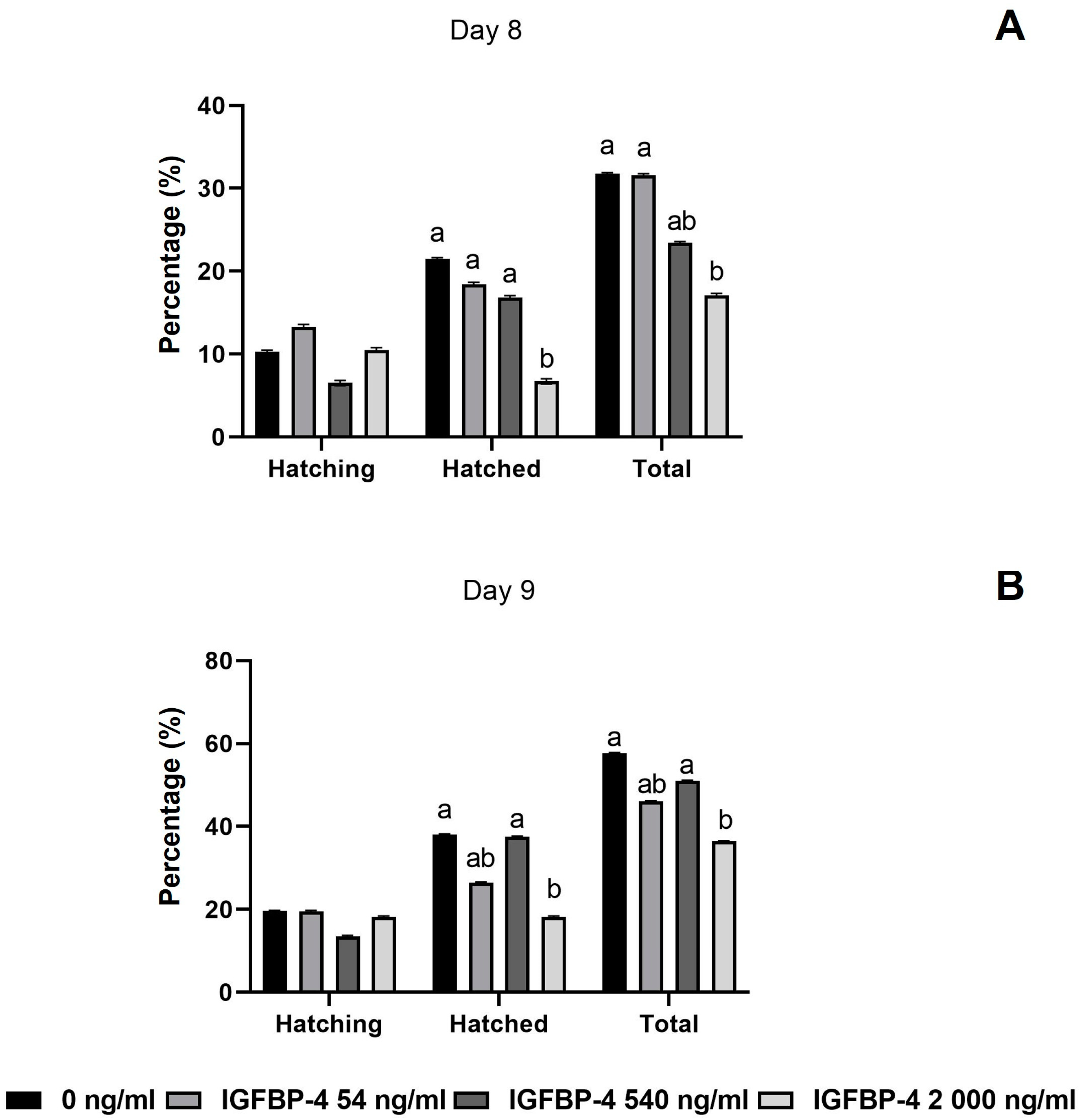
3.3. Developmental Rates of COCs and Embryos
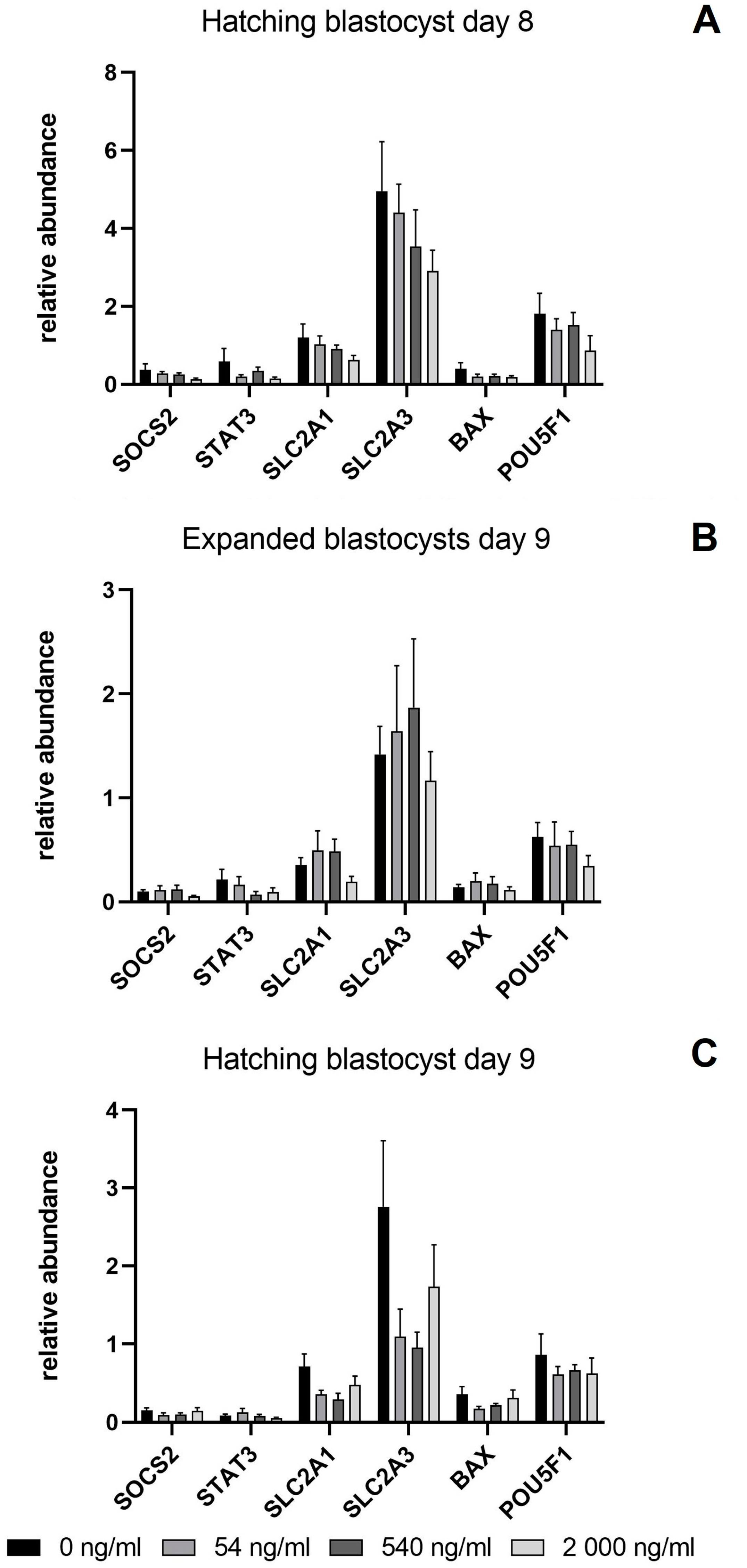
3.4. Relative Abundance of mRNA Transcripts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revelli, A.; Piane, L.D.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular Fluid Content and Oocyte Quality: From Single Biochemical Markers to Metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Spicer, L.J.; Aad, P.Y. Insulin-like Growth Factor (IGF) 2 Stimulates Steroidogenesis and Mitosis of Bovine Granulosa Cells through the IGF1 Receptor: Role of Follicle-Stimulating Hormone and IGF2 Receptor. Biol. Reprod. 2007, 77, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, M.A.; Newman, M.; Christie, M.F.; Cripps, P.J.; Crowe, M.A.; Smith, R.F.; Dobson, H. The Usefulness of a Single Measurement of Insulin-like Growth Factor-1 as a Predictor of Embryo Yield and Pregnancy Rates in a Bovine MOET Program. Theriogenology 2005, 64, 1977–1994. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.C.; Palhão, M.P.; Fernandes, C.A.C.; Sudano, M.J.; Castilho, A.C.S.; Caixeta, E.S. Differential Expression of Insulin-like Growth Factor Family Members in Immature Cumulus–Oocyte Complexes from Dairy Cows with Different Genotypes. Reprod. Domest. Anim. 2017, 52, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Feng, H.L.; Ma, Y.Z.; Cang, M.; Li, H.J.; Yan, Z.; Zhou, P.; Wen, J.X.; Bou, S.; Liu, D.J. Expression of IGF Receptors and Its Ligands in Bovine Oocytes and Preimplantation Embryos. Anim. Reprod. Sci. 2009, 114, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.A.; Wrenzycki, C.; Herrmann, D.; Carnwath, J.W.; Niemann, H. Changes in the Relative Abundance of MRNA Transcripts for Insulin-like Growth Factor (IGF-I and IGF-II) Ligands and Their Receptors (IGF-IR/IGF-IIR) in Preimplantation Bovine Embryos Derived from Different in Vitro Systems. Reproduction 2001, 122, 601–610. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Dominko, T.; Leibfried-Rutledge, M.L.; Nagai, T.; First, N.L. A Combination of EGF and IGF-I Accelerates the Progression of Meiosis in Bovine Follicular Oocytes in Vitro and Fetal Calf Serum Neutralizes the Acceleration Effect. Theriogenology 2000, 54, 1327–1342. [Google Scholar] [CrossRef]
- Rieger, D.; Luciano, A.M.; Modina, S.; Pocar, P.; Lauria, A.; Gandolfi, F. The Effects of Epidermal Growth Factor and Insulin-like Growth Factor I on the Metabolic Activity, Nuclear Maturation and Subsequent Development of Cattle Oocytes in Vitro. J. Reprod. Fertil. 1998, 112, 123–130. [Google Scholar] [CrossRef][Green Version]
- Stefanello, J.R.; Barreta, M.H.; Porciuncula, P.M.; Arruda, J.N.; Oliveira, J.F.; Oliveira, M.A.; Gonçalves, P.B. Effect of Angiotensin II with Follicle Cells and Insulin-like Growth Factor-I or Insulin on Bovine Oocyte Maturation and Embryo Development. Theriogenology 2006, 66, 2068–2076. [Google Scholar] [CrossRef]
- Byrne, A.T.; Southgate, J.; Brison, D.R.; Leese, H.J. Regulation of Apoptosis in the Bovine Blastocyst by Insulin and the Insulin-like Growth Factor (IGF) Superfamily. Mol. Reprod. Dev. 2002, 62, 489–495. [Google Scholar] [CrossRef]
- Wasielak, M.; Bogacki, M. Apoptosis Inhibition by Insulin-Like Growth Factor (IGF)-I During In Vitro Maturation of Bovine Oocytes. J. Reprod. Dev. 2007, 53, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Baylink, D.J.; Mohan, S. Insulin-Like Growth Factor-Binding Proteins in Serum and Other Biological Fluids: Regulation and Functions*. Endocr. Rev. 1997, 18, 801–831. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A. 40 YEARS OF IGF1: IGF-Binding Proteins. J. Mol. Endocrinol. 2018, 61, T11–T28. [Google Scholar] [CrossRef] [PubMed]
- Nuttinck, F.; Charpigny, G.; Mermillod, P.; Loosfelt, H.; Meduri, G.; Freret, S.; Grimard, B.; Heyman, Y. Expression of Components of the Insulin-like Growth Factor System and Gonadotropin Receptors in Bovine Cumulus–Oocyte Complexes during Oocyte Maturation. Domest. Anim. Endocrinol. 2004, 27, 179–195. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, C. Expression and Regulation of MRNAs for Insulin-like Growth Factor (IGF-I), IGF-Binding Protein-2, and LH Receptor in the Process of Follicular Atresia. Sci. China C Life Sci. 2000, 43, 272–279. [Google Scholar] [CrossRef]
- Thomas, F.H.; Campbell, B.K.; Armstrong, D.G.; Telfer, E.E. Effects of IGF-1 Bioavailability on Bovine Preantral Follicular Development in Vitro. Reproduction 2007, 133, 1121–1128. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Baxter, G.; Gutierrez, C.G.; Hogg, C.O.; Glazyrin, A.L.; Campbell, B.K.; Bramley, T.A.; Webb, R. Insulin-Like Growth Factor Binding Protein -2 and -4 Messenger Ribonucleic Acid Expression in Bovine Ovarian Follicles: Effect of Gonadotropins and Developmental Status*. Endocrinology 1998, 139, 2146–2154. [Google Scholar] [CrossRef][Green Version]
- Firth, S.M.; Baxter, R.C. Cellular Actions of the Insulin-Like Growth Factor Binding Proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef]
- Mihm, M.; Austin, E.J. The Final Stages of Dominant Follicle Selection in Cattle. Domest. Anim. Endocrinol. 2002, 23, 155–166. [Google Scholar] [CrossRef]
- Rivera, G.M.; Fortune, J.E. Development of Codominant Follicles in Cattle Is Associated with a Follicle-Stimulating Hormone-Dependent Insulin-Like Growth Factor Binding Protein-4 Protease1. Biol. Reprod. 2001, 65, 112–118. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, F.M.; Gareis, N.C.; Hein, G.J.; Salvetti, N.R.; Amweg, A.N.; Huber, E.; Stassi, A.F.; Ortega, H.H.; Rey, F. Role of Components of the Insulin-like Growth Factor System in the Early Stages of Ovarian Follicular Persistence in Cattle. J. Comp. Pathol. 2017, 157, 201–214. [Google Scholar] [CrossRef]
- Mihm, M.; Austin, E.J.; Good, T.E.M.; Ireland, J.L.H.; Knight, P.G.; Roche, J.F.; Ireland, J.J. Identification of Potential Intrafollicular Factors Involved in Selection of Dominant Follicles in Heifers1. Biol. Reprod. 2000, 63, 811–819. [Google Scholar] [CrossRef]
- Wang, T.H.; Chang, C.L.; Wu, H.M.; Chiu, Y.M.; Chen, C.K.; Wang, H.S. Insulin-like Growth Factor-II (IGF-II), IGF-Binding Protein-3 (IGFBP-3), and IGFBP-4 in Follicular Fluid Are Associated with Oocyte Maturation and Embryo Development. Fertil. Steril. 2006, 86, 1392–1401. [Google Scholar] [CrossRef]
- Xue, Y.; Yan, Y.; Gong, H.; Fang, B.; Zhou, Y.; Ding, Z.; Yin, P.; Zhang, G.; Ye, Y.; Yang, C.; et al. Insulin-like Growth Factor Binding Protein 4 Enhances Cardiomyocytes Induction in Murine-Induced Pluripotent Stem Cells. J. Cell Biochem. 2014, 115, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Crosley, E.J.; Dunk, C.E.; Beristain, A.G.; Christians, J.K. IGFBP-4 and -5 Are Expressed in First-Trimester Villi and Differentially Regulate the Migration of HTR-8/SVneo Cells. Reprod. Biol. Endocrinol. 2014, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.; Tsang, D.; Chalmers, J.A.; Maalouf, M.F.; Brubaker, P.L. Insulin-like Growth Factor-Binding Protein-4 Inhibits Epithelial Growth and Proliferation in the Rodent Intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G206–G219. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Gou, R.; Xu, Q.; Duan, D. Recombinant Insulin-like Growth Factor Binding Protein-4 Inhibits Proliferation and Promotes Differentiation of Neural Progenitor Cells. Neurosci. Lett. 2017, 642, 71–76. [Google Scholar] [CrossRef]
- Meyerholz, M.M.; Mense, K.; Lietzau, M.; Kassens, A.; Linden, M.; Knaack, H.; Wirthgen, E.; Hoeflich, A.; Raliou, M.; Richard, C.; et al. Serum IGFBP4 Concentration Decreased in Dairy Heifers towards Day 18 of Pregnancy. J. Vet. Sci. 2015, 16, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Laeger, T.; Wirthgen, E.; Piechotta, M.; Metzger, F.; Metges, C.C.; Kuhla, B.; Hoeflich, A. Effects of Parturition and Feed Restriction on Concentrations and Distribution of the Insulin-like Growth Factor-Binding Proteins in Plasma and Cerebrospinal Fluid of Dairy Cows. J. Dairy. Sci. 2014, 97, 2876–2885. [Google Scholar] [CrossRef]
- Duarte, A.B.G.; Araújo, V.R.; Chaves, R.N.; da Silva, G.M.; Luz, V.B.; Haag, K.T.; Magalhães-Padilha, D.M.; Almeida, A.P.; Lobo, C.H.; Campello, C.C.; et al. Insulin-like Growth Factor II (IGF-II) and Follicle Stimulating Hormone (FSH) Combinations Can Improve the in Vitro Development of Grown Oocytes Enclosed in Caprine Preantral Follicles. Growth Horm. IGF Res. 2013, 23, 37–44. [Google Scholar] [CrossRef]
- de Loos, F.; van Vliet, C.; van Maurik, P.; Kruip, T.A.M. Morphology of Immature Bovine Oocytes. Gamete Res. 1989, 24, 197–204. [Google Scholar] [CrossRef]
- Ralph, J.H.; Telfer, E.E.; Wilmut, I. Bovine Cumulus Cell Expansion Does Not Depend on the Presence of an Oocyte Secreted Factor. Mol. Reprod. Dev. 1995, 42, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.S.; Chan, J.; Levy, D.E.; Horvath, C.; Sadowski, H.B.; Wang, L.H. Mechanism of STAT3 Activation by Insulin-like Growth Factor I Receptor. J. Biol. Chem. 2000, 275, 15099–15105. [Google Scholar] [CrossRef]
- Himpe, E.; Kooijman, R. Insulin-like Growth Factor-I Receptor Signal Transduction and the Janus Kinase/Signal Transducer and Activator of Transcription (JAK-STAT) Pathway. BioFactors 2009, 35, 76–81. [Google Scholar] [CrossRef]
- Takahashi, T.; Fukuda, K.; Pan, J.; Kodama, H.; Sano, M.; Makino, S.; Kato, T.; Manabe, T.; Ogawa, S. Characterization of Insulin-Like Growth Factor-1–Induced Activation of the JAK/STAT Pathway in Rat Cardiomyocytes. Circ. Res. 1999, 85, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Wrenzycki, C. Alterations of Expression of Developmentally Important Genes in Preimplantation Bovine Embryos by in Vitro Culture Conditions: Implications for Subsequent Development. Theriogenology 2000, 53, 21–34. [Google Scholar] [CrossRef]
- Augustin, R.; Pocar, P.; Navarrete-Santos, A.; Wrenzycki, C.; Gandolfi, F.; Niemann, H.; Fischer, B. Glucose Transporter Expression Is Developmentally Regulated in in Vitro Derived Bovine Preimplantation Embryos. Mol. Reprod. Dev. 2001, 60, 370–376. [Google Scholar] [CrossRef]
- Wu, G.; Schöler, H.R. Role of Oct4 in the Early Embryo Development. Cell Regen. 2014, 3, 7. [Google Scholar] [CrossRef]
- Kuzmany, A.; Havlicek, V.; Wrenzycki, C.; Wilkening, S.; Brem, G.; Besenfelder, U. Expression of MRNA, before and after Freezing, in Bovine Blastocysts Cultured under Different Conditions. Theriogenology 2011, 75, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Boulle, N.; Schneid, H.; Listrat, A.; Holthuizen, P.; Binoux, M.; Groyer, A. Developmental Regulation of Bovine Insulin-like Growth Factor-II (IGF-II) Gene Expression: Homology between Bovine Transcripts and Human IGF-II Exons. J. Mol. Endocrinol. 1993, 11, 117–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Francis, G.L.; Upton, F.M.; Ballard, F.J.; McNeil, K.A.; Wallace, J.C. Insulin-like Growth Factors 1 and 2 in Bovine Colostrum. Sequences and Biological Activities Compared with Those of a Potent Truncated Form. Biochem. J. 1988, 251, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Giroto, A.B.; Franchi, F.F.; Fontes, P.K.; Maioli, M.A.; Nogueira, G.P.; Nogueira, M.F.G.; Castilho, A.C.S. 128 Evidence That Pregnancy-Associated Serum Protein A (PAPP-A) Plays Role on Bovine In Vitro Embryo Production. Reprod. Fertil. Dev. 2018, 30, 204. [Google Scholar] [CrossRef]
- Nicholas, B.; Alberio, R.; Fouladi-Nashta, A.A.; Webb, R. Relationship between Low-Molecular-Weight Insulin-like Growth Factor-Binding Proteins, Caspase-3 Activity, and Oocyte Quality. Biol. Reprod. 2005, 72, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Mense, K.; Meyerholz, M.; Gil Araujo, M.; Lietzau, M.; Knaack, H.; Wrenzycki, C.; Hoedemaker, M.; Piechotta, M. The Somatotropic Axis during the Physiological Estrus Cycle in Dairy Heifers-Effect on Hepatic Expression of GHR and SOCS2. J. Dairy. Sci. 2015, 98, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Mense, K.; Heidekorn-Dettmer, J.; Wirthgen, E.; Brockelmann, Y.; Bortfeldt, R.; Peter, S.; Jung, M.; Höflich, C.; Hoeflich, A.; Schmicke, M. Increased Concentrations of Insulin-like Growth Factor Binding Protein (IGFBP)-2, IGFBP-3, and IGFBP-4 Are Associated with Fetal Mortality in Pregnant Cows. Front. Endocrinol. 2018, 9, 310. [Google Scholar] [CrossRef]
- Sang, L.; Ortiz, W.; Xiao, Y.; Estrada-Cortes, E.; Jannaman, E.A.; Hansen, P.J. Actions of Putative Embryokines on Development of the Preimplantation Bovine Embryo to the Blastocyst Stage. J. Dairy. Sci. 2020, 103, 11930–11944. [Google Scholar] [CrossRef]
- Aguilera, C.; Velásquez, A.E.; Gutierrez-Reinoso, M.A.; Wong, Y.S.; Melo-Baez, B.; Cabezas, J.; Caamaño, D.; Navarrete, F.; Rojas, D.; Riadi, G.; et al. Extracellular Vesicles Secreted by Pre-Hatching Bovine Embryos Produced In Vitro and In Vivo Alter the Expression of IFNtau-Stimulated Genes in Bovine Endometrial Cells. Int. J. Mol. Sci. 2023, 24, 7438. [Google Scholar] [CrossRef]
- Lopes, A.S.; Wrenzycki, C.; Ramsing, N.B.; Herrmann, D.; Niemann, H.; Løvendahl, P.; Greve, T.; Callesen, H. Respiration Rates Correlate with MRNA Expression of G6PD and GLUT1 Genes in Individual Bovine in Vitro-Produced Blastocysts. Theriogenology 2007, 68, 223–236. [Google Scholar] [CrossRef]
- Block, J.; Wrenzycki, C.; Niemann, H.; Herrmann, D.; Hansen, P.J. Effects of Insulin-like Growth Factor-1 on Cellular and Molecular Characteristics of Bovine Blastocysts Produced in Vitro. Mol. Reprod. Dev. 2008, 75, 895–903. [Google Scholar] [CrossRef]
- Bonilla, A.Q.S.; Oliveira, L.J.; Ozawa, M.; Newsom, E.M.; Lucy, M.C.; Hansen, P.J. Developmental Changes in Thermoprotective Actions of Insulin-like Growth Factor-1 on the Preimplantation Bovine Embryo. Mol. Cell Endocrinol. 2011, 332, 170–179. [Google Scholar] [CrossRef]
- Velazquez, M.A.; Hadeler, K.G.; Herrmann, D.; Kues, W.A.; Rémy, B.; Beckers, J.F.; Niemann, H. In Vivo Oocyte IGF-1 Priming Increases Inner Cell Mass Proliferation of in Vitro-Formed Bovine Blastocysts. Theriogenology 2012, 78, 517–527. [Google Scholar] [CrossRef]
- Zaraza, J.; Oropeza, A.; Velazquez, M.A.; Korsawe, K.; Herrmann, D.; Carnwath, J.W.; Niemann, H. Developmental Competence and MRNA Expression of Preimplantation in Vitro-Produced Embryos from Prepubertal and Postpubertal Cattle and Their Relationship with Apoptosis after Intraovarian Administration of IGF-1. Theriogenology 2010, 74, 75–89. [Google Scholar] [CrossRef]
- Wo, D.; Peng, J.; Ren, D.N.; Qiu, L.; Chen, J.; Zhu, Y.; Yan, Y.; Yan, H.; Wu, J.; Ma, E.; et al. Opposing Roles of Wnt Inhibitors IGFBP-4 and Dkk1 in Cardiac Ischemia by Differential Targeting of LRP5/6 and β-Catenin. Circulation 2016, 134, 1991–2007. [Google Scholar] [CrossRef]
- Zhu, W.; Shiojima, I.; Ito, Y.; Li, Z.; Ikeda, H.; Yoshida, M.; Naito, A.T.; Nishi, J.I.; Ueno, H.; Umezawa, A.; et al. IGFBP-4 Is an Inhibitor of Canonical Wnt Signalling Required for Cardiogenesis. Nature 2008, 454, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Boyer, A.; Goff, A.K.; Boerboom, D. WNT Signaling in Ovarian Follicle Biology and Tumorigenesis. Trends Endocrinol. Metab. 2010, 21, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Modina, S.; Abbate, F.; Germanà, G.P.; Lauria, A.; Luciano, A.M. β-Catenin Localization and Timing of Early Development of Bovine Embryos Obtained from Oocytes Matured in the Presence of Follicle Stimulating Hormone. Anim. Reprod. Sci. 2007, 100, 264–279. [Google Scholar] [CrossRef]
- Miyakoshi, N.; Qin, X.; Kasukawa, Y.; Richman, C.; Srivastava, A.K.; Baylink, D.J.; Mohan, S. Systemic Administration of Insulin-like Growth Factor (IGF)-Binding Protein-4 (IGFBP-4) Increases Bone Formation Parameters in Mice by Increasing IGF Bioavailability via an IGFBP-4 Protease-Dependent Mechanism. Endocrinology 2001, 142, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Neira, J.A.; Tainturier, D.; Peña, M.A.; Martal, J. Effect of the Association of IGF-I, IGF-II, BFGF, TGF-Β1, GM-CSF, and LIF on the Development of Bovine Embryos Produced in Vitro. Theriogenology 2010, 73, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-C.; Huang, C.-C.; Huang, L.-S.; Chen, C.-I.; Lee, M.-S.; Liu, J.-Y. Evaluation of Mouse Blastocyst Implantation Rate by Morphology Grading. Chin. J. Physiol. 2004, 47, 43–47. [Google Scholar] [PubMed]
- Yuan, Y.Q.; van Soom, A.; Coopman, F.O.J.; Mintiens, K.; Boerjan, M.L.; van Zeveren, A.; de Kruif, A.; Peelman, L.J. Influence of Oxygen Tension on Apoptosis and Hatching in Bovine Embryos Cultured in Vitro. Theriogenology 2003, 59, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.T. Variability in Different Lots of Commercial Bovine Serum Albumin Affects Cell Multiplication and Hatching of Rabbit Blastocysts in Culture. J. Reprod. Fertil. 1983, 69, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Schiewe, M.C.; Hazeleger, N.L.; Sclimenti, C.; Balmaceda, J.P. Physiological Characterization of Blastocyst Hatching Mechanisms by Use of a Mouse Antihatching Model. Fertil. Steril. 1995, 63, 288–294. [Google Scholar] [CrossRef]
- Coates, A.A.; Menino, A.R. Effects of Blastocoelic Expansion and Plasminogen Activator Activity on Hatching and Zona Pellucida Solubility in Bovine Embryos in Vitro. J. Anim. Sci. 1994, 72, 2936–2942. [Google Scholar] [CrossRef] [PubMed]
- Remacle-Bonnet, M.M.; Garrouste, F.L.; Pommier, G.J. Surface-Bound Plasmin Induces Selective Proteolysis of Insulin-like-Growth-Factor (IGF)-Binding Protein-4 (IGFBP-4) and Promotes Autocrine IGF-II Bio-Availability in Human Colon-Carcinoma Cells. Int. J. Cancer 1997, 72, 835–843. [Google Scholar] [CrossRef]
- Mariela, R.-O.; Verónica, M.; María Jesús, S.-C.; Paula, B.-B.; Dimitrios, R.; Alfonso, G.-A. Effect of Urokinase Type Plasminogen Activator on in Vitro Bovine Oocyte Maturation. Reproduction 2017, 154, 331–340. [Google Scholar] [CrossRef][Green Version]
- Lu, C.-H.; Lee, R.K.-K.; Hwu, Y.-M.; Lin, M.-H.; Yeh, L.-Y.; Chen, Y.-J.; Lin, S.-P.; Li, S.-H. Involvement of the Serine Protease Inhibitor, SERPINE2, and the Urokinase Plasminogen Activator in Cumulus Expansion and Oocyte Maturation. PLoS ONE 2013, 8, e74602. [Google Scholar] [CrossRef] [PubMed]
- Sieuwerts, A.M.; Martens, J.W.M.; Dorssers, L.C.J.; Klijn, J.G.M.; Foekens, J.A. Differential Effects of Fibroblast Growth Factors on Expression of Genes of the Plasminogen Activator and Insulin-like Growth Factor Systems by Human Breast Fibroblasts. Thromb. Haemost. 2002, 87, 674–683. [Google Scholar]
- Yoshimura, Y.; Ando, M.; Nagamatsu, S.; Iwashita, M.; Adachi, T.; Sueoka, K.; Miyazaki, T.; Kuji, N.; Tanaka, M. Effects of Insulin-Like Growth Factor-I on Follicle Growth, Oocyte Maturation, and Ovarian Steroidogenesis and Plasminogen Activator Activity in the Rabbit. Biol. Reprod. 1996, 55, 152–160. [Google Scholar] [CrossRef]

| Gene | Strand | Primer Sequenz 5′-3′ | Amplicon | Accession Number |
|---|---|---|---|---|
| Length (bp) | ||||
| Globin | forward | GCA GCC ACG GTG GCG AGT AT | 257 | X04751 |
| reverse | GTG GGA CAG GAG CTT GAA AT | |||
| SOCS2 | forward | GTG TGG CAA GGT AGC TAG GG | 202 | NM_177523.2 |
| reverse | TAC CAG TGC TGG GAC CTT TC | |||
| STAT3 | forward | TCT ACC CCG ACA TTC CAA AG | 157 | NM_001012671 |
| reverse | GGC AGG TCA ATG GTA TTG CT | |||
| SLC2A1 | forward | CAG GAG ATG AAG GAG GAG AGC | 258 | NM_174602 |
| reverse | CAC AAA TAG CGA CAC GAC AGT | |||
| SLC2A3 | forward | ATC CCT GTG GTC CTT GTC TG | 202 | NM_174603 |
| reverse | GAT AAT CAG TCG GCC CAA GA | |||
| BAX | forward | TCT GCA GGC AAC TTC AAC TG | 199 | NM_173894 |
| reverse | TGG GTG TCC CAA AGT AGG AG | |||
| POU5F1 | forward | GAG GAG TCC CAG GAC ATC AA | 149 | NM_174580 |
| reverse | GTC GTT TGG CTG AAC ACC TT |
| Blastocyst (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| rbIGFBP-4 | n1 | Cleavage (%) | n2 | 168 hpi | n3 | 192 hpi | n4 | 216 hpi |
| 0 ng/mL | 450 | 71.6 ± 0.09 | 400 | 21.0 ± 0.10 | 408 | 26.2 ± 0.09 | 334 | 29.0 ± 0.10 |
| 54 ng/mL | 412 | 71.4 ± 0.09 | 412 | 18.7 ± 0.10 | 371 | 26.4 ± 0.10 | 287 | 30.3 ± 0.11 |
| 540 ng/mL | 414 | 70.8 ± 0.05 | 414 | 21.3 ± 0.06 | 373 | 28.7 ± 0.05 | 288 | 33.3 ± 0.06 |
| 2000 ng/mL | 430 | 69.5 ± 0.09 | 386 | 15.8 ± 0.11 | 388 | 27.1 ± 0.09 | 304 | 32.6 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho de Gutiérrez, A.R.; Calisici, O.; Wrenzycki, C.; Gutiérrez-Añez, J.C.; Hoeflich, C.; Hoeflich, A.; Bajcsy, Á.C.; Schmicke, M. Effect of IGFBP-4 during In Vitro Maturation on Developmental Competence of Bovine Cumulus Oocyte Complexes. Animals 2024, 14, 673. https://doi.org/10.3390/ani14050673
Camacho de Gutiérrez AR, Calisici O, Wrenzycki C, Gutiérrez-Añez JC, Hoeflich C, Hoeflich A, Bajcsy ÁC, Schmicke M. Effect of IGFBP-4 during In Vitro Maturation on Developmental Competence of Bovine Cumulus Oocyte Complexes. Animals. 2024; 14(5):673. https://doi.org/10.3390/ani14050673
Chicago/Turabian StyleCamacho de Gutiérrez, Adriana Raquel, Oguz Calisici, Christine Wrenzycki, Juan Carlos Gutiérrez-Añez, Christine Hoeflich, Andreas Hoeflich, Árpád Csaba Bajcsy, and Marion Schmicke. 2024. "Effect of IGFBP-4 during In Vitro Maturation on Developmental Competence of Bovine Cumulus Oocyte Complexes" Animals 14, no. 5: 673. https://doi.org/10.3390/ani14050673
APA StyleCamacho de Gutiérrez, A. R., Calisici, O., Wrenzycki, C., Gutiérrez-Añez, J. C., Hoeflich, C., Hoeflich, A., Bajcsy, Á. C., & Schmicke, M. (2024). Effect of IGFBP-4 during In Vitro Maturation on Developmental Competence of Bovine Cumulus Oocyte Complexes. Animals, 14(5), 673. https://doi.org/10.3390/ani14050673






