Measurement of Calprotectin (S100A8/A9) in the Saliva of Pigs: Validation Data of A Commercially Available Automated Assay and Changes in Sepsis, Inflammation, and Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Assay
- Precision: the intra- and inter-assay coefficients of variation (CVs) were assessed using saliva samples with high and low CALP concentrations.
- Accuracy: It was indirectly studied by evaluating linearity after serial dilutions with ultrapure water of saliva and samples with a high level of CALP. Additionally, recovery studies were made to see if there was a matrix effect in the determinations. For this, purified CALP (control material from Gentian, Moss, Norway) was used to spike saliva samples to reach three different CALP concentrations.
- The lower limit of quantification (LLQ): it was calculated as the lowest CALP concentration that the assay was able to determine with an intra-assay CV < 20%.
- Limit of detection (LD): based on the lowest concentration of CALP that the assays can distinguish from a specimen of zero value (ultrapure water), calculated as a mean value plus 3 standard deviations of 12 replicate determinations.
2.2. Evaluation of the Sampling in the Daytime
2.3. Experimental Sepsis and Non-Septic Inflammation Induction
2.4. Stress Situation
2.5. Statistical Analysis
3. Results
3.1. Saliva Calprotectin Assay Validations
3.2. Evaluation of the Sampling Time on the Day
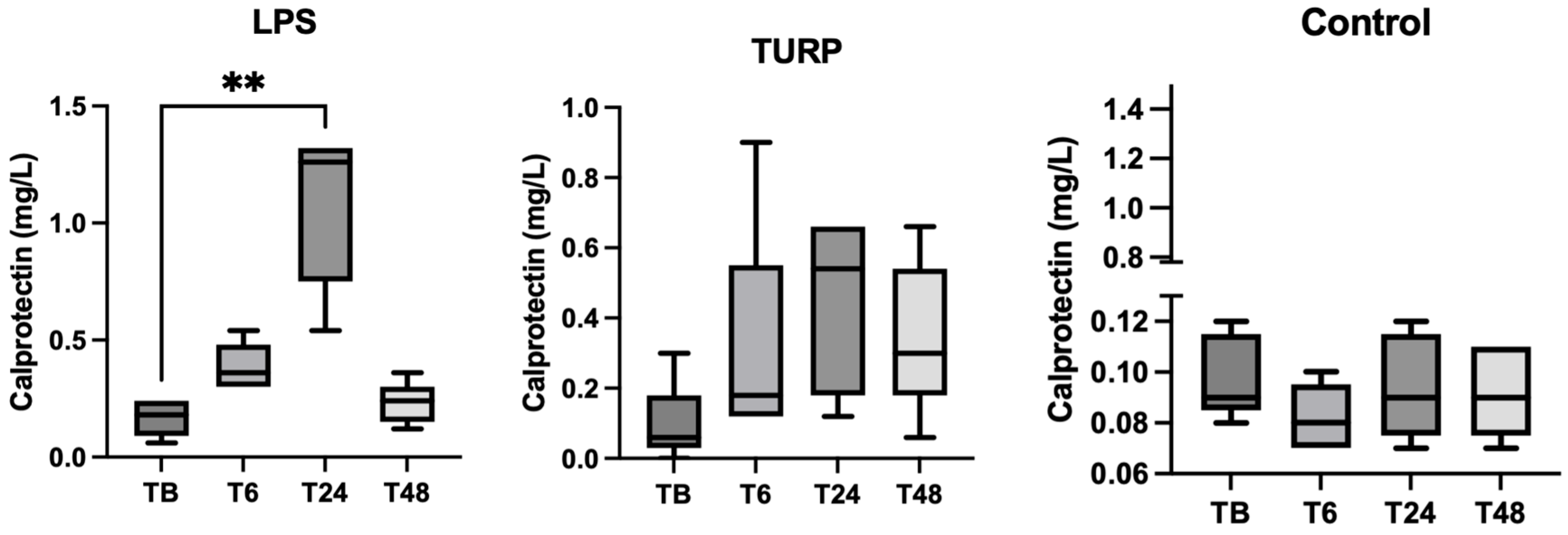
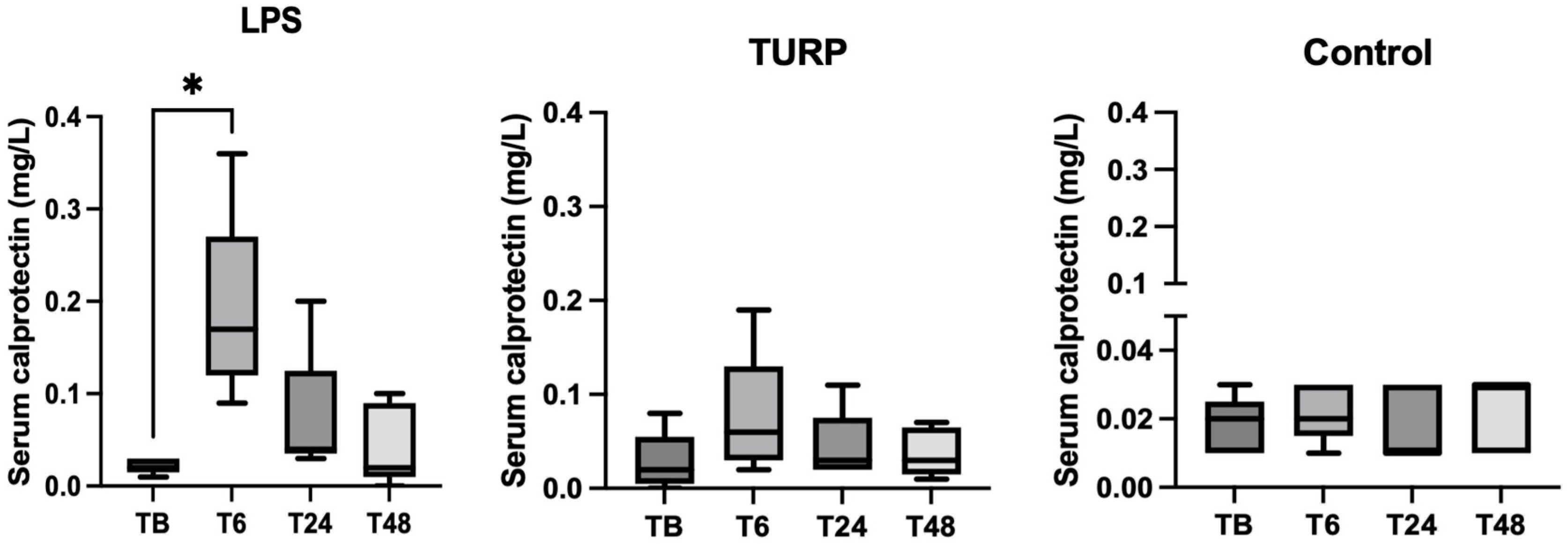
3.3. Experimental Sepsis and Non-Septic Inflammation Induction
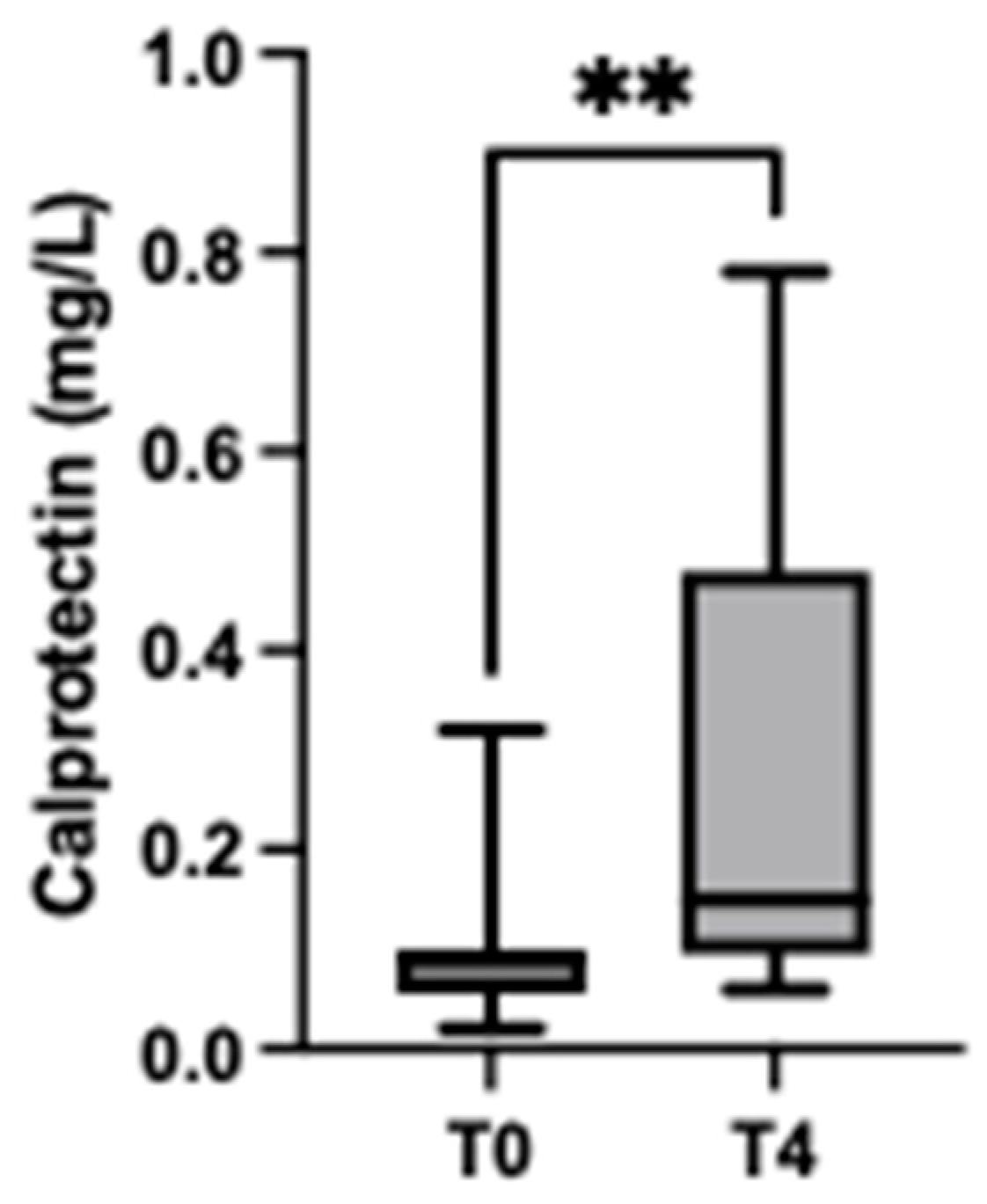
3.4. Stress Situation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manolakis, A.C.; Kapsoritakis, A.N.; Tiaka, E.K.; Potamianos, S.P. Calprotectin, Calgranulin C, and Other Members of the S100 Protein Family in Inflammatory Bowel Disease. Dig. Dis. Sci. 2011, 56, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, L.; Xu, M.; Chen, L.; Lu, W.; Wang, W. Serum Calprotectin as a Prognostic Predictor in Severe Traumatic Brain Injury. Clin. Chim. Acta 2021, 520, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J.; Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Miró, S.; López-Martínez, M.J.; Ortín-Bustillo, A.; Franco-Martínez, L.; Rubio, C.P.; Muñoz-Prieto, A.; Tvarijonaviciute, A.; et al. Basics for the Potential Use of Saliva to Evaluate Stress, Inflammation, Immune System, and Redox Homeostasis in Pigs. BMC Vet. Res. 2022, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.-Y.; Jia, H.-M.; Han, Y.-Z.; Qian, B.-S.; You, P.; Zhang, X.-K.; Li, W.-X.; Huang, L.-F. Calprotectin as a Diagnostic Marker for Sepsis: A Meta-Analysis. Front. Cell Infect. Microbiol. 2022, 12, 1726. [Google Scholar] [CrossRef]
- Majster, M.; Almer, S.; Boström, E.A. Salivary Calprotectin Is Elevated in Patients with Active Inflammatory Bowel Disease. Arch. Oral Biol. 2019, 107, 104528. [Google Scholar] [CrossRef]
- Barbosa, J.A.; Rodrigues, L.A.; Columbus, D.A.; Aguirre, J.C.P.; Harding, J.C.S.; Cantarelli, V.S.; Costa, M.D.O. Experimental Infectious Challenge in Pigs Leads to Elevated Fecal Calprotectin Levels Following Colitis, but Not Enteritis. Porc. Health Manag. 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Bogere, P.; Choi, Y.J.; Heo, J. Optimization of Fecal Calprotectin Assay for Pig Samples. J. Agric. Life Sci. 2019, 53, 93–104. [Google Scholar] [CrossRef]
- Sánchez-Uribe, P.; Romera-Recio, E.; Cabrera-Gómez, C.G.; Hernández-Rodríguez, E.V.; Lamrani, Á.; González-Guijarro, B.; de Pascual-Monreal, C.; Mendonça-Pascoal, L.; Martínez-Alarcón, L.; Ramis, G. Effect of β-Mannanase Addition during Whole Pigs Fattening on Production Yields and Intestinal Health. Animals 2022, 12, 3012. [Google Scholar] [CrossRef]
- Ramis, G.; Pérez-Esteruelas, L.; Gómez-Cabrera, C.G.; de Pascual-Monreal, C.; Gonzalez-Guijarro, B.; Párraga-Ros, E.; Sánchez-Uribe, P.; Claver-Mateos, M.; Mendonça-Pascoal, L.; Martínez-Alarcón, L. Oral and Parenteral Vaccination against Escherichia Coli in Piglets Results in Different Responses. Animals 2022, 12, 2758. [Google Scholar] [CrossRef]
- Christensen, M.; Jacobsen, S.; Ichiyanagi, T.; Kjelgaard-Hansen, M. Evaluation of an Automated Assay Based on Monoclonal Anti-Human Serum Amyloid A (SAA) Antibodies for Measurement of Canine, Feline, and Equine SAA. Vet. J. 2012, 194, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ortín-Bustillo, A.; Contreras-Aguilar, M.D.; Rubio, C.P.; Botia, M.; Cerón, J.J.; López-Arjona, M.; Martínez-Subiela, S.; Escribano, D.; Tecles, F. Evaluation of the Effect of Sampling Time on Biomarkers of Stress, Immune System, Redox Status and Other Biochemistry Analytes in Saliva of Finishing Pigs. Animals 2022, 12, 2127. [Google Scholar] [CrossRef]
- López-Martínez, M.J.; Escribano, D.; Ortín-Bustillo, A.; Franco-Martínez, L.; González-Arostegui, L.G.; Cerón, J.J.; Rubio, C.P. Changes in Biomarkers of Redox Status in Saliva of Pigs after an Experimental Sepsis Induction. Antioxidants 2022, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Escribano, D.; Fuentes-Rubio, M.; Cerón, J.J. Validation of an Automated Chemiluminescent Immunoassay for Salivary Cortisol Measurements in Pigs. J. Vet. Diagn. Investig. 2012, 24, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Pirr, S.; Dauter, L.; Vogl, T.; Ulas, T.; Bohnhorst, B.; Roth, J.; Viemann, D. S100A8/A9 Is the First Predictive Marker for Neonatal Sepsis. Clin. Transl. Med. 2021, 11, e338. [Google Scholar] [CrossRef]
- Simm, M.; Söderberg, E.; Larsson, A.; Castegren, M.; Nilsen, T.; Eriksson, M.; Lipcsey, M. Performance of Plasma Calprotectin as a Biomarker of Early Sepsis: A Pilot Study. Biomark. Med. 2016, 10, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Kamp, K.; Clark-Snustad, K.; Saad, K.; Tolentino, E.; Heitkemper, M.; Dey, N.; Gui, S.; Lee, S.D. Correlation of Fecal, Plasma, Serum, and Salivary Calprotectin to Endoscopic and Histologic Outcomes in Patients with Crohn’s Disease. Gastroenterology 2022, 162, S32. [Google Scholar] [CrossRef]
- López-Arjona, M.; Escribano, D.; Mateo, S.V.; Contreras-Aguilar, M.D.; Rubio, C.P.; Tecles, F.; Cerón, J.J.; Martínez-Subiela, S. Changes in Oxytocin Concentrations in Saliva of Pigs after a Transport and during Lairage at Slaughterhouse. Res. Vet. Sci. 2020, 133, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, Y.; Zhao, W.; Chen, J.; Maas, K.; Hussain, N.; Henderson, W.A.; Cong, X. Trends of Fecal Calprotectin Levels and Associations with Early Life Experience in Preterm Infants. Interdiscip. Nurs. Res. 2022, 1, 36–42. [Google Scholar] [CrossRef]
- González-Moret, R.; Cebolla, A.; Cortés, X.; Baños, R.M.; Navarrete, J.; de la Rubia, J.E.; Lisón, J.F.; Soria, J.M. The Effect of a Mindfulness-Based Therapy on Different Biomarkers among Patients with Inflammatory Bowel Disease: A Randomised Controlled Trial. Sci. Rep. 2020, 10, 6071. [Google Scholar] [CrossRef]
- Sauk, J.S.; Ryu, H.J.; Labus, J.S.; Khandadash, A.; Ahdoot, A.I.; Lagishetty, V.; Katzka, W.; Wang, H.; Naliboff, B.; Jacobs, J.P.; et al. High Perceived Stress Is Associated with Increased Risk of Ulcerative Colitis Clinical Flares. Clin. Gastroenterol. Hepatol. 2023, 21, 741–749.e3. [Google Scholar] [CrossRef]
- Oyaert, M.; Boel, A.; Jacobs, J.; Van den Bremt, S.; De Sloovere, M.; Vanpoucke, H.; Van Hoovels, L. Analytical Performance and Diagnostic Accuracy of Six Different Faecal Calprotectin Assays in Inflammatory Bowel Disease. Clin. Chem. Lab. Med. 2017, 55, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Juricic, G.; Brencic, T.; Tesija Kuna, A.; Njegovan, M.; Honovic, L. Faecal Calprotectin Determination: Impact of Preanalytical Sample Treatment and Stool Consistency on within- and between-Method Variability. Biochem. Med. 2019, 29, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Labaere, D.; Smismans, A.; Van Olmen, A.; Christiaens, P.; D’Haens, G.; Moons, V.; Cuyle, P.-J.; Frans, J.; Bossuyt, P. Comparison of Six Different Calprotectin Assays for the Assessment of Inflammatory Bowel Disease. United Eur. Gastroenterol. J. 2014, 2, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Prieto, A.; Tvarijonaviciute, A.; Escribano, D.; Martínez-Subiela, S.; Cerón, J.J. Use of Heterologous Immunoassays for Quantification of Serum Proteins: The Case of Canine C-Reactive Protein. PLoS ONE 2017, 12, e0172188. [Google Scholar] [CrossRef] [PubMed]

| Method | Comparison | Samples | Mean (mg/L) | SD (mg/L) | CV (%) |
|---|---|---|---|---|---|
| Saliva | Intra-assay | High | 1.54 | 0.03 | 2.14 |
| Low | 0.55 | 0.02 | 4.86 | ||
| Inter-assay | High | 1.67 | 0.04 | 4.26 | |
| Low | 0.43 | 0.03 | 6.23 |
| % Analyte | Expected (mg/L) | Observed (mg/L) | Recovery (%) | |
|---|---|---|---|---|
| 100 | 0 | 0.72 | 0.72 | 100 |
| 75 | 25 | 0.66 | 0.60 | 110 |
| 50 | 50 | 0.54 | 0.48 | 112.5 |
| 25 | 75 | 0.42 | 0.36 | 116.7 |
| 0 | 100 | 0.24 | 0.24 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Martínez, M.J.; Martínez-Subiela, S.; Cerón, J.J.; Ortín-Bustillo, A.; Ramis, G.; López-Arjona, M.; Martínez-Miró, S.; Manzanilla, E.G.; Eckersall, P.D.; Tecles, F.; et al. Measurement of Calprotectin (S100A8/A9) in the Saliva of Pigs: Validation Data of A Commercially Available Automated Assay and Changes in Sepsis, Inflammation, and Stress. Animals 2023, 13, 1190. https://doi.org/10.3390/ani13071190
López-Martínez MJ, Martínez-Subiela S, Cerón JJ, Ortín-Bustillo A, Ramis G, López-Arjona M, Martínez-Miró S, Manzanilla EG, Eckersall PD, Tecles F, et al. Measurement of Calprotectin (S100A8/A9) in the Saliva of Pigs: Validation Data of A Commercially Available Automated Assay and Changes in Sepsis, Inflammation, and Stress. Animals. 2023; 13(7):1190. https://doi.org/10.3390/ani13071190
Chicago/Turabian StyleLópez-Martínez, María José, Silvia Martínez-Subiela, José Joaquín Cerón, Alba Ortín-Bustillo, Guillermo Ramis, Marina López-Arjona, Silvia Martínez-Miró, Edgar García Manzanilla, Peter David Eckersall, Fernando Tecles, and et al. 2023. "Measurement of Calprotectin (S100A8/A9) in the Saliva of Pigs: Validation Data of A Commercially Available Automated Assay and Changes in Sepsis, Inflammation, and Stress" Animals 13, no. 7: 1190. https://doi.org/10.3390/ani13071190
APA StyleLópez-Martínez, M. J., Martínez-Subiela, S., Cerón, J. J., Ortín-Bustillo, A., Ramis, G., López-Arjona, M., Martínez-Miró, S., Manzanilla, E. G., Eckersall, P. D., Tecles, F., Escribano, D., & Muñoz-Prieto, A. (2023). Measurement of Calprotectin (S100A8/A9) in the Saliva of Pigs: Validation Data of A Commercially Available Automated Assay and Changes in Sepsis, Inflammation, and Stress. Animals, 13(7), 1190. https://doi.org/10.3390/ani13071190







