Effects of Heat Stress and Lipopolysaccharides on Gene Expression in Chicken Immune Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Cells
2.2. Cell Collection
2.3. Experimental Design
2.4. KEGG Enrichment Analysis
2.5. Quantitative Detection
2.6. Data and Results Analysis
3. Results
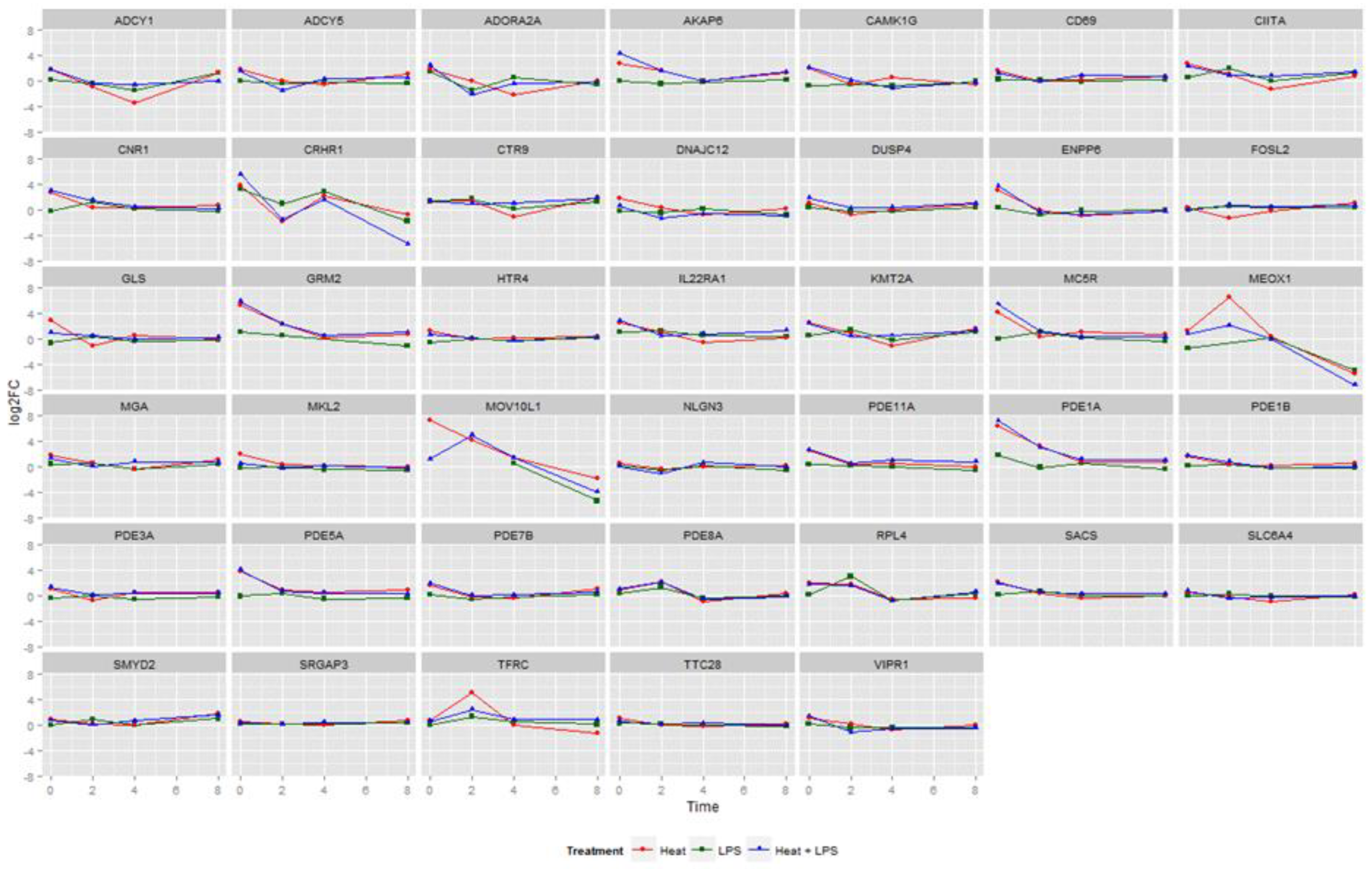
3.1. Changes in Gene Expression in LPS- and Heat Stress-Treated BMDCs
3.2. Gene Expression Trends in LPS and Heat Stress−Treated BMDCs
3.3. KEGG Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene Name | Primer Sequence |
|---|---|
| ADCY1 | ATGCCATTGCCTGTGAAGATGAG GGCGGTGAGCACGATAAGAAC |
| ADCY5 | TAACAGACGCCTCCTCCACAAC GAACATGACAGCCACGCACTC |
| ADORA2A | GGAACAGCCAGCATTAGGACTTC TACTTCTAACGCATACTCACTGATTCG |
| AKAP6 | ATTCCGTTGCTCCTCAGTGTAATG TGTCCTTGCTTTCATCTCCATCTTC |
| CAMK1G | AGGTCCTGACAGCAGTGAAGTAC TCGGGAGTGAGGTAAAGAAGGTTC |
| CD69 | GAGCGATTGGAACAGCAGCAG CCATCTCCTCCTCCGTGTCTAC |
| CIITA | AGCCTGTATAGCCTGGTATCTTGG CTCTTCTCCCGTATCTGAATGTTTGG |
| CNR1 | TCCTCTTGCTGTTCATTGTCTATGC CCATCCTCCGTACTCTGAATGATTATG |
| CRHR1 | AGCCATCGTCCTCACCTATTCC CCAGCACTTCTCGTTGTCGTAG |
| CTR9 | GCACTTCAGGCTGGGAAACTTAG CTCATACAGTCTGGCAAGGTTATAGG |
| DNAJC12 | TTGCTAGGATGTGATGAACTATCTACG GGGTTTCCAGGGTGTTTGTCAG |
| DUSP4 | AGTGCCGAGTCCCTGGATTTG CGCTGCCAAGGTAGAGGAAGG |
| ENPP6 | TGTCAACGTCCTCCTCTTCTCTG ACCTCGGTCCTTCATTTGTATTGTATC |
| FOSL2 | GGACGGAGACGAAGGGATGAG CGCCTGGAGTTTCTCTGTTAGC |
| GLS | TAAATCTGCTGTTTGCCGCCTAC ATAGTCTCTCTGCTCCATATCCATCC |
| GRM2 | ATCATCTGGCTGCTGGTGGAG ATGTTGGAGTCACGGTTGTTGC |
| HTR4 | TCCACTGCGTATTGCTGTAATGC CTTGTTGAACTGCCTTTGCTGAATC |
| IL22RA1 | GCTGCCACAACCATTCCATCC CTCGGAGGTCTGAGGAGCATATC |
| KMT2A | GCAGGAGCGTGAAGAGAACAAC CAGAGATAGGTGGTGTAGGAGGATG |
| MC5R | GCTTTGCCTGGGTACAACTCTG AGGATGAGATGGAGGAAGAATGGAG |
| MEOX1 | AGAGGACAGCGTTCACCAAGG CAGGTTCACAGCGATCTCGTATC |
| MGA | CTTCCTCCGCCTCCTGTGTTC TACTGTTGATGTGCTGTGATAAGATGG |
| MKL2 | GAGACCAGTAACAGCCAACATCAC TTGGTTCTAAGGACACAGGAGGAG |
| MOV10L1 | GATACAAGCGAGTGGACAAGGTTC GTGGTGAATAGCCGTAAGGAAGTC |
| NLGN3 | CGATGTCATGCTCAGTGCTGTG GCCTTGGTGTGGATGAACTTGG |
| PDE11A | TGCCATTGTATGAGTGTCTTGTGAAG ACGAGGATGCTGCCTGAGAAG |
| PDE1A | AGGCTTCTGGACACTGAGGATG TCAGGTCTCCGCTTCACTATTCC |
| PDE1B | AGATGCTGGACACGGAGGATG TGCTGCGGAATTTGGGTTTCTC |
| PDE3A | GCCTCTGGTTATGGACAACTTGG GCCTGTAAGATACCTGACTGAGAATC |
| PDE5A | AATGCTGATGACTGCTTGTGATTTATC TGTTGGTTCTATGTTGAGTTCCTTCC |
| PDE7B | TCAAGAAGATTACCACAGCCAGAAC CCAGCAGTCCAAGCATGATGTC |
| PDE8A | AATAAGGCGGAGAAGACTTGTGAAC CCTCGTGATGTTGTGGATCTGAC |
| RPL4 | GTGACTACAACCTGCCGATGC GGACTCTGCGGTGAATCTTCTTC |
| SACS | ATGAACTGATTCCATCTCGCAAGG TGGGCAGCACAGAAGATAACAAC |
| SLC6A4 | TCAAATGGCTATTCAGGAGTTCAGAG GTGGTTGTGGTGGTGGTTGTG |
| SMYD2 | ACTATCCCTCCTACTCGCTGAATG GATCTTTCCCGTGTGCTACTTCC |
| SRGAP3 | GGATGTGAGCGTGAACCTTCTG GCTTGTCTCTTCTCCTTCATCTTCTC |
| TFRC | GTCAGAGGCAGCACCAAGAAC GCAACGATAGCATCAGGAGTCTC |
| TTC28 | CTGCTTACTCCAGTTCTACCTCAATG CTGATACCATATACGCTTCCTCTTCTG |
| VIPR1 | TGAAAGTGGAGAACCCGAACATTG CACCAGCAGCCAGAAGAAGTTG |
References
- Kpomasse, C.C.; Oke, O.E.; Houndonougbo, F.M.; Tona, K. Broiler production challenges in the tropics: A review. Vet. Med. Sci. 2021, 7, 831–842. [Google Scholar] [CrossRef]
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Zmrhal, V.; Svoradova, A.; Venusova, E.; Slama, P. The Influence of Heat Stress on Chicken Immune System and Mitigation of Negative Impacts by Baicalin and Baicalein. Animals 2023, 13, 2564. [Google Scholar] [CrossRef]
- Cockrem, J.F. Stress, corticosterone responses and avian personalities. J. Ornithol. 2007, 148, 169–178. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Nawaz, A.H.; Amoah, K.; Leng, Q.Y.; Zheng, J.H.; Zhang, W.L.; Zhang, L. Poultry Response to Heat Stress: Its Physiological, Metabolic, and Genetic Implications on Meat Production and Quality Including Strategies to Improve Broiler Production in a Warming World. Front. Vet. Sci. 2021, 8, 699081. [Google Scholar] [CrossRef]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan inhibits oxidative stress and alleviates LPS-induced inflammation and apoptosis of BMECs by activating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2022, 222 Pt B, 2375–2391. [Google Scholar] [CrossRef]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293s–301s. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-K.; Kim, S.J.; Rah, S.-H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.-O.; Park, B.S.; Yoon, T.-Y.; et al. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef]
- Jeljeli, M.; Riccio, L.G.C.; Doridot, L.; Chêne, C.; Nicco, C.; Chouzenoux, S.; Deletang, Q.; Allanore, Y.; Kavian, N.; Batteux, F. Trained immunity modulates inflammation-induced fibrosis. Nat. Commun. 2019, 10, 5670. [Google Scholar] [CrossRef]
- Goel, A. Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1136–1145. [Google Scholar] [CrossRef]
- de Laval, B.; Maurizio, J.; Kandalla, P.K.; Brisou, G.; Simonnet, L.; Huber, C.; Gimenez, G.; Matcovitch-Natan, O.; Reinhardt, S.; David, E.; et al. C/EBPβ-Dependent Epigenetic Memory Induces Trained Immunity in Hematopoietic Stem Cells. Cell Stem Cell 2020, 26, 657–674.e8. [Google Scholar] [CrossRef]
- Swafford, D.; Manicassamy, S. Wnt signaling in dendritic cells: Its role in regulation of immunity and tolerance. Discov. Med. 2015, 19, 303–310. [Google Scholar] [PubMed]
- Lin, X.-J.; Li, Y.-J.; Li, Z.-L.; Zou, F.; Lin, M.-T. Pre-existing lipopolysaccharide may increase the risk of heatstroke in rats. Am. J. Med. Sci. 2009, 337, 265–270. [Google Scholar] [CrossRef]
- Leon, L.R.; Helwig, B.G. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Min, K.S.; Bae, W.J.; Lee, Y.M.; Lee, S.Y.; Lee, E.S.; Kim, E.C. Role of SIRT1 in heat stress- and lipopolysaccharide-induced immune and defense gene expression in human dental pulp cells. J. Endod. 2011, 37, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Wang, Y.; Saelao, P.; Kern, C.; Jin, S.; Gallardo, R.A.; Kelly, T.; Dekkers, J.M.; Lamont, S.J.; Zhou, H. Liver Transcriptome Responses to Heat Stress and Newcastle Disease Virus Infection in Genetically Distinct Chicken Inbred Lines. Genes 2020, 11, 1067. [Google Scholar] [CrossRef]
- Athapaththu, A.M.G.K.; Lee, K.T.; Kavinda, M.H.D.; Lee, S.; Kang, S.; Lee, M.H.; Kang, C.H.; Choi, Y.H.; Kim, G.Y. Pinostrobin ameliorates lipopolysaccharide (LPS)-induced inflammation and endotoxemia by inhibiting LPS binding to the TLR4/MD2 complex. Biomed. Pharmacother. 2022, 156, 113874. [Google Scholar] [CrossRef]
- Lan, X.; Hsieh, J.C.F.; Schmidt, C.J.; Zhu, Q.; Lamont, S.J. Liver transcriptome response to hyperthermic stress in three distinct chicken lines. BMC Genom. 2016, 17, 955. [Google Scholar] [CrossRef]
- Reisinger, N.; Emsenhuber, C.; Doupovec, B.; Mayer, E.; Schatzmayr, G.; Nagl, V.; Grenier, B. Endotoxin Translocation and Gut Inflammation Are Increased in Broiler Chickens Receiving an Oral Lipopolysaccharide (LPS) Bolus during Heat Stress. Toxins 2020, 12, 622. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, L.; Zhang, H.; Zhou, Z.; Ju, L.; Yang, J. CRHR1 antagonist alleviates LPS-induced depression-like behaviour in mice. BMC Psychiatry 2023, 23, 17. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, X.; Su, X.; Wang, Y.; Liu, B.; Zhou, H.; Wang, Y.; Li, F. The role of MEOX1 in non-neoplastic and neoplastic diseases. Biomed. Pharmacother. 2023, 158, 114068. [Google Scholar] [CrossRef]
- Damås, J.K.; Wæhre, T.; Yndestad, A.; Ueland, T.; Müller, F.; Eiken, H.G.; Holm, A.M.; Halvorsen, B.; Frøland, S.S.; Gullestad, L.; et al. Stromal cell-derived factor-1alpha in unstable angina: Potential antiinflammatory and matrix-stabilizing effects. Circulation 2002, 106, 36–42. [Google Scholar] [CrossRef]
- Astle, W.J.; Elding, H.; Jiang, T.; Allen, D.; Ruklisa, D.; Mann, A.L.; Mead, D.; Bouman, H.; Riveros-Mckay, F.; Kostadima, M.A.; et al. The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell 2016, 167, 1415–1429.e19. [Google Scholar] [CrossRef]
- Karagoz, E.; Ulcay, A.; Tanoglu, A.; Kara, M.; Turhan, V.; Erdem, H.; Oncul, O.; Gorenek, L. Clinical usefulness of mean platelet volume and red blood cell distribution width to platelet ratio for predicting the severity of hepatic fibrosis in chronic hepatitis B virus patients. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1320–1324. [Google Scholar] [CrossRef]
- Liu, S.; Huang, S.; Chen, F.; Zhao, L.; Yuan, Y.; Francis, S.S.; Fang, L.; Li, Z.; Lin, L.; Liu, R.; et al. Genomic Analyses from Non-invasive Prenatal Testing Reveal Genetic Associations, Patterns of Viral Infections, and Chinese Population History. Cell 2018, 175, 347–359.e14. [Google Scholar] [CrossRef] [PubMed]
- van den Biggelaar, R.H.G.A.; Arkesteijn, G.; Rutten, V.P.; Van Eden, W.; Jansen, C.A. In vitro Chicken Bone Marrow-Derived Dendritic Cells Comprise Subsets at Different States of Maturation. Front. Immunol. 2020, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Dema, A.; Perets, E.; Schulz, M.S.; Deák, V.A.; Klussmann, E. Pharmacological targeting of AKAP-directed compartmentalized cAMP signalling. Cell Signal 2015, 27, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ladilov, Y. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.R.; Willoughby, D.A. The role of cAMP regulation in controlling inflammation. Clin. Exp. Immunol. 1995, 101, 387–389. [Google Scholar] [CrossRef]
- Tavares, L.P.; Negreiros-Lima, G.L.; Lima, K.M.; E Silva, P.M.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Blame the signaling: Role of cAMP for the resolution of inflammation. Pharmacol. Res. 2020, 159, 105030. [Google Scholar] [CrossRef]
- Yong, H.Y.; Koh, M.S.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert. Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Starnes, T.; Broxmeyer, H.E.; Robertson, M.J.; Hromas, R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J. Immunol. 2002, 169, 642–646. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Zhou, X.; Chen, S.; Liu, A.; Liu, L.; Wang, H.; Wang, Q.; Lan, X. Effects of Heat Stress and Lipopolysaccharides on Gene Expression in Chicken Immune Cells. Animals 2024, 14, 532. https://doi.org/10.3390/ani14040532
Yang G, Zhou X, Chen S, Liu A, Liu L, Wang H, Wang Q, Lan X. Effects of Heat Stress and Lipopolysaccharides on Gene Expression in Chicken Immune Cells. Animals. 2024; 14(4):532. https://doi.org/10.3390/ani14040532
Chicago/Turabian StyleYang, Guang, Xinyi Zhou, Shutao Chen, Anfang Liu, Lingbin Liu, Haiwei Wang, Qigui Wang, and Xi Lan. 2024. "Effects of Heat Stress and Lipopolysaccharides on Gene Expression in Chicken Immune Cells" Animals 14, no. 4: 532. https://doi.org/10.3390/ani14040532
APA StyleYang, G., Zhou, X., Chen, S., Liu, A., Liu, L., Wang, H., Wang, Q., & Lan, X. (2024). Effects of Heat Stress and Lipopolysaccharides on Gene Expression in Chicken Immune Cells. Animals, 14(4), 532. https://doi.org/10.3390/ani14040532





