High Muscle Expression of IGF2BP1 Gene Promotes Proliferation and Differentiation of Chicken Primary Myoblasts: Results of Transcriptome Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Sample Collection
2.2. Total RNA Extraction and RNA-Seq
2.3. RNA-Seq Sequencing Data Analysis
2.4. Bioinformatics Analysis
2.5. cDNA Synthesis and Real-Time Fluorescent Quantitative PCR (QRT-PCR)
2.6. Isolation and Culture of Chicken Primary Myoblasts (CPMs)
2.7. Plasmid, siRNA, and Transfection
2.8. 5-Ethynyl-2′-deoxyuridine (EdU) Assay
2.9. Flow Cytometry Analysis of Cell Cycle
2.10. Statistical Analysis
3. Results
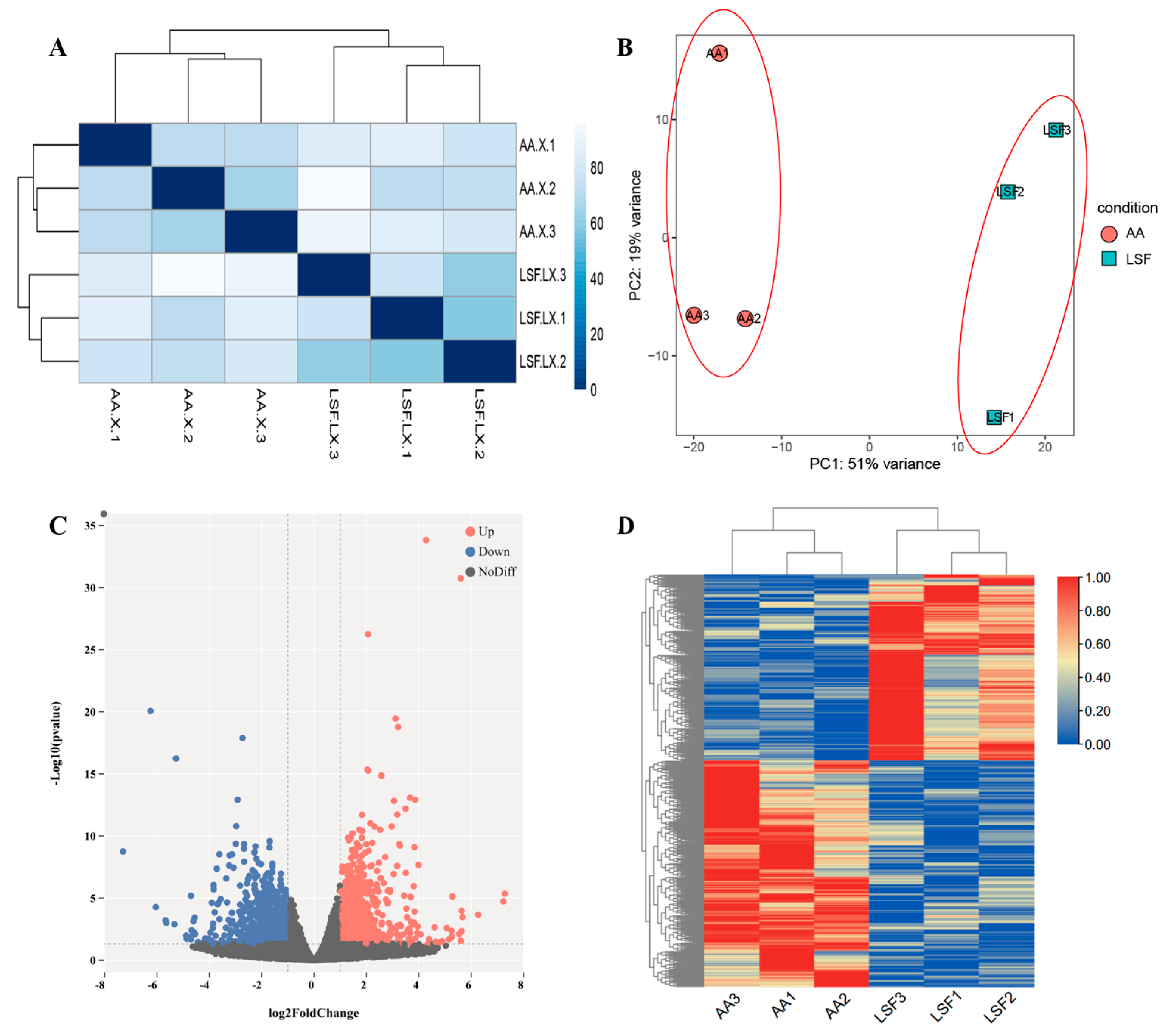
3.1. Summary of RNA-Seq Data
3.2. RNA-Seq Data and Identification of Differentially Expressed Genes (DEGs)
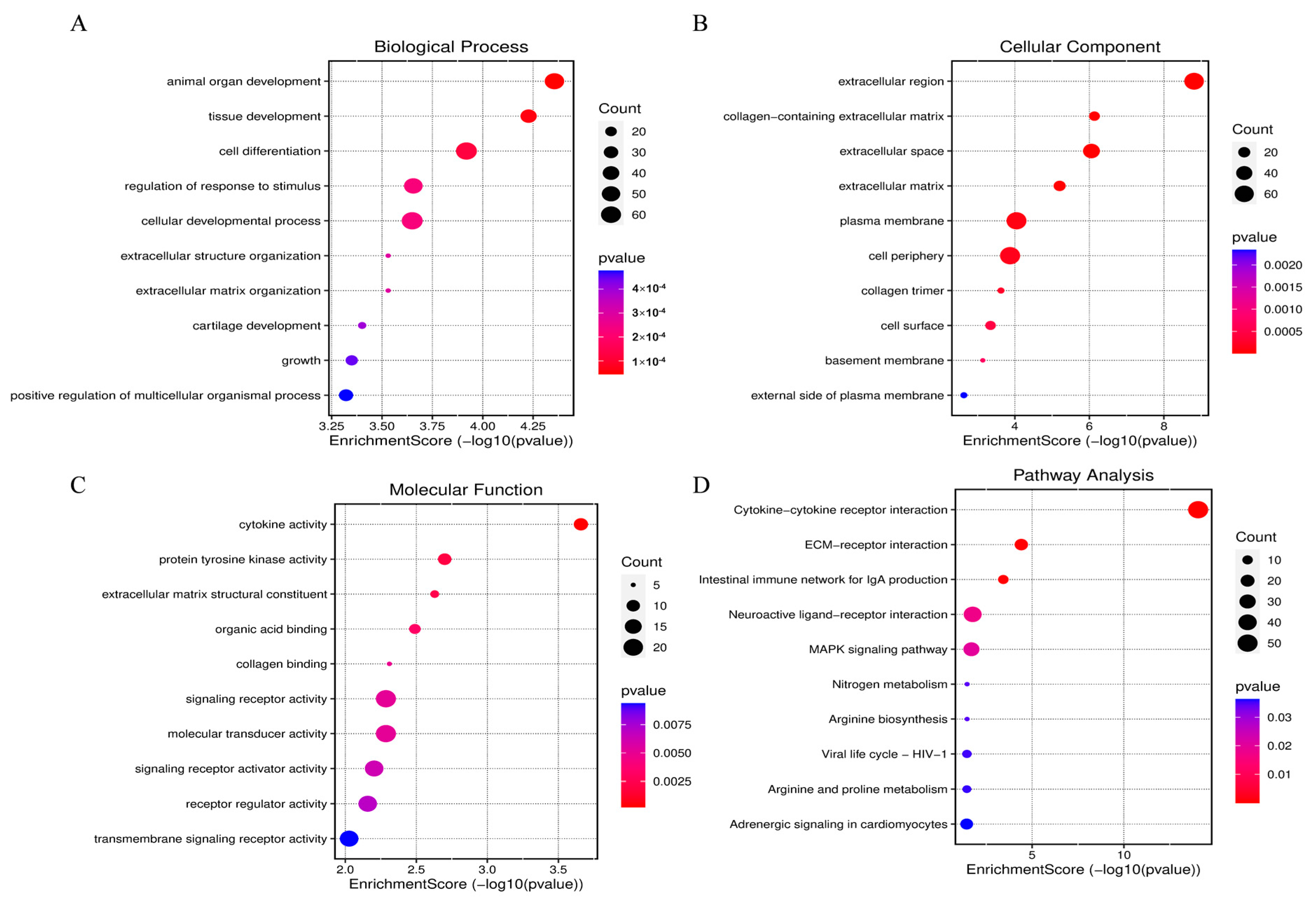
3.3. GO and KEGG Pathway Analysis of DEGs
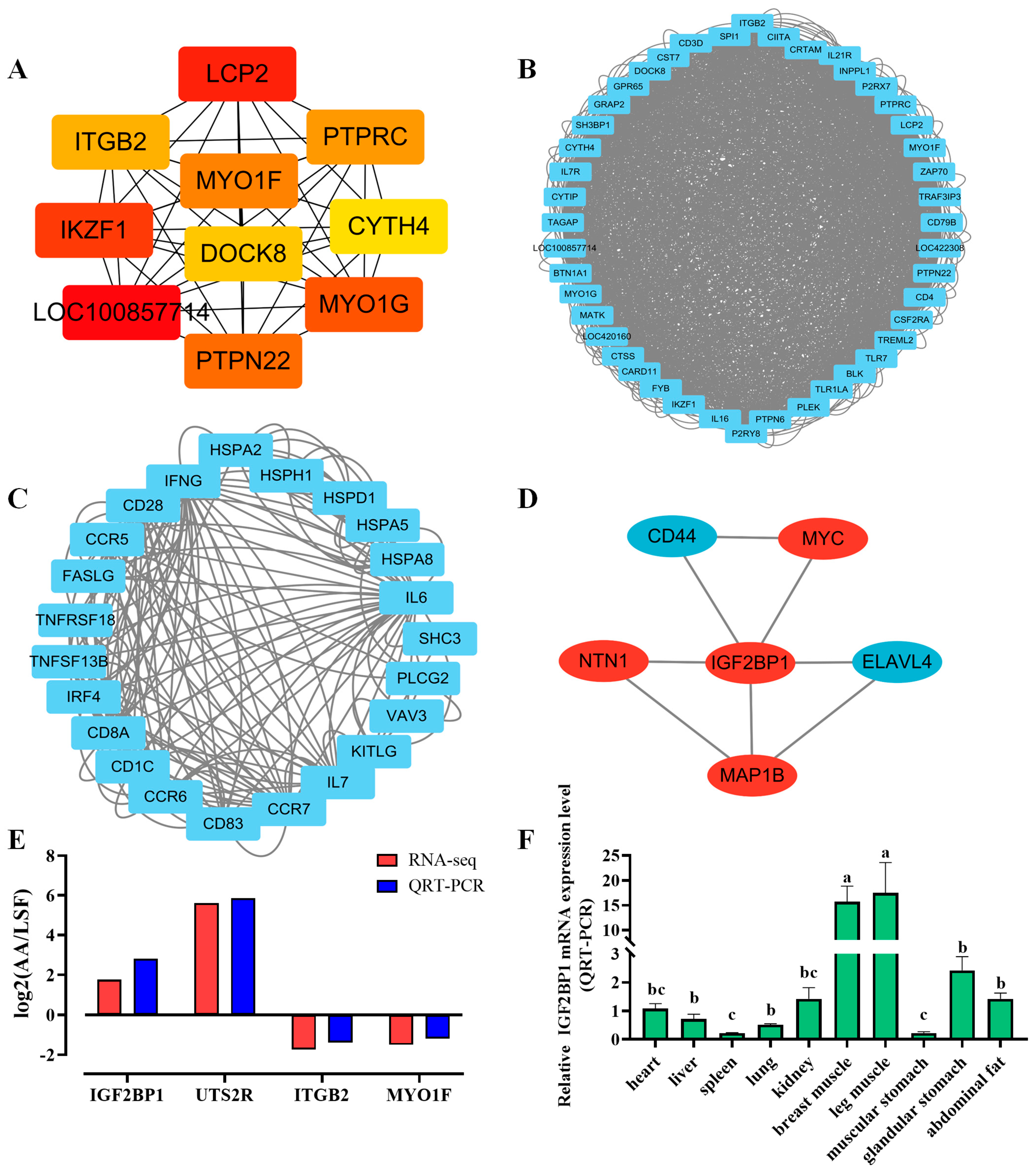
3.4. PPI Interaction Analysis and Selection of Target Genes
3.5. Interfering with IGF2BP1 Inhibits Proliferation and Differentiation of CPM
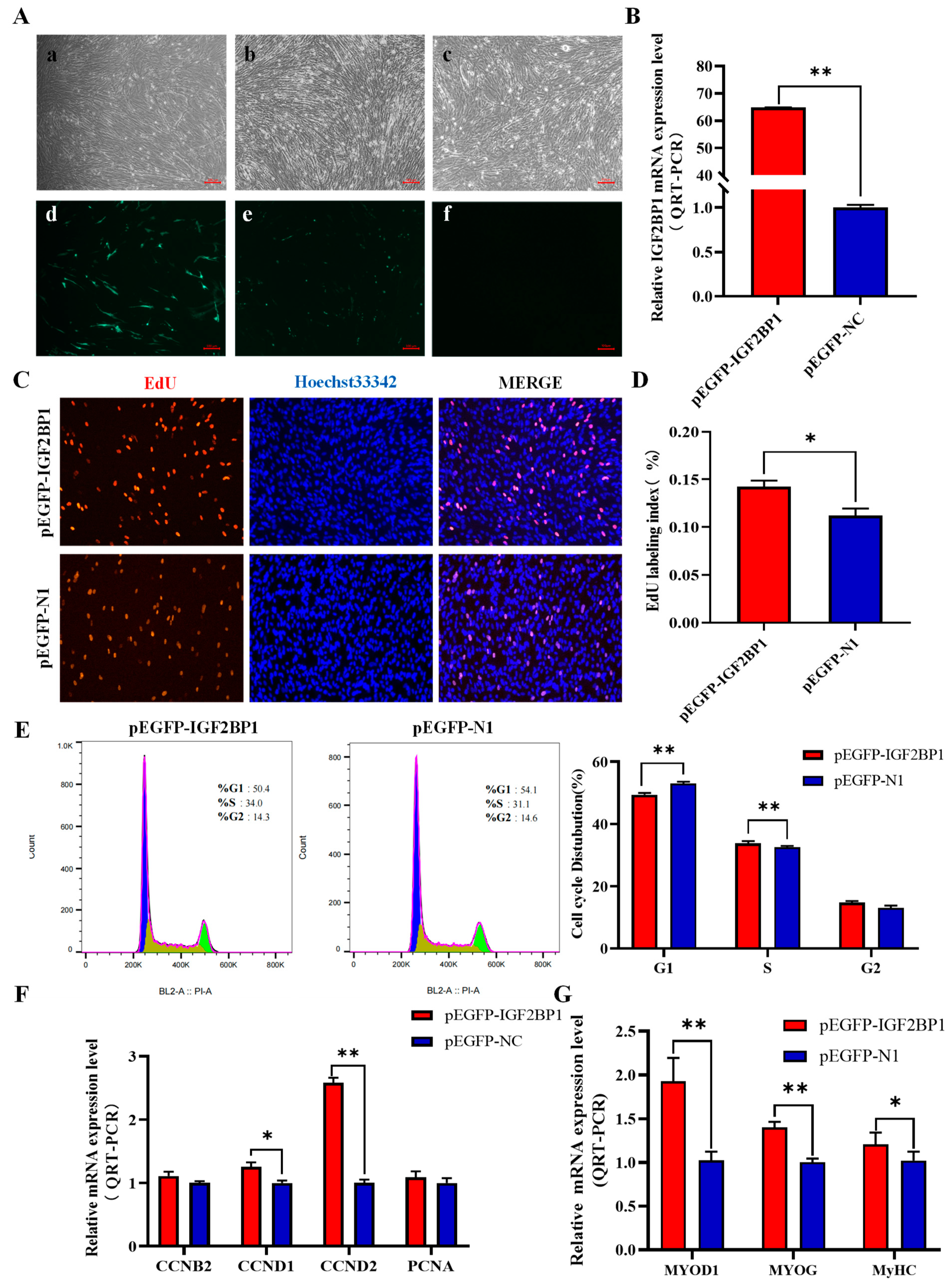
3.6. Overexpression of IGF2BP1 Promotes Proliferation and Differentiation of CPM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, L.; Liu, A.; Wang, Q.; Wang, H.; Dong, D.; Liu, L. Transcriptome analysis of embryonic muscle development in Chengkou Mountain Chicken. BMC Genom. 2021, 22, 431. [Google Scholar] [CrossRef] [PubMed]
- Hayes, V.E.; Hikida, R.S. Naturally-occurring degeneration in chick muscle development: Ultrastructure of the M. complexus. J. Anat. 1976, 122, 67–76. [Google Scholar] [PubMed]
- Khabyuk, J.; Prols, F.; Draga, M.; Scaal, M. Development of ribs and intercostal muscles in the chicken embryo. J. Anat. 2022, 241, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mccarthy, J.J.; Malakoutinia, F. Myonuclear permanence in skeletal muscle memory: A systematic review and meta-analysis of human and animal studies. J. Cachexia Sarcopenia Muscle 2022, 13, 2276–2297. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Xian, M.; Ren, T.; Mo, G.; Zhang, L.; Zhang, X. Mining of chicken muscle growth genes and the function of important candidate gene RPL3L in muscle development. Front. Physiol. 2022, 13, 1033075. [Google Scholar] [CrossRef]
- Ren, P.; Chen, M.; Li, J.; Lin, Z.; Yang, C.; Yu, C.; Zhang, D.; Liu, Y. MYH1F promotes the proliferation and differentiation of chicken skeletal muscle satellite cells into myotubes. Anim. Biotechnol. 2023, 34, 3074–3084. [Google Scholar] [CrossRef] [PubMed]
- Zi, J.; Xu, J.; Luo, J.; Yang, X.; Zhen, Z.; Li, X.; Hu, D.; Guo, Y.; Guo, H.; Ding, X.; et al. PFN1 Inhibits Myogenesis of Bovine Myoblast Cells via Cdc42-PAK/JNK. Cells 2022, 11, 3188. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Dewi, R.E.; Heilshorn, S.C. Microfluidic analysis of extracellular matrix-bFGF crosstalk on primary human myoblast chemoproliferation, chemokinesis, and chemotaxis. Integr. Biol. 2015, 7, 569–579. [Google Scholar] [CrossRef]
- Withanage, M.; Liang, H.; Zeng, E. RNA-Seq Experiment and Data Analysis. Methods Mol. Biol. 2022, 2418, 405–424. [Google Scholar] [CrossRef]
- Peng, L.Y.; Cui, Z.Q.; Wu, Z.M.; Fu, B.D.; Yi, P.F.; Shen, H.Q. RNA-seq profiles of chicken type II pneumocyte in response to Escherichia coli infection. PLoS ONE 2019, 14, e0217438. [Google Scholar] [CrossRef]
- Yu, B.; Liu, J.; Cai, Z.; Wang, H.; Feng, X.; Zhang, T.; Ma, R.; Gu, Y.; Zhang, J. RNA N (6)-methyladenosine profiling reveals differentially methylated genes associated with intramuscular fat metabolism during breast muscle development in chicken. Poult. Sci. 2023, 102, 102793. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Cai, Y.; Li, Y.; Li, T.; Zhang, J.; Hu, Z.; Zhang, J. Characterization and comparative transcriptomic analysis of skeletal muscle in female Pekin duck and Hanzhong Ma duck during different growth stages using RNA-seq. Poult. Sci. 2023, 102, 103122. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Liu, R.; Zhao, G.; Liu, L.; Groenen, M.; Madsen, O.; Zheng, M.; Yang, X.; Crooijmans, R.; Wen, J. RNA-Seq Analysis Reveals Hub Genes Involved in Chicken Intramuscular Fat and Abdominal Fat Deposition During Development. Front. Genet. 2020, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Chennathukuzhi, V.; Stein, J.M.; Abel, T.; Donlon, S.; Yang, S.; Miller, J.P.; Allman, D.M.; Simmons, R.A.; Hecht, N.B. Mice deficient for testis-brain RNA-binding protein exhibit a coordinate loss of TRAX, reduced fertility, altered gene expression in the brain, and behavioral changes. Mol. Cell. Biol. 2003, 23, 6419–6434. [Google Scholar] [CrossRef] [PubMed]
- Ruggiu, M.; Speed, R.; Taggart, M.; Mckay, S.J.; Kilanowski, F.; Saunders, P.; Dorin, J.; Cooke, H.J. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 1997, 389, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Elcheva, I.A.; Gowda, C.P.; Bogush, D.; Gornostaeva, S.; Fakhardo, A.; Sheth, N.; Kokolus, K.M.; Sharma, A.; Dovat, S.; Uzun, Y.; et al. IGF2BP family of RNA-binding proteins regulate innate and adaptive immune responses in cancer cells and tumor microenvironment. Front. Immunol. 2023, 14, 1224516. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, H.; Guo, X.; Zhu, Z.; Cai, H.; Kong, X. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in cancer. J. Hematol. Oncol. 2018, 11, 88. [Google Scholar] [CrossRef]
- Bell, J.L.; Wachter, K.; Muhleck, B.; Pazaitis, N.; Kohn, M.; Lederer, M.; Huttelmaier, S. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): Post-transcriptional drivers of cancer progression? Cell. Mol. Life Sci. 2013, 70, 2657–2675. [Google Scholar] [CrossRef] [PubMed]
- Hammer, N.A.; Hansen, T.; Byskov, A.G.; Rajpert-De, M.E.; Grondahl, M.L.; Bredkjaer, H.E.; Wewer, U.M.; Christiansen, J.; Nielsen, F.C. Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction 2005, 130, 203–212. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, S.; Zhang, X.; Ren, S.; Tang, Z.; Gao, F. Coordinated transcriptional and post-transcriptional epigenetic regulation during skeletal muscle development and growth in pigs. J. Anim. Sci. Biotechnol. 2022, 13, 146. [Google Scholar] [CrossRef]
- Zhan, S.; Zhao, W.; Song, T.; Dong, Y.; Guo, J.; Cao, J.; Zhong, T.; Wang, L.; Li, L.; Zhang, H. Dynamic transcriptomic analysis in hircine longissimus dorsi muscle from fetal to neonatal development stages. Funct. Integr. Genom. 2018, 18, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Leng, J.; Zhang, X.; Capellini, T.D.; Chen, Y.; Yang, L.; Chen, Z.; Zheng, S.; Zhang, X.; Zhan, S.; et al. Identification of IGF2BP1-related lncRNA-miRNA-mRNA network in goat skeletal muscle satellite cells. Anim. Sci. J. 2021, 92, e13631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M.; Cheng, H.; Fan, W.; Yuan, Z.; Gao, Q.; Xu, Y.; Guo, Z.; Zhang, Y.; Hu, J.; et al. Author Correction: An intercross population study reveals genes associated with body size and plumage color in ducks. Nat. Commun. 2018, 9, 3974. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, H.; Tian, Y.; Li, J.; Scheben, A.; Zhang, C.; Li, Y.; Wu, J.; Yang, L.; Fan, X.; et al. The Chicken Pan-Genome Reveals Gene Content Variation and a Promoter Region Deletion in IGF2BP1 Affecting Body Size. Mol. Biol. Evol. 2021, 38, 5066–5081. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, S.; Chen, G.; Deng, X.; Zhang, D.; Wen, H.; Yin, Y.; Lin, Z.; Zhang, X.; Luo, W. Transcriptome Sequencing Reveals Pathways Related to Proliferation and Differentiation of Shitou Goose Myoblasts. Animals 2022, 12, 2956. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Li, Z.; Zhou, Y.; Liu, X.; Han, R.; Wang, Y.; Yan, F.; Sun, G.; Li, H.; Kang, X. Sequencing and characterization of lncRNAs in the breast muscle of Gushi and Arbor Acres chickens. Genome 2018, 61, 337–347. [Google Scholar] [CrossRef]
- Sarsenbek, A.; Wang, T.; Zhao, J.K.; Jiang, W. Comparison of carcass yields and meat quality between Baicheng-You chickens and Arbor Acres broilers. Poult. Sci. 2013, 92, 2776–2782. [Google Scholar] [CrossRef]
- Ahmad, K.; Lee, E.J.; Moon, J.S.; Park, S.Y.; Choi, I. Multifaceted Interweaving Between Extracellular Matrix, Insulin Resistance, and Skeletal Muscle. Cells 2018, 7, 148. [Google Scholar] [CrossRef]
- Ahmed, M.; Ffrench-Constant, C. Extracellular Matrix Regulation of Stem Cell Behavior. Curr. Stem Cell Rep. 2016, 2, 197–206. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harbor Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem. Cells 2013, 31, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Lehka, L.; Redowicz, M.J. Mechanisms regulating myoblast fusion: A multilevel interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yin, H.; Yu, X.; Zhang, Y.; Ma, M.; Li, D.; Zhu, Q. PDLIM5 Affects Chicken Skeletal Muscle Satellite Cell Proliferation and Differentiation via the p38-MAPK Pathway. Animals 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, C.; Ge, S.; Mei, J.; Li, X.; Guo, W. Igf2bp1 is required for hepatic outgrowth during early liver development in zebrafish. Gene 2020, 744, 144632. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, K.; Fainsod, A.; Kalcheim, C.; Yisraeli, J.K. The RNA-binding protein Vg1 RBP is required for cell migration during early neural development. Development 2003, 130, 5649–5661. [Google Scholar] [CrossRef] [PubMed]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Niu, Y.; Wang, Y.; Liu, Y.; Ji, H.; Han, R.; Tian, Y.; Liu, X.; Kang, X.; et al. RRM2 promotes the proliferation of chicken myoblasts, inhibits their differentiation and muscle regeneration. Poult. Sci. 2023, 103, 103407. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Y.; Hou, D.; Zhang, Y.; Zhang, B.; Niu, Y.; Ji, H.; Tian, Y.; Liu, X.; Kang, X.; et al. Transcriptome-Based Identification of the Muscle Tissue-Specific Expression Gene CKM and Its Regulation of Proliferation, Apoptosis and Differentiation in Chicken Primary Myoblasts. Animals 2023, 13, 2316. [Google Scholar] [CrossRef]
- Daczewska, M. Differentiation of skeletal muscles. Semin. Cell Dev. Biol. 2020, 104, 1–2. [Google Scholar] [CrossRef]
- O’Neill, M.C. Growth and differentiation during myogenesis in the chick embryo. Dev. Biol. 1987, 120, 465–480. [Google Scholar] [CrossRef] [PubMed]

| Sample Name | Clean Reads | Clean Base(G) | Q20(%) | Content (%) | Mapping Rate (%) |
|---|---|---|---|---|---|
| AA1 | 82,372,256 | 12.36 | 97.61 | 53.67 | 93.37 |
| AA2 | 76,265,758 | 11.44 | 97.74 | 56.45 | 93.31 |
| AA3 | 90,003,894 | 13.5 | 97.62 | 56.85 | 92.87 |
| LSF1 | 103,112,308 | 15.47 | 97.34 | 53.12 | 92.33 |
| LSF2 | 107,514,536 | 15.67 | 97.18 | 54.05 | 92.37 |
| LSF3 | 98,934,380 | 14.84 | 97.19 | 52.46 | 92.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Yang, Z.; Li, X.; Xiao, C.; Yuan, H.; Yang, X.; Zhou, B.; Zheng, Y.; Zhang, J.; Yang, X. High Muscle Expression of IGF2BP1 Gene Promotes Proliferation and Differentiation of Chicken Primary Myoblasts: Results of Transcriptome Analysis. Animals 2024, 14, 2024. https://doi.org/10.3390/ani14142024
Luo J, Yang Z, Li X, Xiao C, Yuan H, Yang X, Zhou B, Zheng Y, Zhang J, Yang X. High Muscle Expression of IGF2BP1 Gene Promotes Proliferation and Differentiation of Chicken Primary Myoblasts: Results of Transcriptome Analysis. Animals. 2024; 14(14):2024. https://doi.org/10.3390/ani14142024
Chicago/Turabian StyleLuo, Jintang, Zhuliang Yang, Xianchao Li, Cong Xiao, Hong Yuan, Xueqin Yang, Biyan Zhou, Yan Zheng, Jiayi Zhang, and Xiurong Yang. 2024. "High Muscle Expression of IGF2BP1 Gene Promotes Proliferation and Differentiation of Chicken Primary Myoblasts: Results of Transcriptome Analysis" Animals 14, no. 14: 2024. https://doi.org/10.3390/ani14142024
APA StyleLuo, J., Yang, Z., Li, X., Xiao, C., Yuan, H., Yang, X., Zhou, B., Zheng, Y., Zhang, J., & Yang, X. (2024). High Muscle Expression of IGF2BP1 Gene Promotes Proliferation and Differentiation of Chicken Primary Myoblasts: Results of Transcriptome Analysis. Animals, 14(14), 2024. https://doi.org/10.3390/ani14142024





