Vitamin Solutions Effects on Reproduction of Broodstock, Growth Performance, and Survival Rate of Pangasius Catfish Fingerlings
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
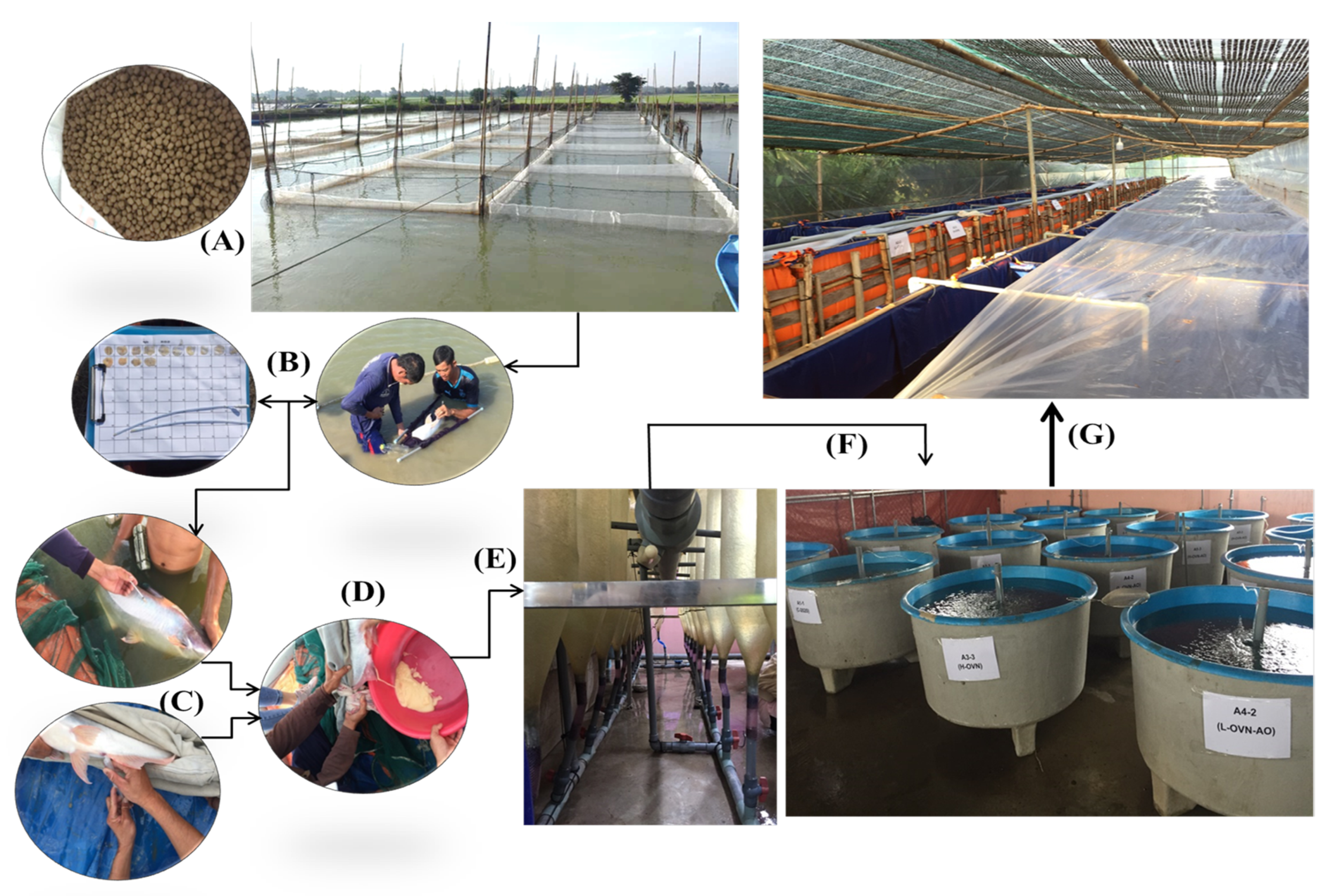
2.1. Study Site and Research Layout
2.2. Pond Preparation and Management of the Broodstock
2.3. Selection Criteria of Broodstock
2.4. Experimental Diet Preparation and Feeding Practice
2.5. Broodstock Experimental Design
2.6. Induced Spawning and Larvae Rearing Practices
2.6.1. Mature Broodstock Fish Selection for Induced Spawning
2.6.2. Induced Stripping of the Broodstock
2.6.3. Egg Incubation and Larvae Fish Nursing Practices
2.7. Larvae to Fingerlings Rearing Experiment
2.8. Water Quality Monitoring
2.9. Chemical Analysis
2.10. Calculation
2.11. Statistical Analysis
3. Results
3.1. Chemical Composition and Essential Amino Acid Content of Feed Ingredients and Test Diets
3.2. Growth Performance Indices of Broodstock
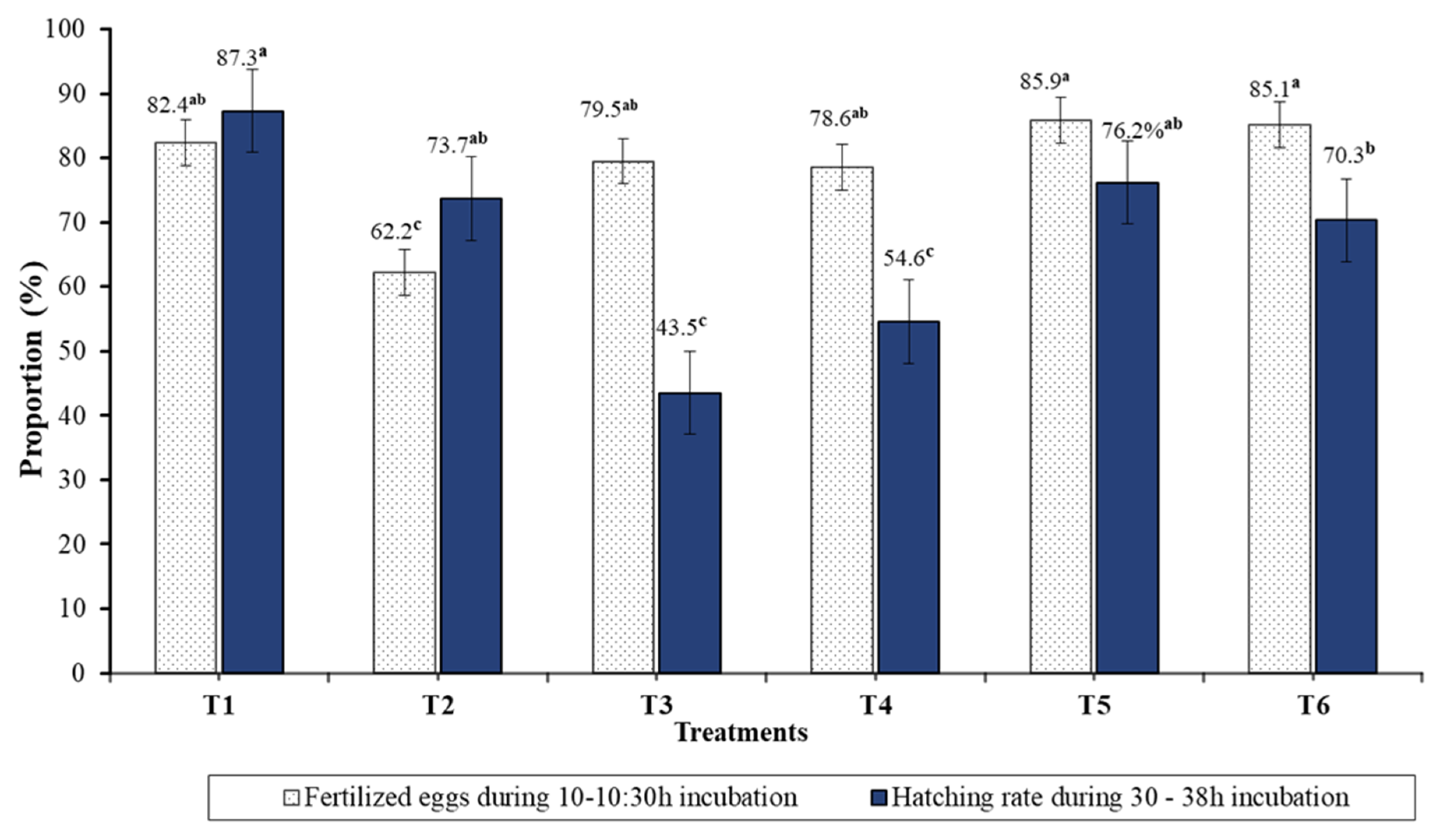
3.3. Reproductive Breeding, Hatching, and Early Life Stage Development
3.4. Growth Performance Indices of Fish Larvae 15 Days after Hatching
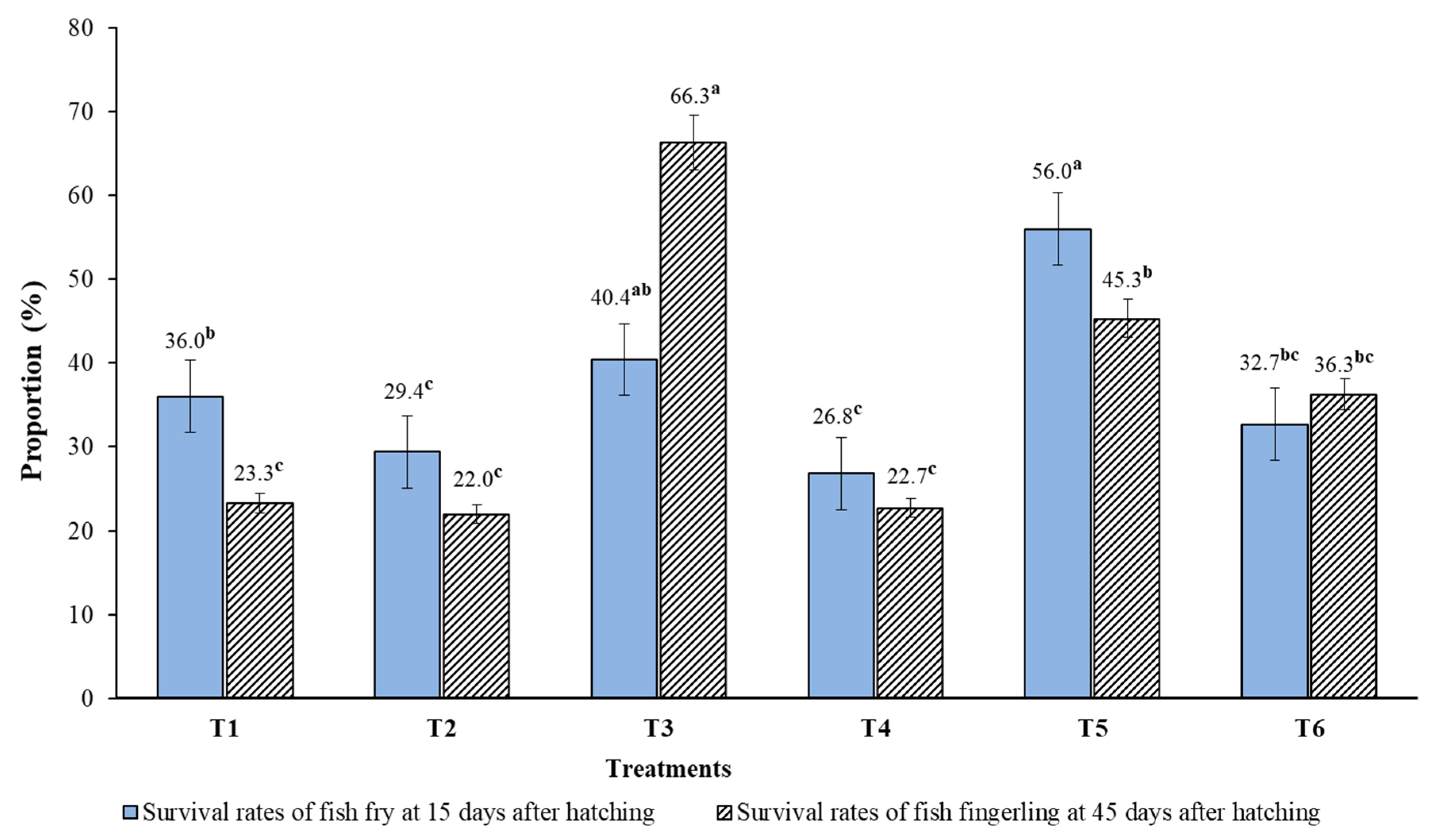
3.5. Growth Performance and Survival Rates of Fish Fry and Fingerlings 30 and 45 Days after Hatching
3.6. Water Quality Monitoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abidin, M.Z.; Hashim, R.; Chong, S.C.A. Influence of dietary protein levels on growth and egg quality in broodstock female Bagrid catfish (Mystus nemurus Cuv. & Val.). Aquac. Res. 2006, 37, 416–418. [Google Scholar] [CrossRef]
- Adewolu, M.A.; Benfey, T.J. Growth, nutrient utilization and body composition of juvenile Bagrid catfish, Chrysichthys nigrodigitatus (Actinopterygii: Siluriformes: Claroteidae), fed different dietary crude protein levels. Acta Ichthyol. Piscat. 2009, 39, 95–101. [Google Scholar] [CrossRef][Green Version]
- Ahmad, A.W.; Hassan, S.; Banat, F. An overview of microalgae biomass as a sustainable aquaculture feed ingredient: Food security and circular economy. Bioengineered 2022, 13, 9521–9547. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Jauncey, K. Approaches to optimizing dietary protein to energy ratio for African catfish Clarias gariepinus (Burchell, 1822). Aquac. Nutr. 2005, 11, 95–101. [Google Scholar] [CrossRef]
- AOAC. Animal feeds. In Official Methods of Analysis. Association of Official Analytical Chemists International, 16th ed.; Cunniff, P.A., Ed.; AOAC: Arlington, VA, USA, 1997; Chapter 4; Volume VI, p. 1102. [Google Scholar]
- Astiasarán, I.; Ansorena, D. Gourmet and Health-Promoting Specialty Oils; Moreau, R.A., Kamal-Eldin, A., Eds.; AOCS Press: Arlington, VA, USA, 2009; pp. 491–513. [Google Scholar] [CrossRef]
- Baidya, A.P.; Senoo, S. Decline in fertilization and hatching rates of Patin, (Pangasius hypophthalmus) after ovulation. Aquac. Sci. 2003, 51, 407–415. [Google Scholar] [CrossRef]
- Berlinsky, D.L.; Kenter, L.W.; Reading, B.J.; Goetz, F.W. Chapter 1—Regulating reproductive cycles for captive spawning. In Fish Physiology; Benfey, T.J., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 38, pp. 1–52. [Google Scholar]
- Bruce, M.; Oyen, F.; Bell, G.; Asturiano, J.F.; Farndalem, B.; Carrillo, M.; Zanuy, S.; Ramos, J.; Bromage, N. Development of broodstock diets for the European Sea Bass (Dicentrarchus labrax) with special emphasis on the importance of n−3 and n−6 highly unsaturated fatty acid to reproductive performance. Aquaculture 1999, 177, 85–97. [Google Scholar] [CrossRef]
- Bui, T.M.; Lam, P.T.; Ingram, B.A.; Thuy, N.T.T.; Gooley, G.J.; Hao, N.V.; Phuong, N.T.; De Silva, S.S. Seed production practices of Striped catfish (Pangasianodon hypophthalmus) in the Mekong Delta region, Vietnam. Aquaculture 2010, 306, 92–100. [Google Scholar] [CrossRef]
- Bui, T.M.; Phuong, N.T.; Nguyen, G.H.; De Silva, S.S. Fry and fingerling transportation in the Striped catfish (Pangasianodon hypophthalmus) farming sector, Mekong Delta, Vietnam: A pivotal link in the production chain. Aquaculture 2013, 388, 70–75. [Google Scholar] [CrossRef]
- Cacot, P. Description of the sexual cycle related to the environment and set up of the artificial propagation in Pangasius bocourti (Sauvage 1880) and Pangasius hypophthalmus (Sauvage 1878) reared in floating cages and in ponds in the Mekong Delta. In The Biological Diversity and Aquaculture of Clariid and Pangasiid catfishes in South East Asia, Proceedings of the Mid-Term Workshop of the ‘Catfish Asia Project’, Cantho, Vietnam, 11–15 May 1998; Can Tho University: Can Tho, Vietnam, 1999; pp. 71–89. [Google Scholar]
- Cacot, P.; Legendre, M.; Dan, T.Q.; Tung, L.T.; Liem, P.T.; Mariojouls, C.; Lazard, J. Induced ovulation of Pangasius bocourti (Sauvage, 1880) with a progressive hCG treatment. Aquaculture 2002, 213, 199–206. [Google Scholar] [CrossRef]
- Chand, B.K.; Singh, M.K.; Mandal, B. Studies on the breeding of Pangasius sutchi using different inducing agents. J. Appl. Aquac. 2011, 23, 32–40. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Han, D.; Zhu, X.; Xie, S.; Han, D.; Hu, Q. Two filamentous microalgae as feed ingredients improved flesh quality and enhanced antioxidant capacity and immunity of the gibel carp (Carassius auratus gibelio). Aquac. Nutr. 2019, 25, 1145–1155. [Google Scholar] [CrossRef]
- Chuapoehuk, W.; Pothisoong, T. Protein requirements of catfish fry, Pangasius sutchi, Fowler. In Finfish Nutrition in Asia: Methodological Approaches to Research Development, Proceedings of the Asian Finfish Nutrition Workshop, Singapore, 23–26 August 1983; EurekaMag: Ottawa, ON, Canada, 1985; pp. 103–106. [Google Scholar]
- Colombo, S.M. Fish Physiology; Benfey, T.J., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 38, pp. 53–82. [Google Scholar] [CrossRef]
- Craig, S.R.; Gardner, T.R.; Carnevali, O. Marine Ornamental Species Aquaculture; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 139–158. [Google Scholar] [CrossRef]
- D-Fish. Vietnam Shrimp 2021: Farming output Increases, Exports Are Estimated at 3.8 Billion USD (Reported on December 10, 2021). Website of the Directorate of Fisheries, Vietnam. Available online: https://tongcucthuysan.gov.vn/vi-vn/Tin-t%E1%BB%A9c/-Tin-v%E1%BA%AFn/doc-tin/016572/2021-12-13/tom-viet-nam-2021-san-luong-nuoi-tang-xuat-khau-uoc-dat-38-ty-usd (accessed on 3 March 2022).
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and algae as sources of medicinal and other biologically active compounds: A Review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef] [PubMed]
- Da, C.T.; Hung, L.T.; Berg, H.; Lindberg, J.E.; Lundh, T. Evaluation of potential feed sources, and technical and economic considerations of small-scale commercial striped catfish (Pangasius hypothalamus) pond farming systems in the Mekong Delta of Vietnam. Aquac. Res. 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Da, C.T.; Lundh, T.; Lindberg, J.E. Evaluation of local feed resources as alternatives to fish meal in terms of growth performance, feed utilisation and biological indices of striped catfish (Pangasianodon hypophthalmus) fingerlings. Aquaculture 2012, 364, 150–156. [Google Scholar] [CrossRef]
- Datta, S.N.; Ansal, M.D. Induced breeding of Asian striped catfish (Pangasianodon hypophthalmus) under farmer participatory mode in Punjab. J. Krishi Vigyan 2020, 9, 202–208. [Google Scholar] [CrossRef]
- Datta, S.N.; Singh, A.; Jassal, G.; Pandey, A. A study on induced breeding, embryonic and larval development of Pangasianodon hypophthalmus in semi-arid agro-climate. J. Environ. Biol. 2018, 39, 671–676. [Google Scholar] [CrossRef]
- Estrada-Godinez, J.A.; De Oca, G.A.R.-M.; Bañuelos-Vargas, M.I.; Martínez-Montaño, E.; Pacheco-Marges, M.d.R.; Román-Reyes, J.C. Effect of feeding rate and hormonal treatments on the condition factor and the reproductive performance of the catfish, Pangasianodon hypophthalmus. J. Appl. Aquac. 2021, 34, 1005–1020. [Google Scholar] [CrossRef]
- Fagbenro, O.A. Quantitative dietary protein requirements of Clarias isheriensis (Sydenham 1980) (Clariidae) fingerlings. J. Appl Ichthyol. 1992, 8, 164–169. [Google Scholar] [CrossRef]
- Fernández-Palacios, H.; Izquierdo, M.S.; Robaina, L.; Valencia, A.; Salhi, M.; Vergara, J. Effect of n − 3 HUFA level in broodstock diets on egg quality of gilthead sea bream (Sparus aurata L.). Aquaculture 1995, 132, 325–337. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Poe, W.E.; Wilson, R.P. Protein and energy requirements of fingerling channel catfish for maintenance and maximum growth. J. Nutr. 1986, 116, 116–2121. [Google Scholar] [CrossRef]
- Ha, H.P.; Nguyen, T.T.T.; Poompuang, S.; Na-Nakorn, U. Microsatellites revealed no genetic differentiation between hatchery and contemporary wild populations of Striped catfish, Pangasianodon hypophthalmus (Sauvage 1878) in Vietnam. Aquaculture 2009, 291, 154–160. [Google Scholar] [CrossRef]
- Haas, S.; Bauer, J.L.; Adakli, A.; Meyer, S.; Lippemeier, S.; Schwarz, K.; Schulz, C. Marine microalgae Pavlova viridis and Nannochloropsis sp. as n-3 PUFA source in diets for juvenile European sea bass (Dicentrarchus labrax L.). J. Appl. Phyc. 2016, 28, 1011–1021. [Google Scholar] [CrossRef]
- Hossain, M.R.A.; Rahman, B.M.S. Thai Pangas (Pangasius sutchi) in Bangladesh: A review. Inter. J. Bus. Soc. Sci. Res. 2014, 1, 98–106. Available online: http://www.ijbssr.com/currentissueview/13090117 (accessed on 12 August 2023).
- Hung, L.T.; Liem, P.T.; Tu, H.T.; Mariojouls, C. Comparing growth and protein requirements for fingerlings of three catfish of the Mekong River (Pangasius bocourti, Pangagasius hypothalmus and Pangasius conchophilus). J. Aquac. Trop. 2002, 17, 325–335. [Google Scholar]
- Hung, L.T.; Thanh, N.T.; Pham, M.A.; Browdy, C.L. A comparison of the effect of dietary fungal phytase and dicalcium phosphate supplementation on growth performances, feed and phosphorus utilization of tra catfish juveniles (Pangasianodon hypophthalmus Sauvage, 1878). Aquac. Nutr. 2015, 21, 10–17. [Google Scholar] [CrossRef]
- Ibrahem, M.D.; Mohamed, M.F.; Ibrahim, M.A. The role of spirulina platensis (Arthrospira platensis) in Growth and Immunity of Nile Tilapia (Oreochromis niloticus) and Its Resistance to bacterial infection. J. Agric. Sci. 2013, 5, 109. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Fernández-Palacios, H.; Tacon, A.G.J. Reproductive Biotechnology in Finfish Aquaculture; Lee, C.-S., Donaldson, E.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 25–42. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Turkmen, S.; Montero, D.; Zamorano, M.J.; Afonso, J.M.; Karalazos, V.; Fernández-Palacios, H. Nutritional programming through broodstock diets to improve utilization of very low fishmeal and fish oil diets in gilthead sea bream. Aquaculture 2015, 449, 18–26. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Forster, I.P.; Dominy, W.G. Effects of supplementing two species of marine algae or their fractions to a formulated diet on growth, survival and composition of shrimp (Litopenaeus vannamei). Aquaculture 2009, 292, 237–243. [Google Scholar] [CrossRef]
- Kabir, M.A.; Ghaedi, A.; Talpur, A.D.; Hashim, R. Effect of dietary protein levels on reproductive development and distribution of amino acids in the body tissues of female Pangasianodon hypophthalmus (Sauvage, 1878) broodstock in captivity. Aquac. Res. 2015, 46, 1736–1747. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, Y.; Ji, W.X. Growth, feed utilization and body composition of Asian catfish (Pangasius hypophthalmus) fed at different dietary protein and lipid levels. Aquac. Nutr. 2011, 17, 578–584. [Google Scholar] [CrossRef]
- Mejri, S.; Audet, C.; Vandenberg, G.W.; Parrish, C.C.; Tremblay, R. Biochemical egg quality in a captive walleye (Sander vitreus) broodstock population relative to ovulation timing following hormonal treatment. Aquaculture 2014, 431, 99–106. [Google Scholar] [CrossRef]
- Mejri, S.; Tremblay, R.; Vandenberg, G.; Moren, M.; Khemis, I.B.; Audet, C. Differences in nutrient content of eggs and larvae as indicators for improvement of broodstock nutrition in walleye (Sander vitreus) production. Can. J. Zool. 2017, 95, 299–310. [Google Scholar] [CrossRef]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. n-3 Oil sources for use in aquaculture—Alternatives to the unsustainable harvest of wild fish. Nutr. Res. Rev. 2008, 21, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Na-Nakorn, U.; Moeikum, T. Genetic diversity of domesticated stocks of striped catfish, Pangasianodon hypophthalmus (Sauvage 1878), in Thailand: Relevance to broodstock management regimes. Aquaculture 2009, 297, 70–77. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Mahata, C.; Al-Jabri, H.; Vatland, A.K.; Kumar, G. Potential of microalgae as a sustainable feed ingredient for aquaculture. J. Biotechnol. 2021, 341, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.K.; Soon, S.C.; Hashim, R. The dietary protein requirement of a bagrid catfish, Mystus nemurus, determined using semipurified diets of varying protein level. Aquac. Nutr. 2001, 7, 45–51. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; National Research Council of the National Academies: Washington, DC, USA, 2011; Volume 363, Available online: http://www.nap.edu/openbook.php?record_id=13039 (accessed on 28 September 2023).
- Pérez, M.J.; Rodríguez, C.; Cejas, J.R.; Martín, M.V.; Jerez, S.; Lorenzo, A. Lipid and fatty acid content in wild white seabream (Diplodus sargus) broodstock at different stages of the reproductive cycle. Comp. Biochem. Physiol. B Bioc Mol. Biol. 2007, 146, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.L.; Tam, B.M.; Thuy, N.T.T.; Geoff, G.J.; Brett, I.A.; Hao, N.V.; Phuong, N.T.; Silva, S.S.D. Current status of farming practices of striped catfish, Pangasianodon hypophthalmus in the Mekong Delta, Vietnam. Aquaculture 2009, 296, 227–236. [Google Scholar] [CrossRef]
- Phumee, P.; Hashim, R.; Aliyu-Paiko, M.; Shu-Chien, A.C. Effects of dietary protein and lipid content on growth performance and biological indices of iridescent Shark (Pangasius hypophthalmus, Sauvage 1878) fry. Aquac. Res. 2009, 40, 456–463. [Google Scholar] [CrossRef]
- Phuong, N.T.; Tam, B.M.; Nguyen, T.A.; De Silva, S. Advances in Aquaculture Hatchery Technology; Allan, G., Burnell, G., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 498–518. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Micr Biotec. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Qin, Y.; He, L.; Wang, Y.; Li, D.; Chen, W.; Ye, J. Growth performance, fatty acid composition, and lipid metabolism are altered in groupers (Epinephelus coioides) by dietary fish oil replacement with palm oil. Anim. Nutr. 2022, 8, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Quintero, H.; Davis, A.D. Broodstock nutrition: Enhancement of egg quality in Channel catfish. In Nutrición Acuícola: Investigación y Desarrollo; Universidad Autónoma de Nuevo León: San Nicolás de los Garza, México, 2015; pp. 259–273. ISBN 978-607-27-0593-7. [Google Scholar]
- Rahman, M.A.; Ullah, M.R.; Kabir, M.A.; Alam, M.A.; Rahman, M.; Hossen, M.F. Artificial propagation of indigenous Yellowtail catfish (Pangasius pangasius): Experiences and challenges. Aquaculture 2020, 523, 735215. [Google Scholar] [CrossRef]
- Sah, U.; Wagle, S.K.; Mehta, S.N.; Mukhiya, Y.K. Preliminary observations on breeding and fry rearing of Pangas (Pangasius hypophthalmus) in eastern terai region of Nepal. Inter. J. Fish. Aquat. Res. 2018, 3, 14–16. [Google Scholar]
- Salhi, M.; Bessonart, M.; Chediak, G.; Bellagamba, M.; Carnevia, D. Growth, feed utilization and body composition of black catfish, Rhamdia quelen, fry fed diets containing different protein and energy levels. Aquaculture 2004, 231, 435–444. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Sink, T.D.; Lochmann, R.T.; Pohlenz, C.; Buentello, A.; Gatlin, D. Effects of dietary protein source and protein–lipid source interaction on Channel catfish (Ictalurus punctatus) egg biochemical composition, egg production and quality, and fry hatching percentage and performance. Aquaculture 2010, 298, 251–259. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Takeuchi, T.; Lu, J.; Yoshizaki, G.; Satoh, S. Effect on the growth and body composition of juvenile tilapia (Oreochromis niloticus) fed raw Spirulina. Fish. Sci. 2002, 68, 34–40. [Google Scholar] [CrossRef]
- Thuy, N.T.T. Patterns of use and exchange of genetic resources of the striped catfish, Pangasianodon hypophthalmus (Sauvage 1878). Rev. Aquac. 2009, 1, 224–231. [Google Scholar] [CrossRef]
- Trong, T.Q.; Hao, N.V.; Griffiths, D. Status of Pangasiid aquaculture in Vietnam. MRC Tech. Pap. 2002, 2, 16. [Google Scholar]
- Va’zquez-Ortiz, F.A.; Caire, G.; Huguere-Ciapara, I.; Herna´ndez, G. High-performance liquid chromatographic determination of free amino acid in shrimp. J. Liq. Chromatogr. Relat. Technol. 1995, 18, 2059–2068. [Google Scholar] [CrossRef]
- Vu, N.U.; Pham, T.H.; Huynh, P.V.; Huynh, T.G. Importance of the freshwater rotifer Brachionus angularis for improved survival rate of early life-history stages of Pangasius catfish (Pangasianodon hypophthalmus). Aquac. Res. 2021, 52, 783–792. [Google Scholar] [CrossRef]
- Watanabe, T.; Vassallo-Agius, R. Broodstock nutrition research on marine finfish in Japan. Aquaculture 2003, 227, 35–61. [Google Scholar] [CrossRef]
- White, R.L.; Ryan, R.A. Long-term cultivation of algae in open-raceway ponds: Lessons from the field. Ind. Biotech. 2015, 11, 213–220. [Google Scholar] [CrossRef]
- Wilson, R.P.; Halver, J.E. Protein and amino acid requirements of fishes. Ann. Rev. Nutr. 1986, 6, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Role of Broodstock Nutrition and its Impacts on Fish Reproductive Output: An Overview. Agric. Rev. 2022, 2464, 1–6. [Google Scholar] [CrossRef]
- Engdaw, F.; Geremew, A. Broodstock nutrition in Nile tilapia and its implications on reproductive efficiency. Front. Aqua. 2024, 3, 187–196. [Google Scholar] [CrossRef]
- Zakeri, M.; Kochanian, P.; Marammazi, J.G.; Yavari, V.; Savari, A.; Haghi, M. Effects of dietary n-3 HUFA concentrations on spawning performance and fatty acids composition of broodstock, eggs and larvae in yellowfin sea bream, Acanthopagrus latus. Aquaculture 2011, 310, 388–394. [Google Scholar] [CrossRef]
- Zakeri, M.; Marammazi, J.G.; Kochanian, P.; Savari, A.; Yavari, V.; Haghi, M. Effects of protein and lipid concentrations in broodstock diets on growth, spawning performance and egg quality of yellowfin sea bream (Acanthopagrus latus). Aquaculture 2009, 295, 99–105. [Google Scholar] [CrossRef]
| Raw Materials | Experimental Diets | |||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | |
| Fish meal (578 g/kg CP) | 75 | 75 | 75 | 75 | 75 | 75 |
| Poultry by-product meal (648 g/kg CP) | 184.1 | 184.1 | 184.1 | 184.1 | 184.1 | 184.1 |
| Wheat flour (158 g/kg CP) | 288.1 | 288.1 | 288.1 | 289.2 | 289.2 | 288 |
| Soybean meal (490 g/kg CP) | 360 | 360 | 360 | 360 | 360 | 360 |
| Soybean oil | 38.9 | 38.9 | 38.9 | 47.6 | 47.6 | 47.2 |
| Fish oil | 40 | 40 | 40 | 17.6 | 17.6 | 17.6 |
| Choline chloride | 5 | 5 | 5 | 5 | 5 | 5 |
| Mineral premix a | 3 | 3 | 3 | 3 | 3 | 3 |
| Vitamin premix (Rovimix 2020) b | 6 | − | − | − | − | − |
| Vitamin premix (L-OVN) c | − | 6 | − | 6 | − | − |
| Vitamin premix (H-OVN) d | − | − | 6 | − | 6 | 6 |
| Algal oil e | − | − | − | 12.6 | 12.6 | 12.6 |
| Fungal oil | − | − | − | − | − | 1.5 |
| Actual chemical composition (g/kg DM) | ||||||
| Dry matter | 901 | 904.1 | 885.2 | 897 | 895.3 | 930.2 |
| Crude protein | 350.1 | 350.5 | 350 | 351.5 | 358.5 | 351.5 |
| Crude fat | 65.2 | 66.8 | 65.2 | 68.4 | 73.8 | 69.8 |
| Crude fiber | 28.9 | 34.4 | 48.8 | 46.4 | 40.9 | 44.5 |
| Ash | 103 | 110 | 109 | 110 | 110 | 118 |
| Amino acid profiles (g/kg DM) | ||||||
| Histidine | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 |
| Isoleucine | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 |
| Leucine | 23.8 | 23.8 | 23.8 | 23.8 | 23.8 | 23.8 |
| Lysine | 17.2 | 17.2 | 17.2 | 17.3 | 17.3 | 17.3 |
| Methionine | 4.6 | 4.6 | 4.6 | 4.6 | 4.6 | 4.6 |
| Phenylalanine | 7.7 | 7.7 | 7.7 | 7.7 | 7.8 | 7.8 |
| Valine | 16.9 | 16.9 | 16.9 | 17 | 17 | 17 |
| Threonine | 12.1 | 12.1 | 12.1 | 12.1 | 12.1 | 12.1 |
| Aspartic acid | 30.4 | 30.4 | 30.4 | 30.4 | 30.4 | 30.4 |
| Glutamic acid | 58.2 | 58.2 | 58.2 | 58.3 | 58.3 | 58.3 |
| Alanine | 29.4 | 29.4 | 29.4 | 29.4 | 29.4 | 29.4 |
| Glycine | 34.9 | 34.9 | 34.9 | 35 | 35 | 35 |
| Proline | 30.6 | 30.6 | 30.6 | 30.7 | 30.7 | 30.7 |
| Serine | 16.6 | 16.6 | 16.6 | 16.6 | 16.6 | 16.6 |
| Tyrosine | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 |
| Feed Ingredients | ||||
|---|---|---|---|---|
| Soybean Meal | Wheat Flour | Fish Meal | Poultry By-Product Meal | |
| Dried matters | 895 | 888 | 911 | 953 |
| CP | 490 | 158 | 578 | 648 |
| Lipid | 12.0 | 13.0 | 70.0 | 72.0 |
| Ash | 58.0 | 15.0 | 185 | 259 |
| Crude fiber | 26.0 | 4.0 | 4.0 | 26.0 |
| Total | 175.8 | 37.1 | 145.1 | 108.7 |
| Treatments | Crude Fat | MA (C14:0) | PA (C16:0) | SA (C18:0) | OLA (C18:1n9) | LA (C18:2n6) | αNA (C18:3n3) | ARA (C20:4n6) | EPA (C20:5n3) | DHA (C22:6n3) |
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 3.01 | 0.043 | 0.556 | 0.393 | 0.820 | 0.597 | 0.056 | 0.010 | 0.015 | 0.053 |
| T2 | 2.59 | 0.032 | 0.518 | 0.237 | 0.879 | 0.560 | 0.038 | 0.016 | 0.010 | 0.010 |
| T3 | 3.18 | 0.040 | 0.622 | 0.302 | 1.065 | 0.661 | 0.047 | 0.010 | 0.010 | 0.024 |
| T4 | 3.30 | 0.042 | 0.648 | 0.306 | 1.075 | 0.665 | 0.048 | 0.011 | 0.016 | 0.066 |
| T5 | 3.01 | 0.038 | 0.590 | 0.261 | 0.953 | 0.641 | 0.047 | 0.013 | 0.024 | 0.082 |
| T6 | 4.41 | 0.047 | 0.817 | 0.389 | 1.395 | 1.016 | 0.076 | 0.018 | 0.033 | 0.121 |
| Indices | Experimental Treatments | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | |||
| Growth performance indices of brooder fish | ||||||||
| Initial body weight (kg) | 4.4 ± 0.7 | 5.0 ± 0.9 | 5.2 ± 0.9 | 4.9 ± 0.8 | 5.0 ± 0.1 | 4.7 ± 0.1 | 0.841 | |
| Final body weight (kg) | 6.2 ± 0.9 b | 6.1 ± 0.7 b | 6.0 ± 0.0 b | 5.6 ± 0.6 c | 7.0 ± 1.1 a | 6.0 ± 0.2 | 0.024 | |
| Weight gain (kg) | 1.8 ± 0.2 ab | 1.0 ± 0.3 b | 0.9 ± 0.9 bc | 0.6 ± 0.3 c | 2.0 ± 0.9 a | 1.3 ± 0.2 ab | 0.027 | |
| Daily weight gain (kg) | 0.04 ± 0.0 ab | 0.02 ± 0.01 b | 0.02 ± 0.02 bc | 0.02 ± 0.01 c | 0.10 ± 0.02 a | 0.02 ± 0.01 ab | 0.027 | |
| Specific growth rate (SGR%) | 0.5 ± 0.4 ab | 0.3 ± 0.2 b | 0.3 ± 0.2 bc | 0.2 ± 0.1 c | 0.6 ± 0.2 a | 0.4 ± 0.0 ab | 0.027 | |
| Food conversion ratio (FCR) | 1.8 ± 0.3 | 1.8 ± 0.4 | 1.7 ± 0.9 | 1.8 ± 0.2 | 1.6 ± 0.1 | 1.7 ± 0.3 | 0.100 | |
| Reproductive performance indices | ||||||||
| Egg size (µm) | Before injecting hCG | 1.0 ± 0.01 | 0.9 ± 0.01 | 0.9 ± 0.01 | 1.0 ± 0.01 | 1.0 ± 0.01 | 0.9 ± 0.1 | 0.071 |
| After injecting hCG | 1.1 ± 0.01 | 1.1 ± 0.01 | 1.0 ± 0.03 | 1.0 ± 0.05 | 1.1 ± 0.03 | 1.0 ± 0.01 | 0.862 | |
| Gonad weight (g/fish) | 750.1 ± 54.8 a | 366.7 ± 100.1 b | 400.0 ± 109.5 ab | 466.7 ± 264.6 ab | 754.02 ± 248.1 a | 500.1 ± 100.6 ab | 0.004 | |
| Gonad somatic index (GSI%) | 12.2 ± 1.0 a | 6.0 ± 1.7 b | 6.7 ± 1.8 ab | 8.4 ± 4.8 ab | 12.8 ± 0.8 a | 8.4 ± 0.1 ab | 0.027 | |
| Relative fecundity index (egg/kg) | 152,158 ± 7467 a | 90,014 ± 2467 b | 104,267 ± 7381 ab | 133,642 ± 1503 a | 199,512 ± 7467 a | 119,748 ± 1166 ab | 0.022 | |
| Total number of eggs in female ovary (egg) | 1,227,000 ± 107.4 a | 546,383 ± 303.7 b | 625,600 ± 452.3 ab | 744,387 ± 119.9 ab | 1,057,500 ± 232.1 a | 712,500 ± 106.9 ab | 0.047 | |
| Indices | Experimental Treatments | |||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | |
| Body indices of fish fry reared for 15 days (n = 15,000 larvae/tank) | ||||||
| BWG (mg) | 12.1 ± 0.1 c | 11.11 ± 0.0 d | 15.6 ± 0.1 a | 14.4 ± 0.2 b | 14.3 ± 0.2 b | 12.9 ± 0.2 c |
| Length (mm) | 14.9 ± 0.4 bc | 14.82 ± 0.4 c | 15.4 ± 0.5 a | 15.2 ± 0.4 ab | 15.1 ± 0.4 bc | 15.0 ± 0.4 bc |
| DWG (mg) | 0.6 ± 0.01 d | 0.60 ± 0.01 e | 0.8 ± 0.01 a | 0.8 ± 0.01 b | 0.8 ± 0.01 b | 0.6 ± 0.0 cd |
| SGR% | 8.5 ± 0.6 c | 10.05 ± 0.7 a | 8.2 ± 0.4 c | 9.2 ± 0.9 b | 9.1 ± 0.6 b | 8.6 ± 1.0 c |
| Body indices of fingerlings reared for 30 days (n = 2000 fingerlings/tank) | ||||||
| BWG (mg) | 63.0 ± 35.2 b | 147.0 ± 24.5 a | 41.0 ± 9.4 d | 23.0 ± 15.2 e | 47.0 ± 11.3 c | 39.0 ± 18.7 d |
| Length (mm) | 25.0 ± 2.2 a | 22.0 ± 2.0 ab | 18.3 ± 0.5 b | 23.6 ± 0.6 a | 18.0 ± 0.4 b | 24.0 ± 1.4 a |
| DWG (mg/d) | 4.9 ± 0.3 b | 11.3 ± 1.5 a | 3.2 ± 0.8 d | 1.7 ± 0.9 e | 3.6 ± 1.5 c | 3.0 ± 19 d |
| SGR (%) | 6.9 ± 1.2 c | 10.6 ± 2.2 a | 4.4 ± 1.6 e | 2.6 ± 1.7 f | 6.4 ± 3.0 d | 4.7 ± 2.1 e |
| Body indices of fingerlings reared for 45 days (n = 2000 fingerlings/tank) | ||||||
| BWG (mg) | 947.0 ± 64.0 a | 313.0 ± 58.7 d | 708.0 ± 53.2 b | 431.0 ± 66.4 c | 304.0 ± 51.2 d | 499.0 ± 98.8 c |
| Length (mm) | 60.1 ± 1.7 a | 41.5 ± 1.2 b | 41.5 ± 0.9 b | 56.5 ± 1.0 a | 37.0 ± 0.7 b | 57.5 ± 1.3 a |
| DWG (mg/d) | 79.0 ± 1.5 a | 26.0 ± 2.3 d | 59.0 ± 3.3 b | 36.0 ± 2.9 c | 25.0 ± 3.2 d | 42.0 ± 5.4 c |
| SGR (%) | 6.8 ± 0.7 b | 2.7 ± 0.6 d | 9.0 ± 1.5 a | 4.0 ± 2.2 c | 4.12 ± 2.2 c | 4.0 ± 1.8 c |
| Total number of surviving fingerlings | 466.7 ± 12.7 b | 439.3 ± 94.7 b | 1326.3 ± 560.5 a | 413.3 ± 242.7 b | 781.3 ± 173.2 b | 603.7 ± 251.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da, C.T.; Xuyen, B.T.K.; Nguyen, T.K.O.; Tang, V.T.; Ha, P.T.T.; Pham, M.T.; Berg, H. Vitamin Solutions Effects on Reproduction of Broodstock, Growth Performance, and Survival Rate of Pangasius Catfish Fingerlings. Animals 2024, 14, 2203. https://doi.org/10.3390/ani14152203
Da CT, Xuyen BTK, Nguyen TKO, Tang VT, Ha PTT, Pham MT, Berg H. Vitamin Solutions Effects on Reproduction of Broodstock, Growth Performance, and Survival Rate of Pangasius Catfish Fingerlings. Animals. 2024; 14(15):2203. https://doi.org/10.3390/ani14152203
Chicago/Turabian StyleDa, Chau Thi, Bui Thi Kim Xuyen, Thi Kieu Oanh Nguyen, Van Tai Tang, Pham Thi Thu Ha, Minh Tan Pham, and Håkan Berg. 2024. "Vitamin Solutions Effects on Reproduction of Broodstock, Growth Performance, and Survival Rate of Pangasius Catfish Fingerlings" Animals 14, no. 15: 2203. https://doi.org/10.3390/ani14152203
APA StyleDa, C. T., Xuyen, B. T. K., Nguyen, T. K. O., Tang, V. T., Ha, P. T. T., Pham, M. T., & Berg, H. (2024). Vitamin Solutions Effects on Reproduction of Broodstock, Growth Performance, and Survival Rate of Pangasius Catfish Fingerlings. Animals, 14(15), 2203. https://doi.org/10.3390/ani14152203






