Long-Term Survival in 241 Cases of Intussusception in Cattle and Factors Associated with Mortality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Data Collection and Selection Procedure
2.2. Statistical Analysis
3. Results
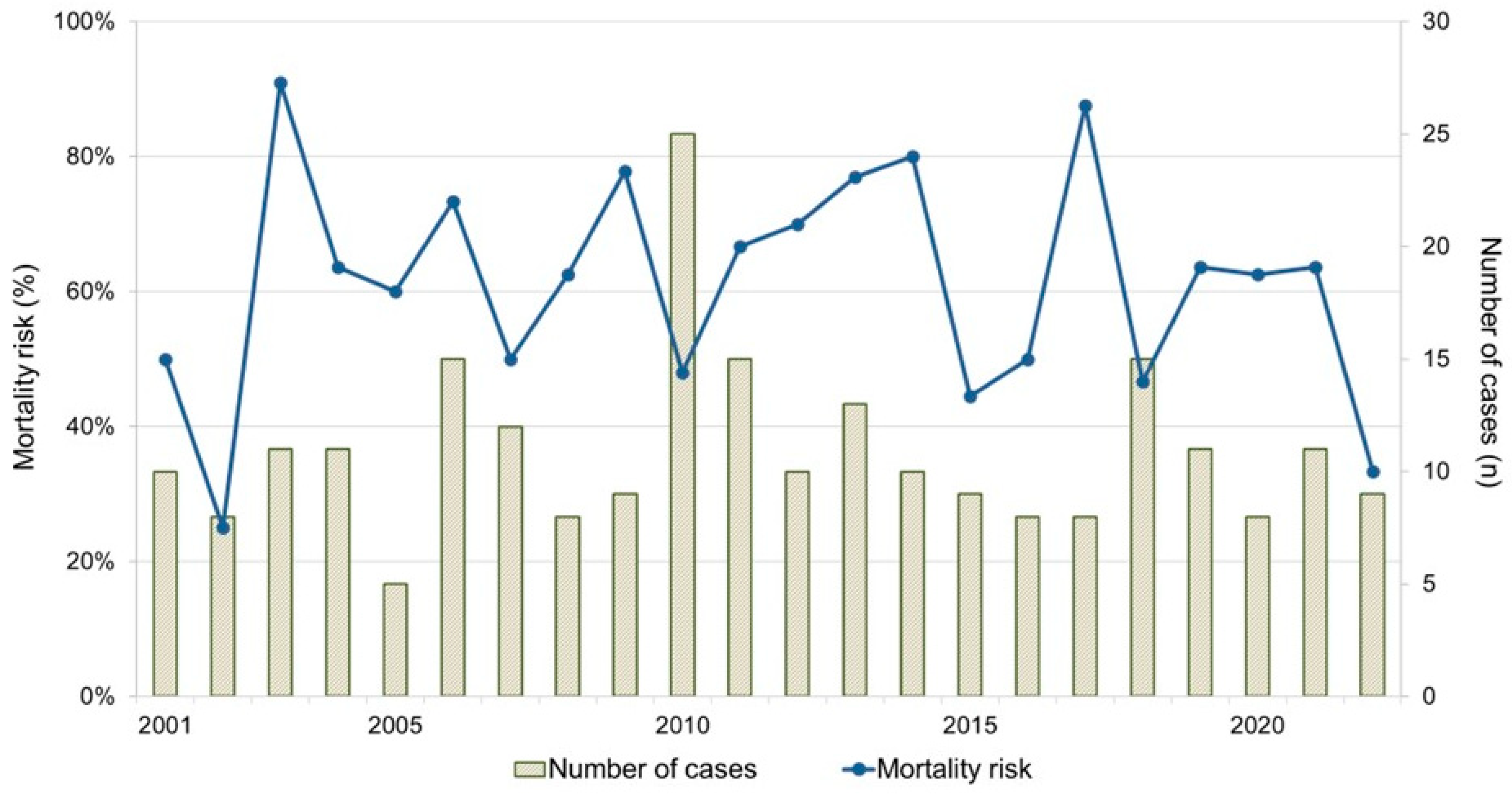
3.1. Animals
3.2. Mortality
3.3. Multivariable Model and Decision Tree
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tafti, A.K. Ileoileal Intussusception Associated with Coccidiosis in Sheep. J. Vet. Intern. Med. 1999, 46, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Shakespeare, A. Intussusception as a Complication of Rectal Prolapse Replacement in a Ewe. Vet. Rec. 2000, 147, 78–79. [Google Scholar] [CrossRef]
- Navarre, C.B.; Pugh, D.G. Diseases of the Gastrointestinal System. In Sheep & Goat Medicine, 1st ed.; Pugh, D.G., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2002; pp. 69–105. [Google Scholar]
- Mushonga, B.; Ntahonshikira, C.; Habarugira, G. Mesenteric Tear (Rent), Jejunal Volvulus, Torsion and Entrapment in a Kalahari Red Nanny Goat: Post Mortem Case Report and Literature Review. Glob. Vet. 2016, 17, 1–4. [Google Scholar] [CrossRef]
- Constable, P.D.; St Jean, G.; Hull, B.L.; Rings, D.M.; Morin, D.E.; Nelson, D.R. Intussusception in Cattle: 336 Cases (1964–1993). J. Am. Vet. Med. Assoc. 1997, 210, 531–536. [Google Scholar] [CrossRef]
- Mitchell, W.C. Intussusception in Goats. Vet. Med. Small Anim. Clin. 1983, 78, 1918. [Google Scholar]
- Pravettoni, D.; Morandi, N.; Rondena, M.; Riccaboni, P.; Zani, D.D.; Scandella, M.; Belloli, A.G. Repeated Occurrence of Jejuno-Jejunal Intussusception in a Calf. Can. Vet. J. 2009, 50, 287–290. [Google Scholar] [PubMed]
- Pearson, H. Intussusception in Cattle. Vet. Rec. 1971, 89, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.B.; Shin, S.M.; Lee, K.C.; Lee, H.B.; Kim, M.S.; Kim, N.S. Surgical Management of an Ileocecocolic Intussusception in a Korean Native Calf: A Case Report. Vet. Med. 2013, 58, 645–649. [Google Scholar] [CrossRef]
- Smith, D.F. Surgery of the Bovine Small Intestine. Vet. Clin. N. Am. Food Anim. Pract. 1990, 6, 449–460. [Google Scholar] [CrossRef]
- Dirksen, G.; Doll, K. Ileus and Subileus in the Young Bovine Animal. Bov. Pract. 1986, 21, 33–40. [Google Scholar] [CrossRef]
- Imran, S.; Tyagi, S.P.; Kumar, A.; Kumar, A.; Sharma, A.; Sharma, S. Usefulness and Limitation of Ultrasonography in the Diagnosis of Intestinal Intussusception in Cows. Vet. Med. Int. 2011, 2011, 584387. [Google Scholar] [CrossRef][Green Version]
- Francoz, D.; Guard, C.L. Obstructive Intestinal Diseases. In Large Animal Internal Medicine, 5th ed.; Smith, B.P., Ed.; Elsevier Mosby: Maryland Heights, MO, USA, 2015; p. 821. [Google Scholar]
- Horne, M.M. Colonic Intussusception in a Holstein Calf. Can. Vet. J. 1991, 32, 493–495. [Google Scholar] [PubMed]
- Braun, U.; Gerspach, C.; Volz, C.; Boesiger, M.; Hilbe, M.; Nuss, K. A Retrospective Review of Small Intestinal Intussusception in 126 Cattle in Switzerland. Vet. Rec. Open 2023, 10, e58. [Google Scholar] [CrossRef] [PubMed]
- Pardon, B.; Ribbens, S.; Van Damme, L.; Vlaminck, L.; Martens, A.; Deprez, P. Use of a National Identification Database to Determine the Lifetime Prognosis in Cattle with Necrotic Laryngitis and the Predictive Value of Venous PCO2. J. Vet. Intern. Med. 2018, 32, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Marmier, O.; Pusterla, N. Ultrasonographic Examination of the Small Intestine of Cows with Ileus of the Duodenum, Jejunum or Ileum. Vet. Rec. 1995, 137, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Marmier, O. Ultrasonographic Examination of the Small Intestine of Cows. Vet. Rec. 1995, 136, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Padel-Gschwind, D.; Stocker, H. Sonographic Examinations of the Intestines in Calves. Schweiz. Arch. Tierheilkd. 2004, 146, 173–181. [Google Scholar] [CrossRef]
- Edwards, G.B. Surgical Management of Intussusception in the Horse. Equine Vet. J. 1986, 18, 313–321. [Google Scholar] [CrossRef]
- Nelson, B.B.; Brounts, S.H. Intussusception in Horses. Compend. Contin. Educ. Vet. 2012, 34, 1–5. [Google Scholar]
- Gleerup, K.B.; Andersen, P.H.; Munksgaard, L.; Forkman, B. Pain Evaluation in Dairy Cattle. Appl. Anim. Behav. Sci. 2015, 171, 25–32. [Google Scholar] [CrossRef]
- Desrochers, A.; Anderson, D.E. Intestinal Surgery. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 645–671. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F. Bovine Gastrointestinal Surgery: Abomasal Volvulus Intestinal Surgery. Bov. Pract. 1984, 19, 230–235. [Google Scholar] [CrossRef]
- Hamilton, G.F.; Tulleners, E.P. Intussusception Involving the Spiral Colon in a Calf. Can. Vet. J. 1980, 21, 32. [Google Scholar] [PubMed]
- Kovács, L.; Jurkovich, V.; Bakony, M.; Szenci, O.; Póti, P.; Tå’Zsér, J. Welfare Implication of Measuring Heart Rate and Heart Rate Variability in Dairy Cattle: Literature Review and Conclusions for Future Research. Animal 2014, 8, 316–330. [Google Scholar] [CrossRef]
- Meylan, M. Prognostic Indicators in Cattle with Right-Sided Displacement of the Abomasum and Abomasal Volvulus. Schweiz. Arch. Tierheilkd. 1999, 141, 413–418. [Google Scholar]
- Braun, U.; Nuss, K.; Warislohner, S.; Reif, C.; Oschlies, C.; Gerspach, C. Diagnostic Reliability of Clinical Signs in Cows with Traumatic Reticuloperitonitis and Abomasal Ulcers. BMC Vet. Res. 2020, 16, 359. [Google Scholar] [CrossRef]
- Farrell, A.; Kersh, K.; Liepman, R.; Dembek, K.A. Development of a Colic Scoring System to Predict Outcome in Horses. Front. Vet. Sci. 2021, 8, 697589. [Google Scholar] [CrossRef]
- Dillane, P.; Krump, L.; Kennedy, A.; Sayers, R.G.; Sayers, G.P. Establishing Blood Gas Ranges in Healthy Bovine Neonates Differentiated by Age, Sex, and Breed Type. J. Dairy. Sci. 2018, 101, 3205–3212. [Google Scholar] [CrossRef]
- Weiss, D.J.; Wardrop, K.J. Schalm’s Veterinary Hematology, 6th ed.; John Wiley & Sons: New York City, NY, USA, 2011. [Google Scholar]
- Muiño, R.; Hernández, J.; Castillo, C. Acute Abdominal Disorders in Dairy Cattle: What Can Clinicians Do under Field Conditions? Ruminants 2021, 1, 46–57. [Google Scholar] [CrossRef]
- Taylor, J.; Fulton, R.; Lehenbauer, T.; Step, D.; Confer, A. The Epidemiology of Bovine Respiratory Disease: What Is the Evidence for Predisposing Factors? Can. Vet. J. 2010, 51, 1351–1359. [Google Scholar] [PubMed]
- Pardon, B.; Hostens, M.; Duchateau, L.; Dewulf, J.; De Bleecker, K.; Deprez, P. Impact of Respiratory Disease, Diarrhea, Otitis and Arthritis on Mortality and Carcass Traits in White Veal Calves. BMC Vet. Res. 2013, 9, 79. [Google Scholar] [CrossRef]
- Pas, M.L.; Bokma, J.; Lowie, T.; Boyen, F.; Pardon, B. Sepsis and Survival in Critically Ill Calves: Risk Factors and Antimicrobial Use. J. Vet. Intern. Med. 2023, 37, 374–389. [Google Scholar] [CrossRef]
- Reeves, M.J.; Gay, J.M.; Hilbert, B.J.; Morris, R.S. Association of Age, Sex and Breed Factors in Acute Equine Colic: A Retrospective Study of 320 Cases Admitted to a Veterinary Teaching Hospital in the U.S.A. Prev. Vet. Med. 1989, 7, 149–160. [Google Scholar] [CrossRef]
- Sundrum, A. Metabolic Disorders in the Transition Period Indicate that the Dairy Cows’ Ability to Adapt Is Overstressed. Animals 2015, 5, 978–1020. [Google Scholar] [CrossRef]
- Bhatt, N.; Singh, N.P.; Mishra, A.K.; Kandpal, D.; Jamwal, S. A Detailed Review of Transportation Stress in Livestock and Its Management Techniques. Int. J. Livest. Res. 2021, 11, 30–41. [Google Scholar]
- Anderson, D.E.; Constable, P.D.; St Jean, G.; Hull, B.L. Small-Intestinal Volvulus in Cattle: 35 Cases (1967–1992). J. Am. Vet. Med. Assoc. 1993, 203, 1178–1183. [Google Scholar] [CrossRef]
- Wittek, T.; Grosche, A.; Locher, L.F.; Fürll, M. Diagnostic Accuracy of D-dimer and Other Peritoneal Fluid Analysis Measurements in Dairy Cows with Peritonitis. J. Vet. Intern. Med. 2010, 24, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Boulay, G.; Francoz, D.; Doré, E.; Dufour, S.; Veillette, M.; Badillo, M.; Bélanger, A.M.; Buczinski, S. Preoperative Cow-Side Lactatemia Measurement Predicts Negative Outcome in Holstein Dairy Cattle with Right Abomasal Disorders. J. Dairy. Sci. 2014, 97, 212–221. [Google Scholar] [CrossRef]



| Variable | Mean | Median | SD | Min.–Max. | Number of Cases (n) |
|---|---|---|---|---|---|
| pH | 7.42 | 7.43 | 0.11 | 6.67–7.83 | 213 |
| pCO2 (mmHg) | 47.9 | 46.4 | 10.2 | 25.0–91.9 | 213 |
| Base excess (mmol/L) | 5.4 | 5.2 | 8.6 | −30.8–34.2 | 228 |
| HCO3− (mmol/L) | 30.0 | 29.4 | 8.1 | 5.5–60.3 | 212 |
| Packed cell volume (%) | 37.3 | 37.0 | 6.4 | 20.0–55.0 | 225 |
| Sodium (mmol/L) | 133.2 | 133.1 | 5.9 | 115.0–156.0 | 138 |
| Potassium (mmol/L) | 3.72 | 3.50 | 0.96 | 1.82–7.34 | 138 |
| Ionized calcium (mmol/L) | 1.01 | 1.01 | 0.12 | 0.71–1.28 | 143 |
| Chloride (mmol/L) | 87.6 | 89.5 | 10.1 | 65.0–105.0 | 98 |
| Glucose (mg/dL) | 111.3 | 107.0 | 48.4 | 28.0–331.0 | 95 |
| L-lactate (mmol/L) | 6.5 | 4.3 | 5.7 | 1.2–22.7 | 29 |
| Variable | Cattle (n) | Mean ± SD; Median (Min.–Max.) | p-Value | |
|---|---|---|---|---|
| Slaughtered | Mortality * | |||
| Age at admission (days) | 241 | 871.3 ± 671.4; 680.5 (10.0–3016.0) | 578.9 ± 729.4; 289.0 (4.0–4239.0) | 0.004 |
| Days of clinical signs (days) | 208 | ±1.7; 2.0 (0.0–10.0) | 2.5 ± 2.3; 2.0 (0.0–14.0) | 0.11 |
| Body weight (kg) | 154 | 432.0 ± 218.2; 483.0 (50.0–890.0) | 325.2 ± 258.1; 258.1 (42.0–800.0) | 0.05 |
| Heart rate (bpm) | 235 | 81.2 ± 21.1; 80.0 (40.0–152.0) | 93.6 ± 28.1; 93.0 (29.0–180.0) | 0.001 |
| Rectal temperature (°C) | 236 | 38.5 ± 0.80; 38.6 (34.1–40.4) | 38.6 ± 0.66; 38.7 (36.8–39.9) | 0.20 |
| Respiratory rate (breaths/min) | 207 | 39.3 ± 15.0; 40.00 (12.0–80.0) | 40.0 ± 17.5; 36.0 (12.0–88.0) | 0.58 |
| pH | 213 | 7.43 ± 0.09; 7.44 (7.21–7.63) | 7.41 ± 0.12; 7.43 (6.67–7.83) | 0.26 |
| pCO2 (mmHg) | 213 | 46.6 ± 9.1; 44.9 (25.0–74.4) | 48.7 ± 10.8; 47.1 (25.6–91.9) | 0.26 |
| Base excess (mmol/L) | 228 | 6.1 ± 8.0; 5.3 (−12.3–34.2) | 4.9 ± 8.9; 5.2 (−30.8–29.0) | 0.41 |
| HCO3− (mmol/L) | 212 | 30.4 ± 7.9; 29.2 (14.8–60.3) | 29.7 ± 8.3; 29.5 (5.5–51.2) | 0.61 |
| Packed cell volume (%) | 225 | 38.4 ± 5.4; 38.0 (27.0–49.0) | 36.6 ± 7.0; 36.0 (20.0–55.0) | 0.14 |
| Sodium (mmol/L) | 138 | 133.0 ± 4.8; 132.9 (120.0–142.0) | 133.3 ± 6.6; 133.1 (115.0–156.0) | 0.61 |
| Potassium (mmol/L) | 138 | 3.46 ± 0.72; 3.37 (1.82–5.23) | 3.91 ± 1.07; 3.62 (1.90–7.34) | 0.02 |
| Ionized calcium (mmol/L) | 143 | 1.01 ± 0.12; 1.02 (0.77–1.28) | 1.01 ± 0.12; 1.01 (0.71–1.25) | 0.74 |
| Chloride (mmol/L) | 98 | 87.7 ± 9.3; 89.0 (65.0–105.0) | 87.5 ± 10.7; 90.0 (65.0–102.0) | 0.72 |
| Glucose (mg/dL) | 95 | 118.2 ± 37.6; 114.0 (58.0–205.0) | 106.3 ± 54.7; 103.0 (28.0–331.0) | 0.44 |
| L-lactate (mmol/L) | 29 | 5.9 ± 5.2; 3.7 (1.5–20.4) | 7.1 ± 6.2; 4.8 (1.2–22.7) | 0.53 |
| Intussusception length (cm) | 63 | 62.0 ± 35.1; 50.0 (20.0–150.0) | 60.1 ± 64.5; 40.0 (0.5–300.0) | 0.85 |
| Variable | Categories | Observed Mortality | p-Value | HR | 95% CI |
|---|---|---|---|---|---|
| Gender | Female (ref) | 108/197 (54.8%) | |||
| Male | 39/44 (88.6%) | <0.001 | 2.2 | 1.4–2.0 | |
| Breed | Belgian Blue (ref) | 128/214 (59.8%) | |||
| Others | 19/27 (70.4%) | 0.30 | 1.3 | 0.80–2.1 | |
| Age at entry (days) | >226 (ref) | 77/160 (48.1%) | |||
| <226 | 70/81 (86.4%) | <0.001 | 2.3 | 1.7–3.4 | |
| Season | Spring (ref) | 40/74 (54.1%) | 0.44 | ||
| Summer | 44/62 (71.0%) | 0.10 | 1.5 | 0.93–2.2 | |
| Fall | 31/51 (60.8%) | 0.52 | 1.3 | 0.73–1.9 | |
| Winter | 32/54 (59.3%) | 0.59 | 1.1 | 0.71–1.8 | |
| Season hot/cold | Spring/summer (ref) | 84/136 (61.8%) | |||
| Autumn/winter | 63/105 (60.0%) | 0.87 | 1.0 | 0.70–1.4 | |
| Weight (kg) | >305.5 (ref) | 46/84 (45.1%) | |||
| <305.5 | 56/70 (54.9%) | 0.007 | 1.5 | 1.2–2.5 | |
| Time to referral (days) | <4.5 (ref) | 107/185 (57.8%) | |||
| >4.5 | 18/23 (78.3%) | 0.04 | 1.3 | 1.0–2.8 | |
| Medication at home: SAID | No (ref) | 124/211 (58.8%) | |||
| Yes | 20/22 (90.9%) | 0.006 | 1.8 | 1.2–3.1 | |
| Medication at home: spasmolytic | No (ref) | 99/167 (59.3%) | |||
| Yes | 45/66 (68.2%) | 0.25 | 1.3 | 0.87–1.8 | |
| Medication at home: NSAID | No (ref) | 99/156 (63.5%) | |||
| Yes (ref) | 45/77 (58.4%) | 0.63 | 1.0 | 0.64–1.3 | |
| Medication at home: antibiotic | No (ref) | 95/163 (58.3%) | |||
| Yes | 49/70 (70.0%) | 0.12 | 1.3 | 0.93–1.9 | |
| Medication at home: NSAID+SAID | No (ref) | 135/223 (60.5%) | |||
| Yes | 9/10 (90.0%) | 0.10 | 1.6 | 0.90–3.5 | |
| No medication at home | No (ref) | 85/136 (62.5%) | |||
| Yes | 59/97 (60.8%) | 0.62 | 0.9 | 0.66–1.3 | |
| Colic | No (ref) | 3/6 (50.0%) | |||
| Yes | 54/98 (55.1%) | 0.91 | 0.3 | 0.29–3.0 | |
| Temperature (°C) | >38.05 (ref) | 115/182 (63.2%) | |||
| <38.05 | 28/54 (51.9%) | 0.25 | 0.8 | 0.52–1.2 | |
| Heart rate (bpm) | <95 (ref) | 72/144 (50.0%) | |||
| >95 | 70/91 (76.9%) | <0.001 | 1.8 | 1.4–2.6 | |
| Respiratory rate (breaths/minute) | <58 (ref) | 103/175 (58.9%) | |||
| >58 | 23/32 (71.9%) | 0.10 | 1.3 | 0.93–2.3 | |
| Pale mucosa | No (ref) | 113/198 (57.1%) | |||
| Yes | 28/33 (84.8%) | 0.02 | 1.6 | 1.1–2.5 | |
| Capillary refill time (s) | ≤2 (ref) | 113/185 (61.1%) | |||
| >2 | 5/7 (71.4%) | 0.61 | 1.4 | 0.51–3.1 | |
| Skin turgor (s) | ≤2 (ref) | 86/137 (62.8%) | |||
| >2 | 44/75 (58.7%) | 0.78 | 1.0 | 0.66–1.4 | |
| Decubitus | No (ref) | 132/216 (60.6%) | |||
| Yes | 6/10 (60.0%) | 1.0 | 1.3 | 0.44–2.3 | |
| Lumbar reflex | Positive (ref) | 53/81 (65.4%) | |||
| Negative | 29/52 (55.8%) | 0.38 | 0.8 | 0.52–1.3 | |
| Tensed or bloated abdomen | No (ref) | 21/39 (53.8%) | |||
| Yes | 60/88 (68.2%) | 0.05 | 1.7 | 0.99–2.8 | |
| Presence of feces in rectum | Yes (ref) | 31/48 (64.6%) | |||
| No | 79/128 (61.7%) | 0.83 | 0.85 | 0.69–1.6 | |
| Melena present | No (ref) | 1/5 (20.0%) | |||
| Yes | 34/55 (61.8%) | 0.21 | 1.3 | 0.49–26.3 | |
| Borborygmus present on auscultation | Yes (ref) | 29/46 (63.0%) | |||
| No | 77/133 (57.9%) | 0.95 | 1.0 | 0.64–1.5 | |
| Rumen contractility on auscultation | Yes (ref) | 4/10 (40.0%) | |||
| No | 65/124 (52.4%) | 0.59 | 0.86 | 0.48–3.6 | |
| Sloshing sounds on auscultation | No (ref) | 44/70 (62.9%) | |||
| Yes | 64/112 (57.1%) | 0.46 | 0.83 | 0.59–1.3 | |
| Ping sounds on auscultation | No (ref) | 54/98 (55.1%) | |||
| Yes | 20/32 (62.5%) | 0.67 | 1.0 | 0.66–1.9 | |
| Transrectal palpation: dilated intestinal loops | No (ref) | 14/25 (56.0%) | |||
| Yes | 50/105 (47.6%) | 0.26 | 0.74 | 0.39–1.3 | |
| Transrectal palpation: donut-shaped structure | No (ref) | 47/98 (48.0%) | |||
| Yes | 17/32 (53.1%) | 0.91 | 0.95 | 0.55–1.7 | |
| pH | <7.415 (ref) | 59/89 (66.3%) | |||
| >7.415 | 68/124 (54.8%) | 0.15 | 0.79 | 0.55–1.1 | |
| pCO2 (mmHg) | <44.95 (ref) | 46/90 (51.1%) | |||
| >44.95 | 80/123 (65.0%) | 0.15 | 1.3 | 0.91–1.9 | |
| Base excess (mmol/L) | <2.85(ref) | 57/87 (65.5%) | |||
| >2.85 | 81/141 (57.4%) | 0.33 | 1.0 | 0.60–1.2 | |
| HCO3− (mmol/L) | >22.1 (ref) | 103/181 (56.9%) | |||
| <22.1 | 23/31 (74.2%) | 0.20 | 1.2 | 0.86–2.1 | |
| Packed cell volume (%) | <36.5 (ref) | 76/108 (70.4%) | |||
| >36.5 | 59/117 (50.4%) | 0.03 | 0.68 | 0.49–0.96 | |
| L-lactate (mmol/L) | <6.425 (ref) | 8/19 (42.1%) | |||
| >6.425 | 7/10 (70.0%) | 0.19 | 2.0 | 0.71–5.6 | |
| Sodium (mmol/L) | <139.70 (ref) | 69/123 (56.1%) | |||
| >139.70 | 11/15 (73.3%) | 0.26 | 1.2 | 0.76–2.7 | |
| Potassium (mmol/L) | <4645 (ref) | 60/116 (51.7%) | |||
| >4645 | 20/22 (90.9%) | 0.001 | 2.0 | 1.4–3.9 | |
| Ionized calcium (mmol/L) | >0.905 (ref) | 69/119 (59.0%) | |||
| <0.905 | 13/26 (50.0%) | 0.37 | 1.2 | 0.41–1.4 | |
| Chloride (mmol/L) | >89.50 (ref) | 33/49 (67.3%) | |||
| <89.50 | 25/49 (51.0%) | 0.25 | 0.62 | 0.44–1.2 | |
| Glucose (mg/dL) | <138.5 (ref) | 50/78 (64.1%) | |||
| >138.5 | 5/17 (29.4%) | 0.05 | 0.49 | 0.16–1.0 | |
| Ultrasound: Intestinal motility | Yes (ref) | 44/66 (66.7%) | |||
| No | 44/71 (62.0%) | 0.70 | 0.88 | 0.71–1.6 | |
| Ultrasound: Intestinal dilatation | No (ref) | 8/10 (80.0%) | |||
| Yes | 127/214 (59.3%) | 0.25 | 0.60 | 0.32–1.3 | |
| Ultrasound: Free fluid | No (ref) | 24/36 (66.7%) | |||
| Yes | 79/134 (59.0%) | 0.46 | 0.78 | 0.53–1.3 | |
| Ultrasound: Donut-shaped structure | No (ref) | 40/70 (57.1%) | |||
| Yes | 28/52 (53.8%) | 0.47 | 0.69 | 0.51–1.4 | |
| Location of intussusception | Small intestines (ref) | 61/114 (53.5%) | |||
| Large intestines | 33/39 (84.6%) | <0.001 | 2.0 | 1.4–3.4 | |
| Length of intussusception (cm) | <35 (ref) | 15/22 (68.2%) | |||
| >35 | 18/41 (43.9%) | 0.12 | 0.52 | 0.29–1.2 | |
| Peritonitis seen during surgery | No (ref) | 12/24 (50.0%) | |||
| Yes | 31/47 (66.0%) | 0.28 | 1.1 | 0.74–2.8 | |
| Adhesion noticed during surgery | No (ref) | 13/27 (48.1%) | |||
| Yes | 24/36 (66.7%) | 0.19 | 1.1 | 0.79–3.1 | |
| Postoperative antibiotics | Broad spectrum (ref) | 43/104 (41.3%) | |||
| Small spectrum | 5/20 (25.0%) | 0.27 | 0.53 | 0.23–1.5 | |
| Postoperative flunixin vs. other NSAIDs | Flunixin (ref) | 53/122 | |||
| Other NSAID | 9/18 | 0.53 | 1.3 | 0.62–2.5 |
| Variable | Mean | Median | SD | Min.–Max. | Number of Cases (n) |
|---|---|---|---|---|---|
| Survival time (days): | |||||
| All cases | 322.3 | 7.0 | 520.5 | 0–2797 | 241 |
| Male | 30.5 | 1.0 | 103.6 | 0–598 | 41 |
| Female | 354.4 | 10.0 | 544.9 | 0–2797 | 188 |
| Slaughtered | 718.0 | 579.0 | 543.2 | 98–2607 | 82 |
| Deceased | 61.3 | 0 | 297.1 | 0–2797 | 147 |
| Dismissed and non-slaughtered | 623.1 | 323.0 | 784.4 | 19–2797 | 14 |
| Dismissed and still alive | 815.9 | 663.0 | 473.4 | 381–1916 | 12 |
| Age of mortality (days): | |||||
| All cases | 1003.1 | 848.0 | 931.5 | 5–5121 | 229 |
| Male | 161.9 | 64.0 | 186.9 | 7–637 | 41 |
| Female | 1186.6 | 1090.5 | 928.1 | 5–5121 | 188 |
| Slaughtered | 1653.7 | 1630.5 | 679.3 | 260–3580 | 82 |
| Deceased | 640.2 | 324.0 | 853.6 | 5–5121 | 147 |
| Dismissed and non-slaughtered | 1476.3 | 1104.0 | 1400.4 | 54–5121 | 14 |
| Dismissed and still alive | 1246.9 | 1226.0 | 491.5 | 540–2162 | 12 |
| Variable | Mortality | HR | 95% CI | p-Value | Se (%) | Sp (%) | Acc (%) | |
|---|---|---|---|---|---|---|---|---|
| Model 1 | ||||||||
| n = 223 | Heart rate > 95 bpm | 70/91 (76.9%) | 2.0 | 1.4–2.9 | <0.001 | 60.4 | 49.4 | 56.1 |
| PCV < 36.5% | 75/108 (69.4%) | 1.5 | 1.1–2.1 | 0.025 | ||||
| Model 2 | ||||||||
| n = 208 | Male gender | 39/44 (88.6%) | 2.1 | 1.4–3.2 | <0.001 | 88.0 | 65.6 | 74.5 |
| Time to referral > 4.5 d. | 70/91 (76.9%) | 1.8 | 1.1–2.9 | 0.026 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantillon, L.; Pas, M.L.; Vlaminck, L.; Pardon, B. Long-Term Survival in 241 Cases of Intussusception in Cattle and Factors Associated with Mortality. Animals 2024, 14, 676. https://doi.org/10.3390/ani14050676
Chantillon L, Pas ML, Vlaminck L, Pardon B. Long-Term Survival in 241 Cases of Intussusception in Cattle and Factors Associated with Mortality. Animals. 2024; 14(5):676. https://doi.org/10.3390/ani14050676
Chicago/Turabian StyleChantillon, Laurens, Mathilde Laetitia Pas, Lieven Vlaminck, and Bart Pardon. 2024. "Long-Term Survival in 241 Cases of Intussusception in Cattle and Factors Associated with Mortality" Animals 14, no. 5: 676. https://doi.org/10.3390/ani14050676
APA StyleChantillon, L., Pas, M. L., Vlaminck, L., & Pardon, B. (2024). Long-Term Survival in 241 Cases of Intussusception in Cattle and Factors Associated with Mortality. Animals, 14(5), 676. https://doi.org/10.3390/ani14050676





