Recovery of Copper and Zinc from Livestock Bio-Sludge with An Environmentally Friendly Organic Acid Extraction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of the Livestock Sludge
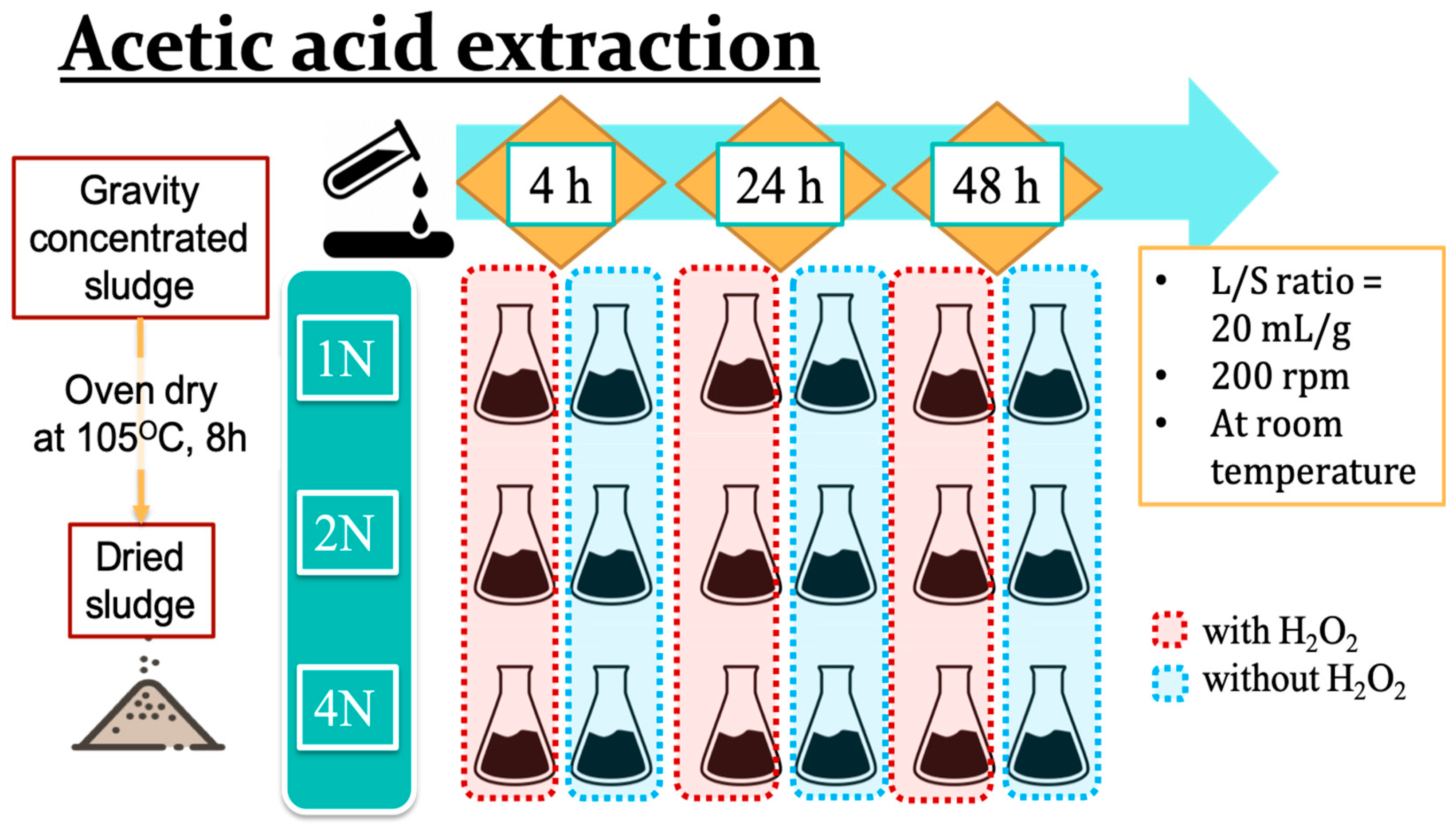
2.2. Extraction of Copper and Zinc from the Sludge by Adding Acetic Acid and Hydrogen Peroxide
2.3. Quantitative Analysis of Heavy Metals
2.4. Analysis of Liquid Samples
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analysis and Extraction of Heavy Metals from Gravity-Concentrated Sludge Samples
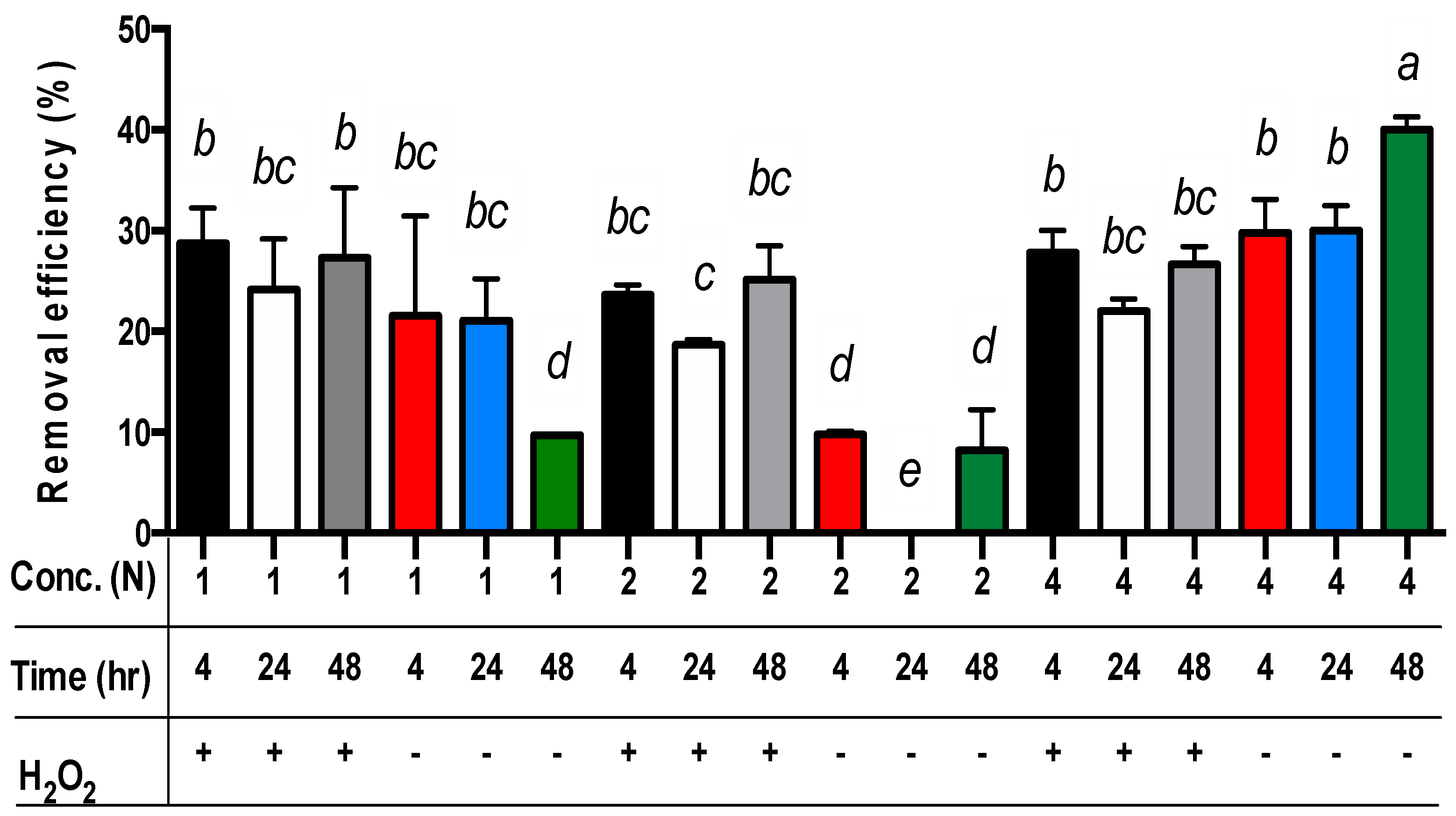
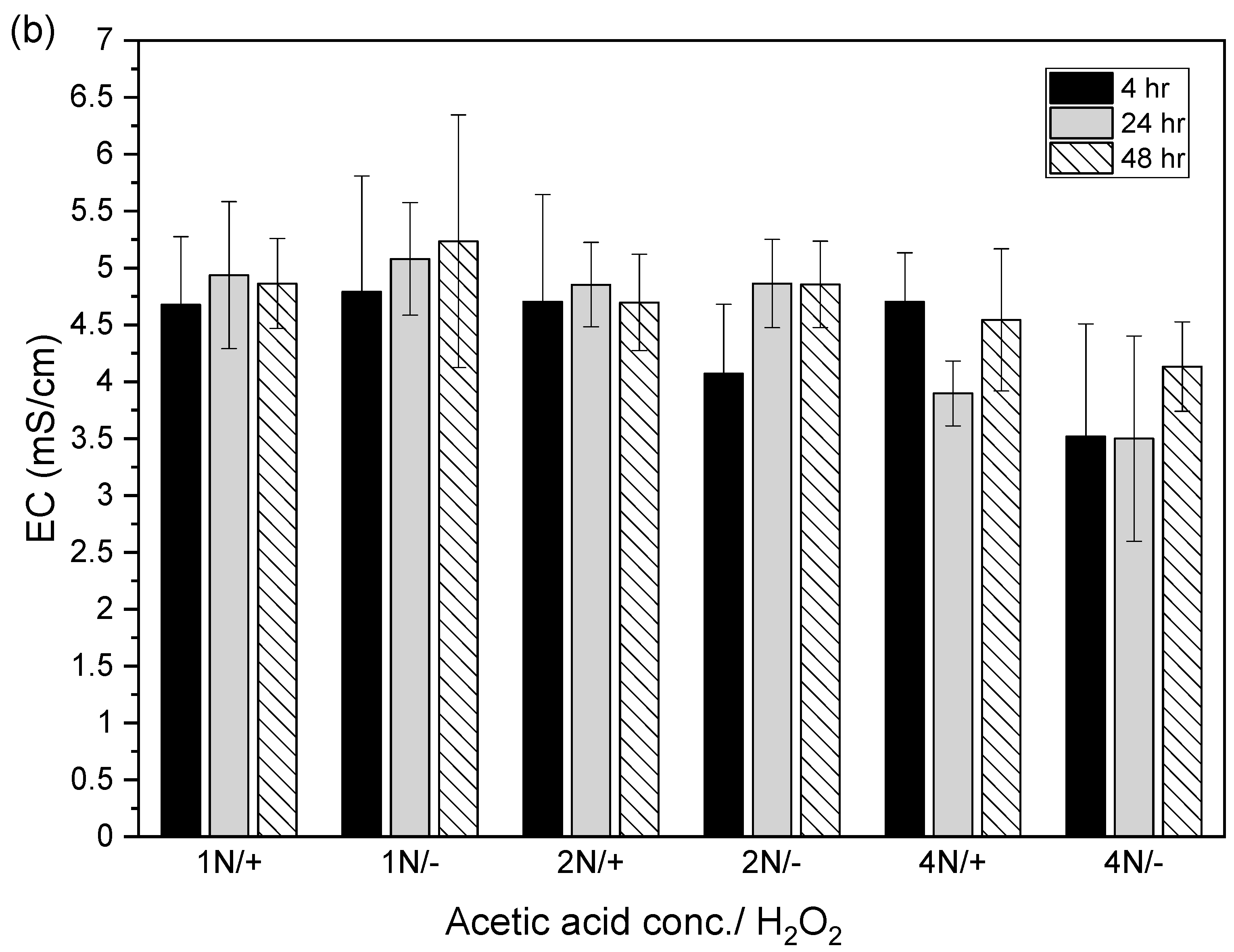
3.2. Different Combinations of Acetic Acid with Hydrogen Peroxide for the Extraction of Copper from Sludge Vary with Reaction Periods
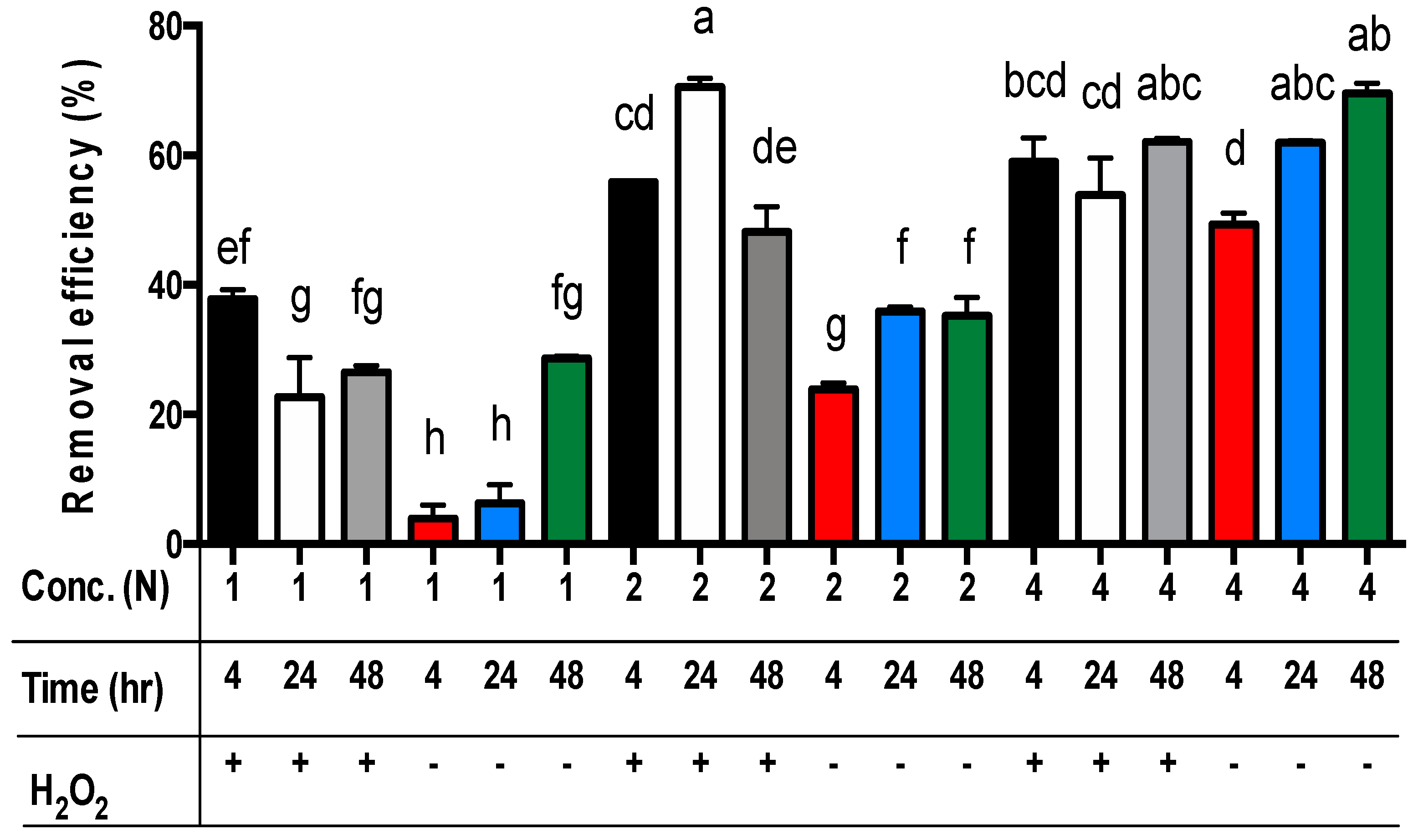
3.3. Different Combinations of Acetic Acid with Hydrogen Peroxide for the Extraction of Zinc from Sludge Vary with Reaction Periods
3.4. Different Combinations of Acetic Acid with Hydrogen Peroxide for the Extraction of Copper and Zinc Simultaneously from Sludge Vary with Reaction Periods
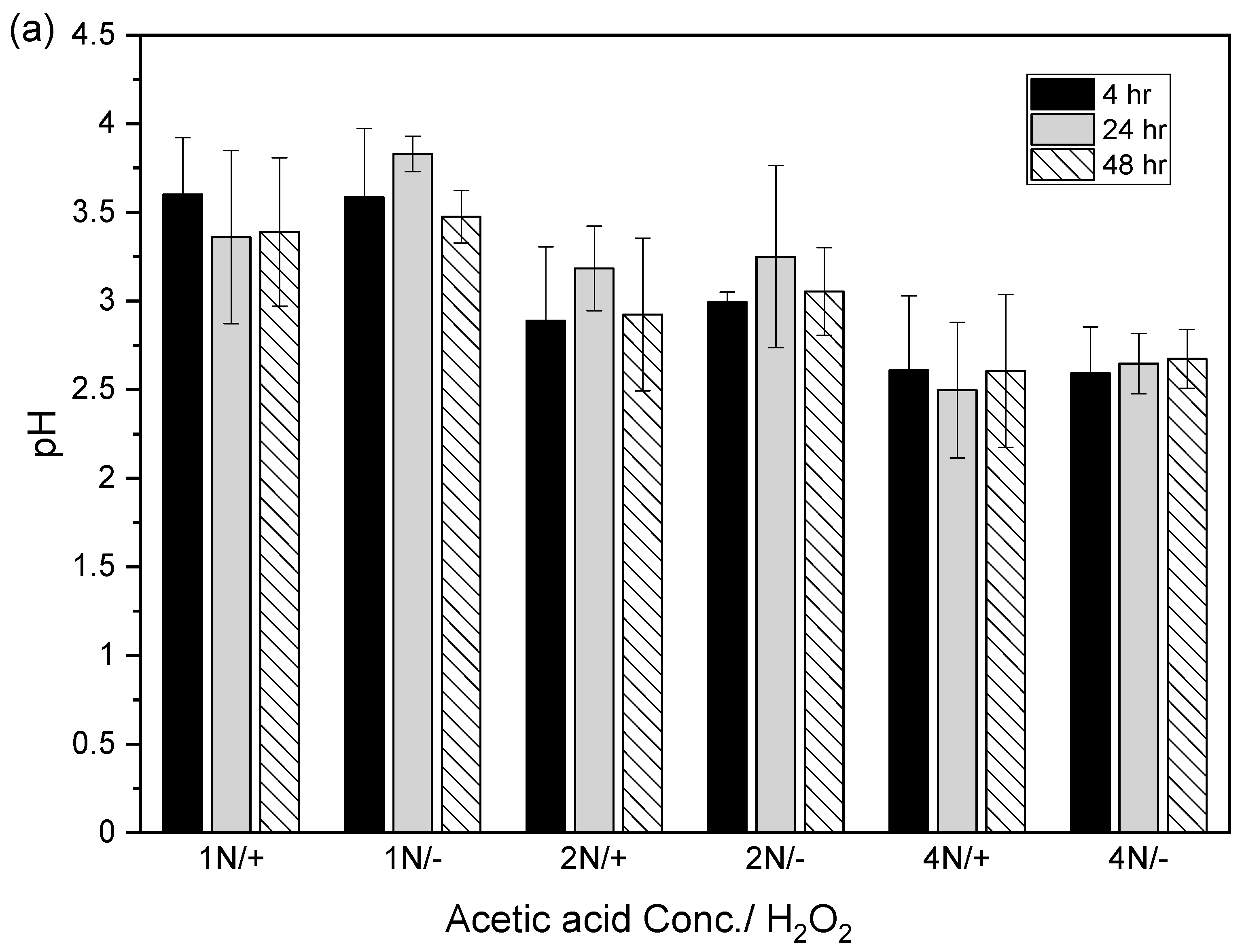
3.5. Limitation Factors for Extracting Copper and Zinc from Sludge with A Combination of Acetic Acid and Hydrogen Peroxide
3.5.1. Sources and Composition of the Sludge
3.5.2. Extraction Time
3.5.3. Types of Acids Used
3.5.4. The Concentrations of Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaleckas, E.; Paulauskas, V.; Sendžikienė, E. Fractionation of heavy metals in sewage sludge and their removal using low-molecular-weight organic acids. J. Environ. 2012, 21, 189–198. [Google Scholar] [CrossRef]
- Ren, X.; Yan, R.; Wang, H.C.; Kuo, Y.Y.; Chae, K.J.; Kim, I.S.; Park, Y.J.; Wang, A.J. Citric acid and ethylene diamine tetra-acetic acid as effective washing agents to treat sewage sludge for agricultural reuse. Waste Manag. 2015, 46, 440–448. [Google Scholar] [CrossRef] [PubMed]
- del Dacera, D.M.; Babel, S. Use of citric acid for heavy metals extraction from contaminated sewage sludge for land application. Water Sci. Technol. 2006, 54, 129–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fytianos, K.; Charantoni, E.; Voudrias, E. Leaching of heavy metals from municipal sewage sludge. Environ. Int. 1998, 24, 467–475. [Google Scholar] [CrossRef]
- Veeken, A.H.M.; Hamelers, H.V.M. Removal of heavy metals from sewage sludge by extraction with organic acids. Water Sci. Technol. 1999, 40, 129–136. [Google Scholar] [CrossRef]
- He, Z.; Song, J.; Zhang, N.; Zhang, P.; Xu, Y. Variation characteristics and ecological risk of heavy metals in the south Yellow Sea surface sediments. Environ. Monit. Assess. 2009, 157, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Lin, J.G. Bioleaching of heavy metals from livestock sludge by indigenous sulfur-oxidizing bacteria: Effects of sludge solids concentration. Chemosphere 2004, 54, 283–289. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, J.; Fanning, S.; Wang, L.; Li, M.; Maheshwari, N.; Sun, J.; Li, F. Effects of metal and metalloid pollutants on the microbiota composition of feces obtained from twelve commercial pig farms across China. Sci. Total Environ. 2019, 647, 577–586. [Google Scholar] [CrossRef]
- Svane, S.; Karring, H. A comparison of the transition metal concentrations in the faeces, urine, and manure slurry from different livestock animals related to environmentally relevant microbial processes. Cogent Chem. 2019, 5, 1644702. [Google Scholar] [CrossRef]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Poulsen, H.D. Zinc and copper as feed additives, growth factors or unwanted environmental factors. J. Anim. Feed Sci. 1998, 7, 135–142. [Google Scholar] [CrossRef]
- Ciesinski, L.; Guenther, S.; Pieper, R.; Kalisch, M.; Bednorz, C.; Wieler, L.H. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE 2018, 13, e0191660. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, H.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Tsai, W.T. Regulatory promotion and benefit analysis of biogas-power and biogas-digestate from anaerobic digestion in Taiwan’s livestock industry. Fermentation 2018, 4, 57. [Google Scholar] [CrossRef]
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferier, R.C.; Li, H.; Luo, W.; et al. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef]
- Jaime-Pérez, N.; Kaftan, D.; Bína, D.; Bokhari, S.N.H.; Shreedhar, S.; Küpper, H. Mechanisms of sublethal copper toxicity damage to the photosynthetic apparatus of Rhodospirillum rubrum. Biochim. Biophys. Acta 2019, 1860, 640–650. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha, N.; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Babel, S.; del Mundo Dacera, D. Heavy metal removal from contaminated sludge for land application: A review. Waste Manag. 2006, 26, 988–1004. [Google Scholar] [CrossRef]
- Di Palma, L.; Mecozzi, R. Heavy metals mobilization from harbor sediments using EDTA and citric acid as chelating agents. J. Hazard. Mater. 2007, 147, 768–775. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Lo, S.L. Removal of metals from industrial sludge by extraction with different acids. J. Environ. Sci. Health A 2004, 39, 2205–2219. [Google Scholar] [CrossRef]
- Badmus, M.A.O.; Andu, T.O.K.; Anyata, B.U. Removal of heavy metal from industrial wastewater using hydrogen peroxide. Afr. J. Biotechnol. 2007, 6, 238–242. [Google Scholar]
- Sparks, D.L. Environmental Soil Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 0080494803. [Google Scholar]
- Wang, H.; Shen, L.; Wang, H. Technology research for the removal of heavy metals from municipal sludge. Appl. Mech. Mater. 2013, 295, 1353–1358. [Google Scholar] [CrossRef]
- Yuan, S.; Xi, Z.; Jiang, Y.; Wan, J.; Wu, C.; Zheng, Z.; Lu, X. Desorption of copper and cadmium from soils enhanced by organic acids. Chemosphere 2007, 68, 1289–1297. [Google Scholar] [CrossRef]
- del Dacera, D.M.; Babel, S. Removal of heavy metals from contaminated sewage sludge using Aspergillus niger fermented raw liquid from pineapple wastes. Bioresour. Technol. 2008, 99, 1682–1689. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wasterwater, 23rd ed.; Bridgewater, L.L., American Public Health Association, American Water Works Association, Water Environment Federation, Eds.; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- Su, J.J.; Chen, Y.J.; Chang, Y.C. A study of a pilot-scale biogas bio-filter system for utilization on pig farms. J. Agric. Sci. 2014, 152, 217–224. [Google Scholar] [CrossRef]
- Su, J.J.; Chou, Y.C. Biodiesel production by acid methanolysis of slaughterhouse sludge cake. Animals 2019, 9, 1029. [Google Scholar] [CrossRef]
- Popenda, A. Effect of redox potential on heavy metals and arsenic behavior in dredged sediments. Desalin. Water Treat. 2013, 52, 3918–3927. [Google Scholar] [CrossRef]
- Hammaini, A.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Biosorption of heavy metals by activated sludge and their desorption characteristics. J. Environ. Manag. 2007, 84, 419–426. [Google Scholar] [CrossRef]
- Suzuki, K.; Waki, M.; Yasuda, T.; Fukumoto, Y.; Kuroda, K.; Sakai, T.; Suzuki, N.; Suzuki, R.; Matsuba, K. Distribution of phosphorus, copper, and zinc in activated sludge treatment process of swine wastewater. Bioresour. Technol. 2010, 101, 9399–9404. [Google Scholar] [CrossRef]
- Guo, Z.H.; Xiao, X.Y.; Chen, T.B.; Liao, X.; Song, J.; Wu, B. Heavy Metal Pollution of Soils and Vegetables from Midstream and Downstream of Xiangjiang River. J. Geogr. Sci. 2008, 63, 3–11. [Google Scholar] [CrossRef]
| Heavy Metals | Unit (mg/kg) |
|---|---|
| Cu | 528 ± 12 * |
| Zn | 1347 ± 54 |
| P | 42,468 ± 5176 |
| K | 1914 ± 378 |
| Fe | 5798 ± 604 |
| Mn | 493 ± 57 |
| Pb | 8.1 ± 7.7 |
| Cd | N.D. |
| Cr | 18.5 ± 1.9 |
| Moisture (%) | 95.8 ± 1.8 |
| COD (mg/L) | 11,357 ± 6246 |
| pH | 7.02 ± 0.13 |
| EC (mS/cm) | 1.07 ± 0.023 |
| Source | Degrees of Freedom | Type III Sum of Squares | Mean Squares | F-Value | p Value |
|---|---|---|---|---|---|
| Acid | 2 | 55,809.95185 | 27,904.97593 | 86.34 | <0.0001 |
| H2O2 | 1 | 12,345.88082 | 12,345.88082 | 38.20 | <0.0001 |
| Acid × H2O2 | 2 | 37,254.25877 | 18,627.12939 | 57.63 | <0.0001 |
| Time | 2 | 5053.31842 | 2526.65921 | 7.82 | 0.0017 |
| Acid × Time | 4 | 7672.72960 | 1918.18240 | 5.94 | 0.0011 |
| H2O2 × Time | 2 | 339.12025 | 169.56013 | 0.52 | 0.5968 |
| Acid × H2O2 × Time | 4 | 6353.09868 | 1588.27467 | 4.91 | 0.0033 |
| Concentration (N) | Means | Difference | |
|---|---|---|---|
| 4 | 29.6 a | - | - |
| 1 | 22.6 b | 6.95 * | - |
| 2 | 13.7 c | 15.9 * | 8.91 * |
| Time (h) | Means | Difference | |
| 4 | 23.7 a | - | - |
| 48 | 23.2 a | 0.498 | - |
| 24 | 18.8 b | 4.89 * | 4.39 |
| w or w/o H2O2 | Means | Difference | |
| w | 24.7 a | - | - |
| w/o | 18.8 b | 5.97 * | - |
| Sources | Degrees of Freedom | Type III Sum of Squares | Mean Squares | F-Value | p Values |
|---|---|---|---|---|---|
| Acid | 2 | 90.79026373 | 45.39513186 | 184.99 | <0.0001 |
| H2O2 | 1 | 20.76977726 | 20.76977726 | 84.64 | <0.0001 |
| Acid × H2O2 | 2 | 12.51283781 | 6.25641891 | 25.50 | <0.0001 |
| Time | 2 | 4.17825025 | 2.08912513 | 8.51 | 0.0011 |
| Acid × Time | 4 | 8.55051418 | 2.13762855 | 8.71 | <0.0001 |
| H2O2 × Time | 2 | 10.53066852 | 5.26533426 | 21.46 | <0.0001 |
| Acid × H2O2 × Time | 4 | 4.340426795 | 1.07606699 | 4.39 | 0.0061 |
| Concentration (N) | Means | Difference | |
|---|---|---|---|
| 4 | 53.1 a | - | - |
| 2 | 46.9 b | 6.25 * | - |
| 1 | 21.3 c | 31.85 * | 25.6 * |
| Time (h) | Means | Difference | |
| 48 | 46.2 a | - | - |
| 24 | 40.6 b | 5.60 * | - |
| 4 | 36.9 b | 9.34 * | 3.74 |
| W or w/o H2O2 | Means | Difference | |
| W | 48.9 a | - | - |
| w/o | 36.5 b | 12.37 * | - |
| Parameters | SS (n = 3) | SS (n = 4) | PM (n = 3) | PS (n = 6) | PS | LS (n = 6) |
|---|---|---|---|---|---|---|
| pH | 6.10 ± 0.07 | 7.3 ± 0.1 | 8.10 ± 0.21 | 7.9 ± 0.1 | 6.42 | 7.02 ± 0.13 |
| EC * (mS/cm) | 1.31 ± 0.10 | - | 2.14 ± 0.07 | - | 2.91 | 1.07 ± 0.023 |
| Cu (mg/kg) | 347 ± 10 | 1735 ± 286 | 321 ± 13 | 382 ± 28 | 760 | 528 ± 12 |
| Zn (mg/kg) | 3242 ± 52 | 2110 ± 78 | 689 ± 44 | 3283 ± 425 | 4000 | 1347 ± 54 |
| Pb (mg/kg) | 195 ± 9.5 | 54.7 ± 6.2 | 52 ± 1.8 | - | ||
| Mn (mg/kg) | 1400 ± 15 | 493 ± 57 | ||||
| Cr (mg/kg) | 373 ± 24, | |||||
| References | [6] | [2] | [6] | [7] | [32] | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, K.-W.; Chen, W.-C.; Su, J.-J. Recovery of Copper and Zinc from Livestock Bio-Sludge with An Environmentally Friendly Organic Acid Extraction. Animals 2024, 14, 342. https://doi.org/10.3390/ani14020342
Yen K-W, Chen W-C, Su J-J. Recovery of Copper and Zinc from Livestock Bio-Sludge with An Environmentally Friendly Organic Acid Extraction. Animals. 2024; 14(2):342. https://doi.org/10.3390/ani14020342
Chicago/Turabian StyleYen, Kuang-Wei, Wei-Chen Chen, and Jung-Jeng Su. 2024. "Recovery of Copper and Zinc from Livestock Bio-Sludge with An Environmentally Friendly Organic Acid Extraction" Animals 14, no. 2: 342. https://doi.org/10.3390/ani14020342
APA StyleYen, K.-W., Chen, W.-C., & Su, J.-J. (2024). Recovery of Copper and Zinc from Livestock Bio-Sludge with An Environmentally Friendly Organic Acid Extraction. Animals, 14(2), 342. https://doi.org/10.3390/ani14020342










