α-Pinene Improves Follicle Morphology and Increases the Expression of mRNA for Nuclear Factor Erythroid 2-Related Factor 2 and Peroxiredoxin 6 in Bovine Ovarian Tissues Cultured In Vitro
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Source of Ovaries
2.2. In Vitro Culture of Ovarian Tissue
2.3. Evaluation of Follicle Growth and Survival
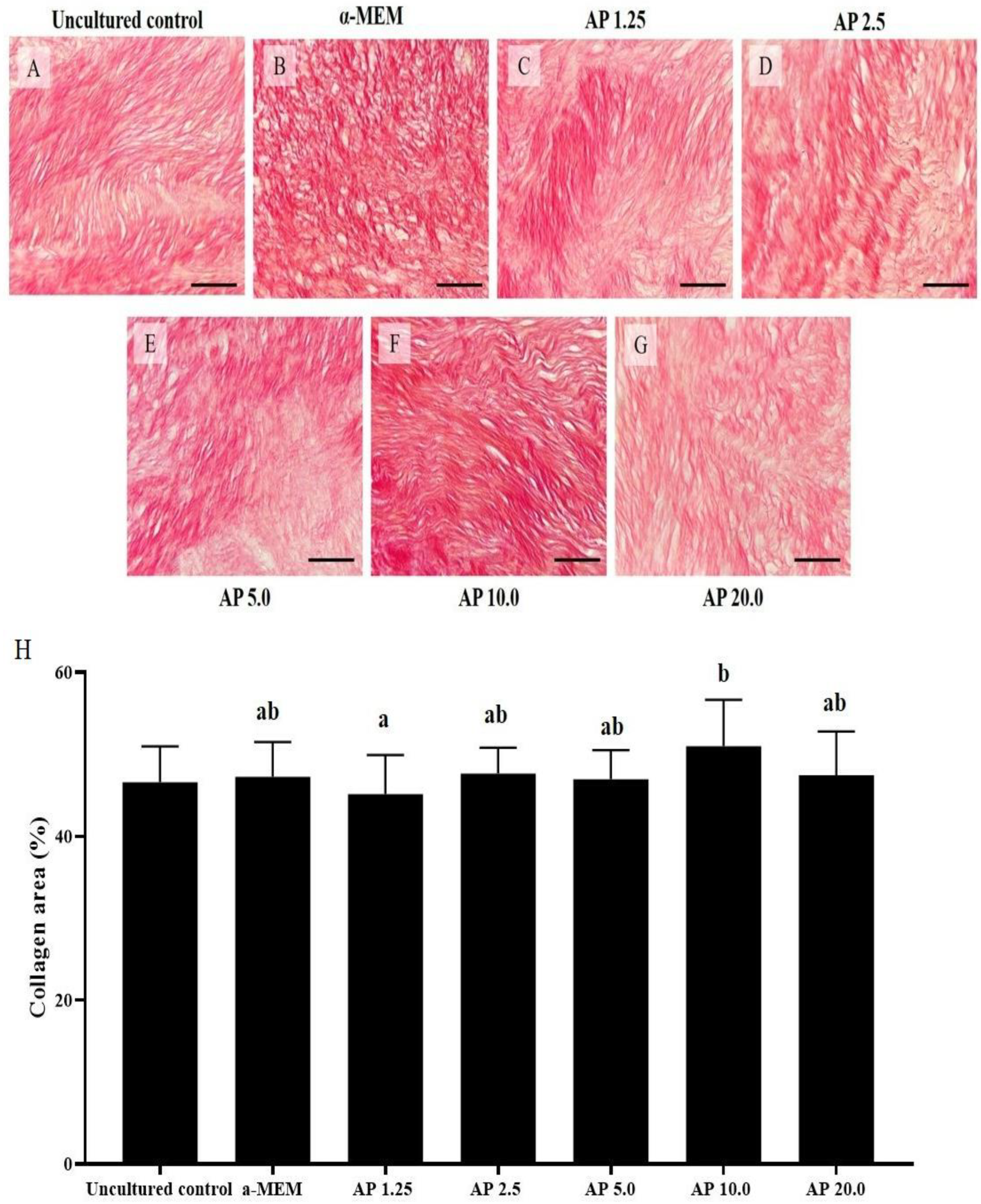
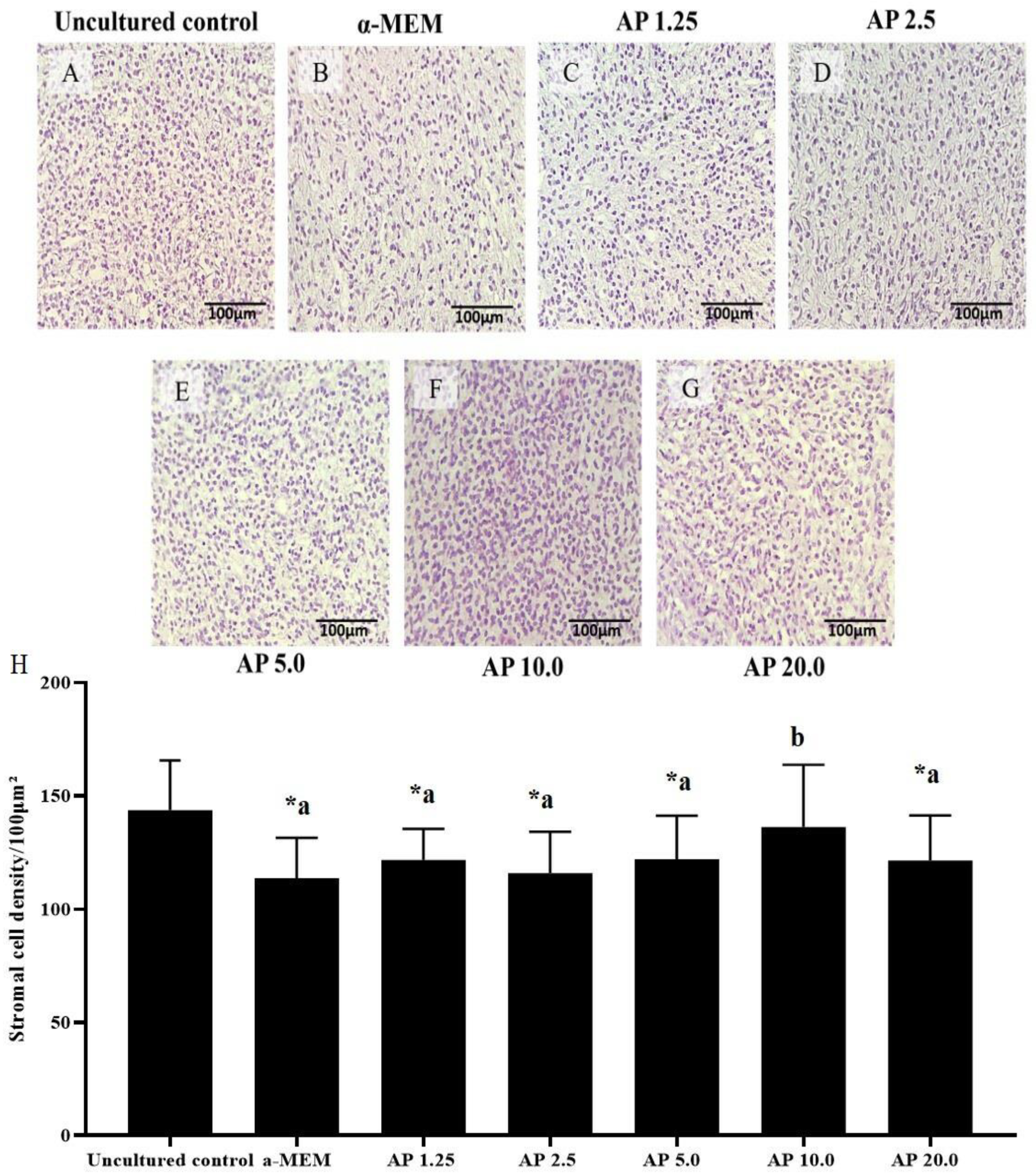
2.4. Evaluation of Stromal Cell Density and Collagen
2.5. Expression of mRNA for SOD, CAT, PRDX6, GPX1 and NRF2
2.6. Statistical Analysis
3. Results
3.1. Effects of α-Pinene on Follicular Morphology
3.2. Effects of α-Pinene on Activation and Development of Primordial Follicles
3.3. Assessment of Collagen Fibers and Stromal Cell Density
3.4. Levels of mRNA for CAT, SOD, GPX1, PRDX6 and NRF2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheng, X.; Zhou, J.; Kang, N.; Liu, W.; Yu, L.; Zhang, Z.; Zhang, Y.; Yue, Q.; Yang, Q.; Zhang, X.; et al. Temporal and spatial dynamics mapping reveals follicle development regulated by different stromal cell populations. J. bioRxiv. 2022. [Google Scholar] [CrossRef]
- Picton, H.M.; Harris, S.E.; Muruvi, W.; Chambers, E.L. The in vitro growth and maturation of follicles. Reproduction 2008, 136, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Grosbois, J.; Bailie, E.C.; Kelsey, T.W.; Anderson, R.A.; Telfer, E.E. Spatio-temporal remodelling of the composition and architecture of the human ovarian cortical extracellular matrix during in vitro culture. Hum. Reprod. 2023, 38, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Sá, N.A.R.; Bruno, J.B.; Guerreiro, D.D.; Cadenas, J.; Alves, B.G.; Cibin, F.W.S.; Leal-Cardoso, J.H.; Gastal, E.L.; Figueiredo, J.R. Anethole reduces oxidative stress and improves in vitro survival and activation of primordial follicles. Braz. J. Med. Biol. Res. 2018, 51, 7129. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, B.N.; Matos-Brito, B.G.; Paulino, L.R.F.M.; Silva, B.R.; Aguiar, A.W.M.; Almeida, E.F.M.; Souza, A.L.P.; Vasconcelos, G.L.; De Assis, E.I.T.; Silva, A.W.B.; et al. Effects of melatonin on morphology and development of primordial follicles during in vitro culture of bovine ovarian tissue. Reprod. Domest. Anim. 2019, 54, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.D.C.; Vasconcelos, E.M.; Azevedo, V.A.N.; Teixeira de Assis, E.I.; Paulino, L.R.F.M.; Silva, A.W.B.; Silva, J.R.V.; Batista, A.L.P.S. Aloe vera increases collagen fibers in extracellular matrix and mRNA expression of peroxiredoxin-6 in bovine ovarian cortical tissues cultured in vitro. Zygote 2022, 30, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.R.; Costa, F.C.; Neto, M.F.D.L.; Caetano Filho, F.F.; de Assis, E.I.; Aguiar, F.L.; Silva, A.W.B.; Martins, S.D.; Araújo, V.R.; Matos, M.H.T.; et al. Melatonin acts through different mechanisms to control oxidative stress and primordial follicle activation and survival during in vitro culture of bovine ovarian tissue. Domest. Anim. Endocrinol. 2024, 86, 106824. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A. Role of antioxidants in assisted reproductive techniques. World J. Mens. Health 2017, 3, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Sovernigo, T.C.; Adona, P.R.; Monzani, P.S.; Guemra, S.; Barros, F.; Lopes, F.G.; Leal, C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.L.D.; Jayaweera, A.S.; Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Major selected monoterpenes α-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and cytoprotective potential of the essential oil Pistacia lentiscus var. chia and its major components myrcene and α-pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.R.; Lima, L.F. Artificial ovary technology: Applications, state of art, limitations and prospects. Bra. J. Anim. Reprod. 2017, 41, 248–253. [Google Scholar]
- Silva, J.R.; Lucci, C.M.; Carvalho, F.C.; Báo, S.N.; Costa, S.H.; Santos, R.R.; Figueiredo, J.R. Effect of coconut water and braun-collins solutions at different temperatures and incubation times on the morphology of goat preantral follicles preserved in vitro. Theriogenoloy 2000, 54, 809–822. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rittié, L. Method for picrosirius red-polarization detection of collagen fibers in tissue sections. Fibrosis 2017, 1627, 395–407. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Naturforsch. C 2016, 71, 191–199. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.A.; Elfeki, A.; Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef]
- Elmann, A.; Mordechay, S.; Rindner, M.; Larkov, O.; Elkabetz, M.; Ravid, U. Protective effects of the essential oil of Salvia fructicosa and its constituents on astrocytic susceptibility to hydrogen peroxide-induced cell death. J. Agric. Food Chem. 2009, 57, 6636–6641. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Bao, H.; Liu, Z.; Man, X.; Liu, H.; Hou, Y.; Luo, Q.; Wang, S.; Fu, Q.; Zhang, H. hUMSCs regulate the differentiation of ovarian stromal cells via TGF-β1/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in POI rats. Stem Cell Res. Ther. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Parkes, W.S.; Amargant, F.; Zhou, L.T.; Villanueva, C.E.; Duncan, F.E.; Pritchard, M.T. Hyaluronan and collagen are prominent extracellular matrix components in bovine and porcine ovaries. Genes 2021, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Belotti, E.M.; Amweg, N.A.; Matiller, V.; Varela, M.L.; Stassi, A.F.; Velázquez, M.M.L.; Ortega, H.H.; Rey, F.; Salvetti, N.R. Effects of adrenocorticotrophic hormone on the expression of matrix metalloproteinases and their inhibitors in the bovine ovary. Reprod. Fert. Dev. 2020, 32, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.; Kanimozhi, G.; Madahavan, N.R.; Agilan, B.; Ganesan, M.; Prasad, N.R.; Rathinaraj, P. Alpha-pinene attenuates UVA-induced photoaging through inhibition of matrix metalloproteinases expression in mouse skin. Life Sci. 2019, 15, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Gagliardi, A.; Landi, C.; Focarelli, R.; De Leo, V.; Luddi, A.; Bini, L.; Piomboni, P. Protein pathways working in human follicular fluid: The future for tailored IVF? Expert Rev. Mol. Med. 2016, 18, e9. [Google Scholar] [CrossRef] [PubMed]
- Karimkhani, M.M.; Nasrollahzadeh, M.; Maham, M.; Jamshidi, A.; Kharazmi, M.S.; Dehnad, D.; Jafari, S.M. Extraction and purification of α-pinene; a comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 16, 4286–4311. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Paramio, M.T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef]
- Paulino, L.R.F.M.; De Assis, E.I.T.; Azevedo, V.A.N.; Silva, B.R.; Cunha, E.V.; Silva, J.R.V. Why is it so difficult to have competent oocytes from in vitro cultured preantral follicles? Reprod. Sci. 2022, 29, 3321–3334. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The role of oxidative stress and natural antioxidants in ovarian aging. Front. Pharmacol. 2021, 11. [Google Scholar] [CrossRef]
- Silva, R.L.S.S.; Barberino, R.S.; Matos, M.H.T. Impact of antioxidant supplementation during in vitro culture of ovarian preantral follicles: A review. Theriogenology 2023, 207, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Bonay, M.; Deramaudt, T.B. Nrf2: New insight in cell apoptosis. Cell Death Dis. 2015, 6, 1897. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Switching of redox signaling by Prdx6 expression decides cellular fate by hormetic phenomena involving Nrf2 and reactive oxygen species. Cells 2022, 8, 1266. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Vasquez-Medina, J.P.; Dodia, C.; Sorokina, E.M.; Tao, J.Q.; Feinstein, S.I. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, X.; Zheng, L.; Li, Z.; Zhao, X.; Lai, W.; Shen, H.; Lv, J.; Yang, G.; Wang, Q.; et al. Peroxiredoxin 6 is a crucial factor in the initial step of mitochondrial clearance and is upstream of the PINK1-parkin pathway. Antioxid. Redox Signal 2016, 24, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, E.M.; Feinstein, S.I.; Zhou, S.; Fisher, A.B. Intracellular targeting of peroxiredoxin 6 to lysosomal organelles requires MAPK activity and binding to epsilon. Am. J. Physiol. Cell Physiol. 2011, 300, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Leyens, G.; Knoops, B.; Donnay, I. Expression of peroxiredoxins in bovine oocytes and embryos produced in vitro. Mol. Reprod. Dev. 2004, 69, 43–251. [Google Scholar] [CrossRef]
- Zhong, Q.; Xiao, X.; Qiu, Y.; Xu, Z.; Chen, C.; Chong, B.; Zhao, X.; Hai, S.; Li, S.; Na, Z.; et al. Protein posttranslational modifications in health and diseases: Functions, regulatory mechanisms, and therapeutic implications. MedComm 2023, 4, 261. [Google Scholar] [CrossRef]





| Gene | Primer Sequence (5′→3′) | Sense (S) Anti-Sense (As) | GenBank Accession No. |
|---|---|---|---|
| GAPDH | TGTTTGTGATGGGCGTGAACCAATG GCGCGTGGACAGTGGTCATAA | S AS | GI: 402744670 |
| PRDX6 | GCACCTCCTCTTACTTCCCGGA TGCGGCCGATGGTAGTAT | S AS | GI: 59858298 |
| GPX1 | AACGTAGCATCGCTCTGAGG GATGCCCAAACTGGTTGCAG | S AS | GI: 156602645 |
| SOD | GTGAACAACCTCAACGTCGC GGGTTCTCCACCACCGTTAG | S AS | GI: 31341527 |
| CAT | AAGTTCTGCATCGCCACTCA GGGGCCCTACTGTCAGACTA | S AS | GI: 402693375 |
| NRF2 | GACCCAGTCCAACCTTTGTC GACCCGGACTTACAGGTACT | S AS | GI: 0304941 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, V.A.N.; De Assis, E.I.T.; Silva, A.W.B.; Costa, F.D.C.; Souza, L.F.; Silva, J.R.V. α-Pinene Improves Follicle Morphology and Increases the Expression of mRNA for Nuclear Factor Erythroid 2-Related Factor 2 and Peroxiredoxin 6 in Bovine Ovarian Tissues Cultured In Vitro. Animals 2024, 14, 1443. https://doi.org/10.3390/ani14101443
Azevedo VAN, De Assis EIT, Silva AWB, Costa FDC, Souza LF, Silva JRV. α-Pinene Improves Follicle Morphology and Increases the Expression of mRNA for Nuclear Factor Erythroid 2-Related Factor 2 and Peroxiredoxin 6 in Bovine Ovarian Tissues Cultured In Vitro. Animals. 2024; 14(10):1443. https://doi.org/10.3390/ani14101443
Chicago/Turabian StyleAzevedo, Venância Antonia Nunes, Ernando Igo Teixeira De Assis, Anderson Weiny Barbalho Silva, Francisco Das Chagas Costa, Layana Freitas Souza, and José Roberto Viana Silva. 2024. "α-Pinene Improves Follicle Morphology and Increases the Expression of mRNA for Nuclear Factor Erythroid 2-Related Factor 2 and Peroxiredoxin 6 in Bovine Ovarian Tissues Cultured In Vitro" Animals 14, no. 10: 1443. https://doi.org/10.3390/ani14101443
APA StyleAzevedo, V. A. N., De Assis, E. I. T., Silva, A. W. B., Costa, F. D. C., Souza, L. F., & Silva, J. R. V. (2024). α-Pinene Improves Follicle Morphology and Increases the Expression of mRNA for Nuclear Factor Erythroid 2-Related Factor 2 and Peroxiredoxin 6 in Bovine Ovarian Tissues Cultured In Vitro. Animals, 14(10), 1443. https://doi.org/10.3390/ani14101443






