Complex Relationships between Milking-Induced Changes in Teat Structures and Their Pre-Milking Dimensions in Holstein Cows
Abstract
Simple Summary
Abstract
1. Introduction
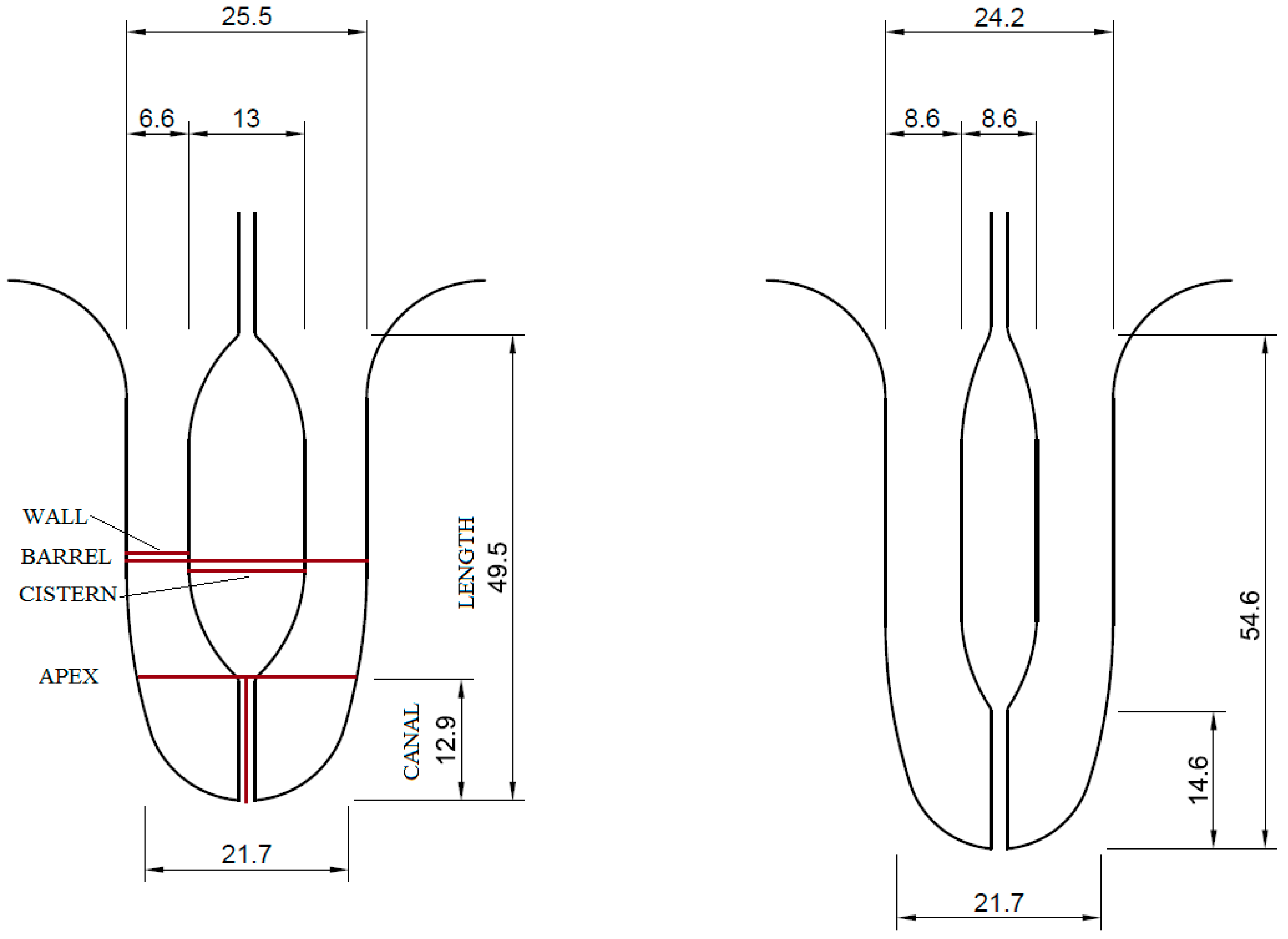
2. Materials and Methods
3. Results
3.1. Basic Statistics
3.2. Significance of Effects in the Model Equation
3.2.1. Effect of Lactation Number, Lactation Stage, and Teat Position
3.2.2. Effect of Teat Length on Milking-Induced Changes
3.2.3. Effect of Teat Thickness at the Barrel on Milking-Induced Changes
3.2.4. Effect of Teat Thickness at the Apex on Milking-Induced Changes
3.2.5. Effect of Teat Cistern Width on Milking-Induced Changes
3.2.6. Effect of Teat Wall Thickness on Milking-Induced Changes
3.2.7. Effect of Teat Canal Length on Milking-Induced Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neijenhuis, F.; Klungel, G.H.; Hogeveen, H. Recovery of cow teats after milking as determined by ultrasonographic scanning. J. Dairy Sci. 2001, 84, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Penry, J.F.; Upton, J.; Mein, G.A.; Rasmussen, M.D.; Ohnstad, I.; Thompson, P.D.; Reinemann, D.J. Estimating teat canal cross-sectional area to determine the effects of teat-end and mouthpiece chamber vacuum on teat congestion. J. Dairy Sci. 2017, 100, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.M.; Heuwieser, W.; Virkler, P.D.; Nydam, D.V.; Wieland, M. Machine milking–induced changes in teat canal dimensions as assessed by ultrasonography. J. Dairy Sci. 2019, 102, 2657–2669. [Google Scholar] [CrossRef]
- Hamann, J.; Mein, G.A. Measurement of machine induced changes in the thickness of the bovine teat. J. Dairy Res. 1990, 57, 495–505. [Google Scholar] [CrossRef]
- Paulrud, C.O.; Clausen, S.; Andersen, P.E.; Rasmussen, M.D. Infrared thermography and ultrasonography to indirectly monitor the influence of liner type and overmilking on teat tissue recovery. Acta Vet. Scand. 2005, 46, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hamann, J.; Nipp, B.; Persson, K. Teat tissue reactions to milking: Changes in blood flow and thickness in the bovine teat. Milchwissenschaft 1994, 49, 243–247. [Google Scholar]
- Besier, J.; Lind, O.; Bruckmaier, R.M. Dynamics of teat-end vacuum during machine milking: Types, causes and impacts on teat condition and udder health—A literature review. J. Appl. Anim. Res. 2016, 44, 263–272. [Google Scholar] [CrossRef]
- Gleeson, D.E.; O’Callaghan, E.J.; Rath, M.V. Effect of liner design, pulsator setting, and vacuum level on bovine teat tissue changes and milking characteristics as measured by ultrasonography. Ir. Vet. J. 2004, 57, 289–296. [Google Scholar] [CrossRef]
- Hamann, J.; Mein, G.A. Teat thickness changes may provide biological test for effective pulsation. J. Dairy Res. 1996, 63, 179–189. [Google Scholar] [CrossRef]
- Wieland, M.; Virkler, P.D.; Borkowski, A.H.; Älveby, N.; Wood, P.; Nydam, D.V. An observational study investigating the association of ultrasonographically assessed machine milking-induced changes in teat condition and teat-end shape in dairy cows. Animal 2019, 13, 341–348. [Google Scholar] [CrossRef]
- Klein, D.; Flock, M.; Khol, J.L.; Franz, S.; Stuger, H.P.; Baumgartner, W. Ultrasonographic measurements of the bovine teat: Breed differences and the significance of the measurements for udder health. J. Dairy Res. 2005, 72, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Bobić, T.; Mijić, P.; Vučković, G.; Gregić, M.; Baban, M.; Gantner, V. Morphological and milkability breed differences of dairy cows. Mljekarstvo 2014, 64, 71–78. Available online: https://hrcak.srce.hr/121600 (accessed on 23 May 2022).
- Tančin, V.; Ipema, B.; Hogewerf, P.; Mačuhová, J. Sources of variation in milk flow characteristics at udder and quarter levels. J. Dairy Sci. 2006, 89, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Němcová, E.; Štípková, M.; Zavadilová, L.; Bouška, J.; Vacek, M. The relationship between somatic cell count, milk production and six linearly scored type traits in Holstein cows. Czech J. Ani. Sci. 2007, 52, 437. [Google Scholar] [CrossRef]
- Guarín, J.F.; Ruegg, P.L. Pre-and postmilking anatomical characteristics of teats and their associations with risk of clinical mastitis in dairy cows. J. Dairy Sci. 2016, 99, 8323–8329. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Weinfurtner, M.; Bruckmaier, R.M. Teat anatomy and its relationship with quarter and udder milk flow characteristics in dairy cows. J. Dairy Sci. 2004, 87, 3280–3289. [Google Scholar] [CrossRef]
- Zwertvaegher, I.; De Vliegher, S.; Verbist, B.; Van Nuffel, A.; Baert, J.; Van Weyenberg, S. Short communication: Associations between teat dimensions and milking-induced changes in teat dimensions and quarter milk somatic cell counts in dairy cows. J. Dairy Sci. 2013, 96, 1075–1080. [Google Scholar] [CrossRef]
- Zecconi, A.; Hamann, J.; Bronzo, V.; Ruffo, G. Machine-induced teat tissue reactions and infection risk in a dairy herd free of contagious mastitis pathogens. J. Dairy Res. 1992, 59, 265–271. [Google Scholar] [CrossRef]
- Wieland, M.; Nydam, D.V.; Älveby, N.; Wood, P.; Virkler, P.D. Teat-end shape and udder-level milking characteristics and their associations with machine milking-induced changes in teat tissue condition. J. Dairy Sci. 2018, 101, 11447–11454. [Google Scholar] [CrossRef]
- Gašparík, M.; Szencziová, I.; Ducháček, J.; Tóthová-Tarová, E.; Stádník, L.; Nagy, M.; Vrhel, M. Supplementary material and dataset for the study complex relations between milking-induced changes in teat structures and their pre-milking dimensions in Holstein cows. Mendeley Data 2022. [Google Scholar] [CrossRef]
- Odorčić, M.; Rasmussen, M.D.; Paulrud, C.O.; Bruckmaier, R.M. Milking machine settings, teat condition and milking efficiency in dairy cows. Animal 2019, 13 (Suppl. S1), 94–99. [Google Scholar] [CrossRef] [PubMed]
- Nørstebø, H.; Rachah, A.; Dalen, G.; Rønningen, O.; Whist, A.C.; Reksen, O. Milk-flow data collected routinely in an automatic milking system: An alternative to milking-time testing in the management of teat-end condition? Acta Vet. Scand. 2018, 60, 2. [Google Scholar] [CrossRef]
- Haeussermann, A.; Britten, J.; Britten, A.; Pahl, C.; Älveby, N.; Hartung, E. Effect of a multi-sided concave liner barrel design on thickness and roughness of teat-end hyperkeratosis. J. Dairy Res. 2016, 83, 188–195. [Google Scholar] [CrossRef]
- Mein, G.; Reinemann, D.; O’Callaghan, E.; Ohnstad, L. Where the rubber meets the teat and what happens to milking characteristics. In IDF Symposium; 100 Years with Liners and Pulsators; IDF: Bruges, Belgium, 2003. [Google Scholar]
- Borkhus, M.; Ronningen, O. Factors affecting mouthpiece chamber vacuum in machine milking. J. Dairy Res. 2003, 70, 283–288. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, E.J. Influence of liner design on interactions of the teat and liner. Ir. J. Agric. Food Res. 2001, 40, 169–176. [Google Scholar]
- Wieland, M.; Shirky, S.; Gioia, G.; Sipka, A.; Virkler, P.D.; Nydam, D.V.; Porter, I.R. Blood perfusion of teat tissue in dairy cows: Changes associated with pre-milking stimulation and machine milking. J. Dairy Sci. 2020, 103, 6588–6599. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, C.; Van der Vekens, E.; Stoffel, M.H.; Schweizer, D.; Bruckmaier, R.M. Increased teat wall thickness in response to machine milking. J. Dairy Sci. 2021, 104, 9082–9092. [Google Scholar] [CrossRef]
- Reinemann, D.J.; Rasmussen, M.D.; Mein, G.A. Instrument requirements and methods for measuring vacuum in milking machines. Trans. ASAE 2001, 44, 975–981. [Google Scholar] [CrossRef]
- Ambord, S.; Bruckmaier, R.M. Milk flow-dependent vacuum loss in high-line milking systems: Effects on milking characteristics and teat tissue condition. J. Dairy Sci. 2010, 93, 3588–3594. [Google Scholar] [CrossRef]
- Szencziová, I.; Strapák, P.; Stádnik, L.; Ducháček, J.; Beran, J. Relationship of udder and teat morphology to milking characteristics and udder health determined by ultrasonographic examinations in dairy cows. Ann. Anim. Sci. 2013, 13, 783–795. [Google Scholar] [CrossRef]
- Strapák, P.; Strapáková, E.; Rušinová, M.; Szencziová, I. The influence of milking in the teat canal of dairy cows determined by ultrasonographic measurements. Czech J. Ani. Sci. 2017, 62, 75–81. [Google Scholar] [CrossRef]
- Pařilová, M.; Stádník, L.; Ježková, A.; Štolc, L. Effect of milking vacuum level and overmilking on cow’s teat characteristics. Acta Univ. Agric. Silvic. Mendel. Brun. 2011, 59, 193–202. [Google Scholar] [CrossRef]
- Edwards, J.P.; O’Brien, B.; Lopez-Villalobos, N.; Jago, J.G. Overmilking causes deterioration in teat-end condition of dairy cows in late lactation. J. Dairy Res. 2013, 80, 344–348. [Google Scholar] [CrossRef] [PubMed]
| Effect | Level | LENGTH% 1 | BARREL% 2 | CISTERN% 3 | WALL% 4 | APEX% 5 | CANAL% 6 |
|---|---|---|---|---|---|---|---|
| Lactation Number | 1 | 12.69 ± 1.039 A | −5.10 ± 0.622 | −38.05 ± 1.865 A | 35.50 ± 2.187 A | 0.58 ± 0.578 | 17.66 ± 1.259 A |
| 2+ | 9.32 ± 0.706 B | −5.22 ± 0.422 | −31.82 ± 1.267 B | 27.34 ± 1.486 B | −0.56 ± 0.393 | 11.43 ± 0.855 B | |
| Lactation Stage | early | 3.25 ± 1.037 A | −6.29 ± 0.621 | −42.65 ± 1.862 A | 40.68 ± 2.184 A | −0.24 ± 0.578 | 20.70 ± 1.257 A |
| mid | 14.28 ± 1.037 B | −4.58 ± 0.621 | −31.60 ± 1.862 B | 25.63 ± 2.184 B | 0.22 ± 0.578 | 10.64 ± 1.257 B | |
| end | 15.47 ± 1.037 B | −4.61 ± 0.621 | −30.56 ± 1.862 B | 27.95 ± 2.184 B | 0.05 ± 0.578 | 12.30 ± 1.257 B | |
| Teat Position | Front | 8.06 ± 0.858 A | −5.32 ± 0.513 | −35.16 ± 1.539 | 27.96 ± 1.805 A | 0.67 ± 0.477 a | 14.20 ± 1.039 |
| Rear | 13.94 ± 0.858 B | −4.99 ± 0.513 | −34.71 ± 1.539 | 34.88 ± 1.805 B | −0.65 ± 0.477 b | 14.89 ± 1.039 |
| Effect | Group (mm) | LENGTH% 1 | BARREL% 2 | CISTERN% 3 | WALL% 4 | APEX% 5 | CANAL% 6 |
|---|---|---|---|---|---|---|---|
| LENGTH 7 | <43.5 Short | 19.83 ± 1.163 A | −3.75 ± 0.859 A | −29.60 ± 2.467 A | 25.13 ± 2.937 A | 0.08 ± 0.785 | 12.22 ± 1.714 A |
| 43.5–52.5 Medium | 7.78 ± 1.081 B | −5.42 ± 0.803 | −39.18 ± 2.296 B | 37.57 ± 2.738 B | 0.37 ± 0.731 | 17.01 ± 1.597 B | |
| >52.5 Long | 1.63 ± 1.390 C | −6.97 ± 0.991 B | −37.67 ± 2.889 | 33.18 ± 3.416 | −0.56 ± 0.914 | 14.96 ± 1.994 | |
| BARREL 8 | <24.2 Thin | 13.42 ± 1.312 A | 0.95 ± 0.675 A | −24.86 ± 2.367 A | 26.46 ± 2.884 A | 3.55 ± 0.736 A | 11.86 ± 1.677 A |
| 24.2–26.5 Medium | 11.32 ± 1.252 | −5.08 ± 0.642 B | −36.92 ± 2.253 B | 31.65 ± 2.745 | −0.48 ± 0.700 B | 13.92 ± 1.596 | |
| >26.5 Thick | 8.65 ± 1.270 B | −10.82 ± 0.658 C | −42.35 ± 2.303 B | 35.66 ± 2.810 B | −2.79 ± 0.717 C | 17.58 ± 1.635 B | |
| APEX 9 | <21 Thin | 12.32 ± 1.242 | −2.33 ± 0.774 A | −29.83 ± 2.351 A | 28.95 ± 2.827 | 3.86 ± 0.703 A | 14.33 ± 1.632 |
| 21–22.5 Medium | 10.34 ± 1.182 | −5.87 ± 0.734 B | −37.81 ± 2.230 B | 33.50 ± 2.681 | −1.51 ± 0.667 B | 14.94 ± 1.548 | |
| >22.5 Thick | 9.62 ± 1.563 | −9.48 ± 0.943 C | −39.67 ± 2.901 B | 32.36 ± 3.468 | −4.81 ± 0.857 C | 14.24 ± 2.003 |
| Effect | Group (mm) | LENGTH% 1 | BARREL% 2 | CISTERN% 3 | WALL% 4 | APEX% 5 | CANAL% 6 |
|---|---|---|---|---|---|---|---|
| CISTERN 7 | <11 Narrow | 14.31 ± 1.426 A | 0.27 ± 0.716 A | −17.06 ± 2.472 A | 13.49 ± 2.921 A | 3.10 ± 0.797 A | 6.48 ± 1.809 A |
| 11–14.5 Medium | 10.81 ± 1.045 | −4.57 ± 0.516 B | −38.24 ± 1.887 B | 33.39 ± 2.222 B | −0.15 ± 0.603 B | 15.00 ± 1.412 B | |
| >14.5 Wide | 8.78 ± 1.351 B | −10.45 ± 0.673 C | −42.79 ± 2.384 B | 41.75 ± 2.813 C | −2.10 ± 0.766 C | 19.99 ± 1.758 C | |
| WALL 8 | <5.7 Thin | 9.77 ± 1.350 | −6.77 ± 0.831 A | −40.21 ± 2.429 A | 50.59 ± 2.498 A | −0.79 ± 0.785 A | 19.77 ± 1.734 A |
| 5.7 to 7 Medium | 11.26 ± 1.122 | −5.96 ± 0.710 A | −39.18 ± 2.056 A | 32.71 ± 2.105 B | −0.56 ± 0.668 A | 15.59 ± 1.492 B | |
| >7 Thick | 11.75 ± 1.263 | −2.73 ± 0.787 B | −24.89 ± 2.291 B | 13.08 ± 2.352 C | 1.44 ± 0.742 B | 8.67 ± 1.647 C | |
| CANAL 9 | <11.8 Short | 8.67 ± 1.900 | −8.76 ± 1.167 A | −45.12 ± 3.461 A | 46.78 ± 3.951 A | 1.15 ± 1.094 | 35.41 ± 2.006 A |
| 11.8–14 Medium | 11.28 ± 1.044 | −5.30 ± 0.731 B | −35.77 ± 2.045 B | 32.17 ± 2.316 B | −0.03 ± 0.656 | 14.73 ± 1.216 B | |
| >14 Long | 11.81 ± 1.423 | −2.69 ± 0.924 C | −27.04 ± 2.673 C | 20.59 ± 3.041 C | −0.59 ± 0.850 | 1.62 ± 1.566 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gašparík, M.; Szencziová, I.; Ducháček, J.; Tóthová Tarová, E.; Stádník, L.; Nagy, M.; Kejdová Rysová, L.; Vrhel, M.; Legarová, V. Complex Relationships between Milking-Induced Changes in Teat Structures and Their Pre-Milking Dimensions in Holstein Cows. Animals 2023, 13, 1085. https://doi.org/10.3390/ani13061085
Gašparík M, Szencziová I, Ducháček J, Tóthová Tarová E, Stádník L, Nagy M, Kejdová Rysová L, Vrhel M, Legarová V. Complex Relationships between Milking-Induced Changes in Teat Structures and Their Pre-Milking Dimensions in Holstein Cows. Animals. 2023; 13(6):1085. https://doi.org/10.3390/ani13061085
Chicago/Turabian StyleGašparík, Matúš, Iveta Szencziová, Jaromír Ducháček, Eva Tóthová Tarová, Luděk Stádník, Melinda Nagy, Lucie Kejdová Rysová, Marek Vrhel, and Veronika Legarová. 2023. "Complex Relationships between Milking-Induced Changes in Teat Structures and Their Pre-Milking Dimensions in Holstein Cows" Animals 13, no. 6: 1085. https://doi.org/10.3390/ani13061085
APA StyleGašparík, M., Szencziová, I., Ducháček, J., Tóthová Tarová, E., Stádník, L., Nagy, M., Kejdová Rysová, L., Vrhel, M., & Legarová, V. (2023). Complex Relationships between Milking-Induced Changes in Teat Structures and Their Pre-Milking Dimensions in Holstein Cows. Animals, 13(6), 1085. https://doi.org/10.3390/ani13061085







