Epigenetics in Canine Mammary Tumors: Upregulation of miR-18a and miR-18b Oncogenes Is Associated with Decreased ERS1 Target mRNA Expression and ERα Immunoexpression in Highly Proliferating Carcinomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mammary Samples and Histopathology
2.2. Immunohistochemistry
2.3. Molecular Investigations
2.3.1. Total RNA Extraction from FFPE Mammary Samples
2.3.2. cDNA Synthesis and Real-Time Quantitative PCR (RT-qPCR) for miRNAs
2.3.3. cDNA Synthesis and RT-qPCR for ESR1 mRNA
2.4. Statistical Analysis
3. Results
3.1. Samples and Immunohistochemistry
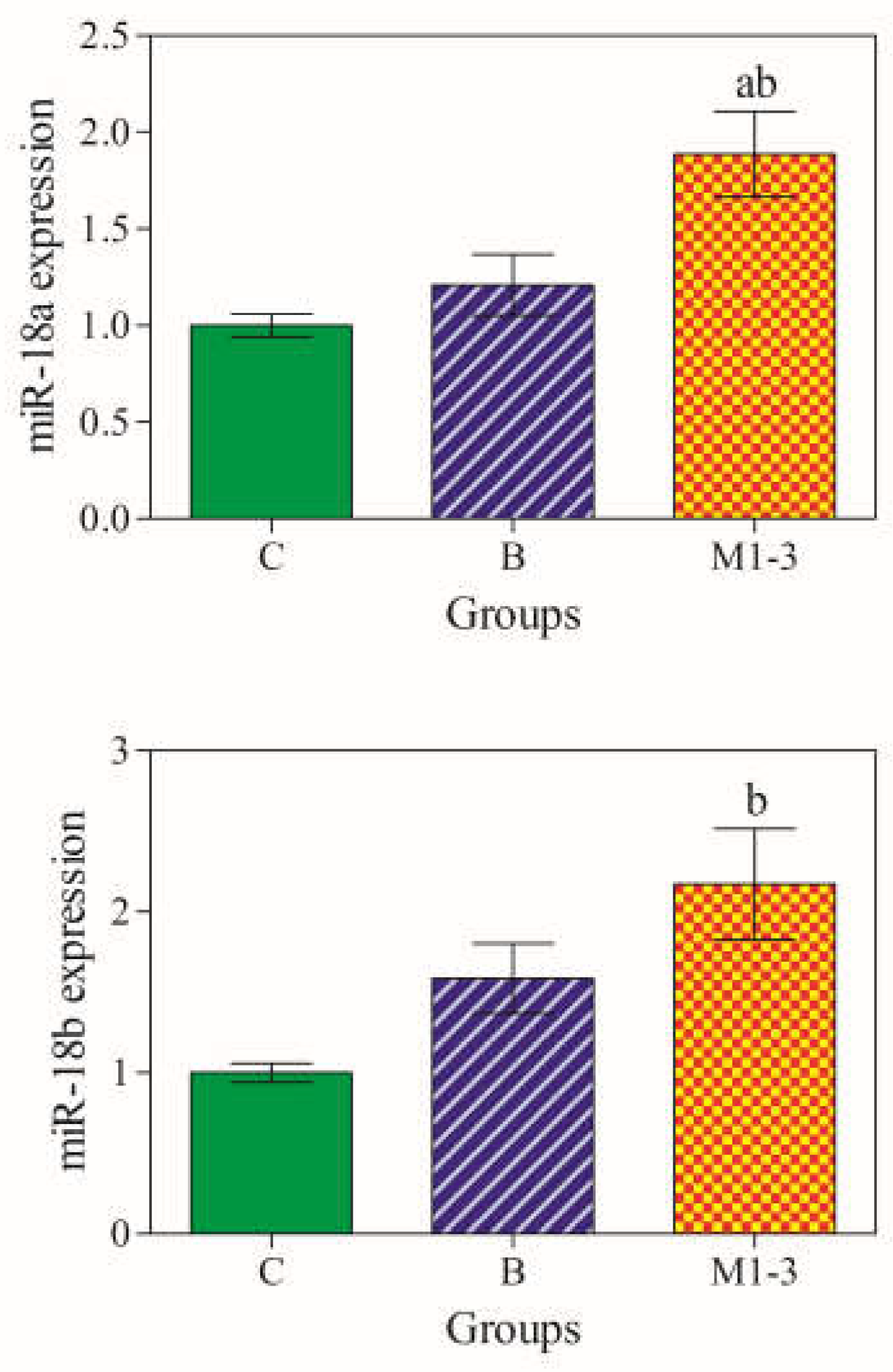
3.2. miR-18a and miR-18b gene Expression Levels
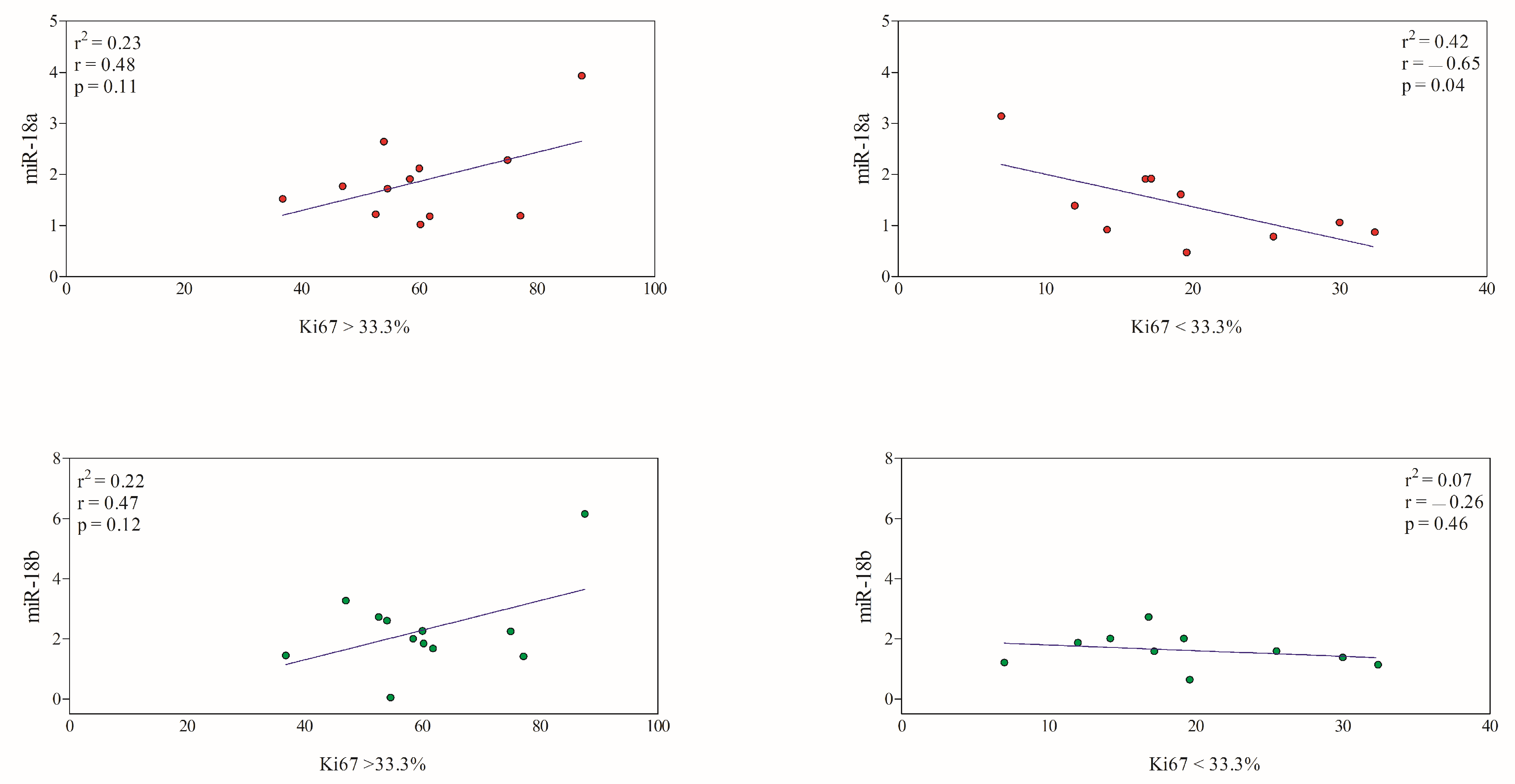
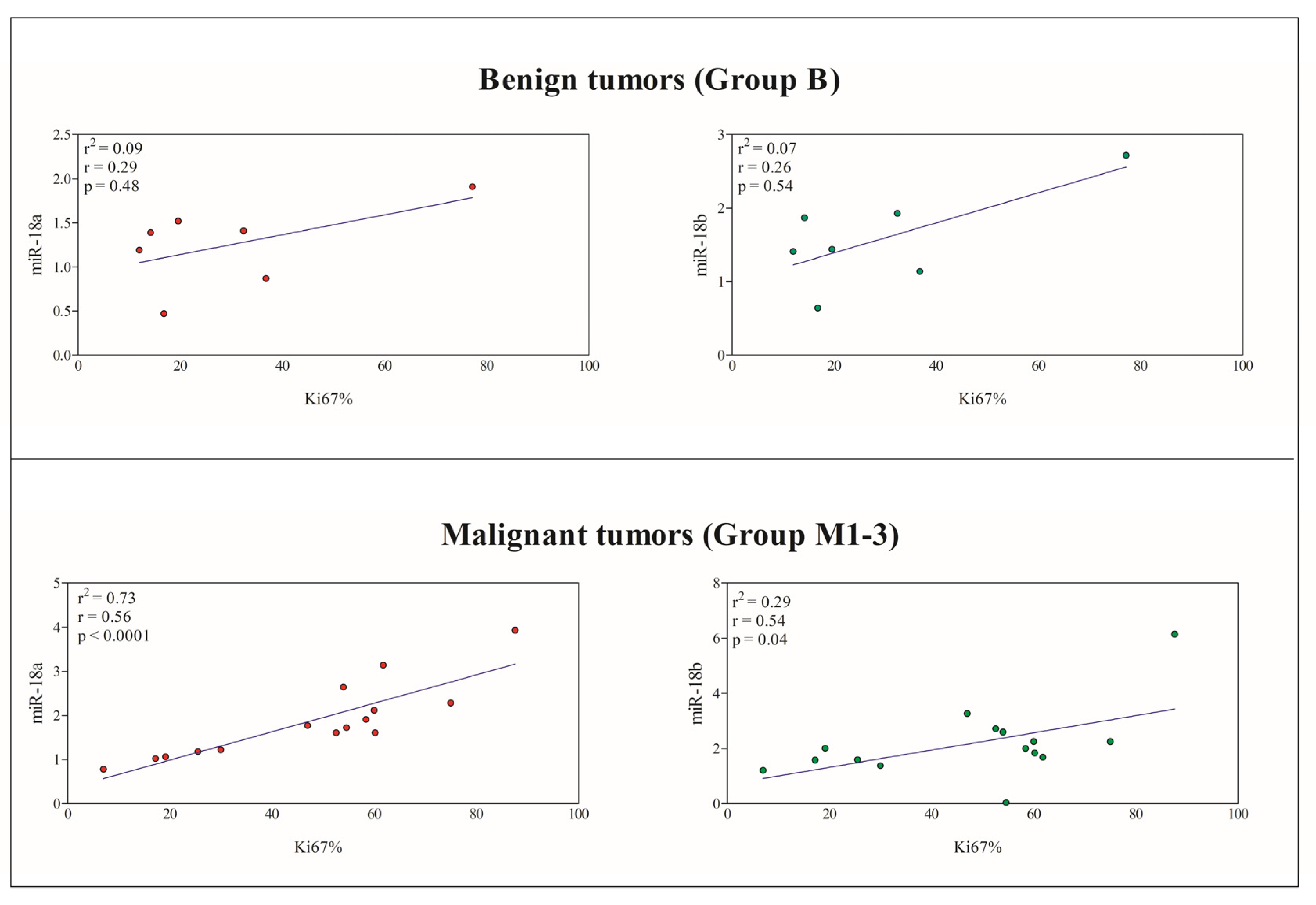
3.3. Correlation between miRNAs and Immunohistochemical Prognostic Factors
3.4. ESR1 Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Yoo, C.B.; Jones, P.A. Epigenetic therapy of cancer: Past, present and future. Nat. Rev. Drug Discov. 2006, 5, 37–50. [Google Scholar] [CrossRef]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N. The mechanisms of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat. Struct. Mol. Biol. 2012, 19, 586–593. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Ramchandran, R.; Chaluvally-Raghavan, P. miRNA-mediated RNA activation in mammalian cells. Adv. Exp. Med. Biol. 2017, 983, 81–89. [Google Scholar] [PubMed]
- Deng, S.; Calin, G.A.; Croce, C.M.; Coukos, G.; Zhang, L. Mechanisms of microRNA deregulation in human cancer. Cell Cycle 2008, 7, 2643–2646. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–277. [Google Scholar] [CrossRef]
- Rahman, M.M.; Brane, A.C.; Tollefsbol, T.O. MicroRNAs and Epigenetic strategies to reverse breast cancer. Cells 2019, 8, 1214. [Google Scholar] [CrossRef]
- Mandujano-Tinoco, E.A.; Garcia-Venzor, A.; Melendez-Zajgla, J.; Maldonado, V. New emerging roles of microRNAs in breast cancer. Breast Cancer Res. Treat. 2018, 171, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Petroušková, P.; Hudáková, N.; Maloveská, M.; Humenik, F.; Cizkova, D. Non-exosomal and Exosome-derived miRNAs as promising biomarkers in canine mammary cancer. Life 2022, 12, 524. [Google Scholar] [CrossRef]
- Kristiansen, V.M.; Peña, L.; Diez Cordova, L.; Illera, J.C.; Skjerve, E.; Breen, A.M.; Cofone, M.A.; Langeland, M.; Teige, J.; Goldschmidt, M.; et al. Effect of ovariohysterectomy at the time of tumor removal in dogs with mammary carcinomas: A randomized controlled trial. J. Vet. Intern. Med. 2016, 30, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, F.L.; Perez-Alenza, M.D.; Silvan, G.; Pena, L.; Lopes, C.; Illera, J.C. Role of steroid hormones and prolactin in canine mammary cancer. J. Steroid Biochem. Mol. Biol. 2005, 94, 181–187. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Shofer, F.S.; Goldschmidt, M.H. Effect of spaying and timing of spaying on survival of dogs with mammary carcinoma. J. Vet. Intern. Med. 2000, 14, 266–270. [Google Scholar] [CrossRef]
- Torres, C.G.; Iturriaga, M.P.; Cruz, P. Hormonal Carcinogenesis in Canine Mammary Cancer. Molecular mechanisms of Estradiol Involved in Malignant Progression. Animals 2021, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Illera, J.C.; Perez-Alenza, M.D.; Nieto, A.; Jimenez, M.A.; Silvan, G.; Dunner, S.; Peña, L. Steroids and receptors in canine mammary cancer. Steroids 2006, 71, 541–548. [Google Scholar] [CrossRef]
- Yager, J.D.; Davidson, N.E. Estrogen Carcinogenesis in Breast Cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Durham, A.C.; Radaelli, E.; Kristiansen, V.; Peña, L.; Goldschmidt, M.H.; Stefanovski, D. The estrogen effect; clinical and histopathological evidence of dichotomous influences in dogs with spontaneous mammary carcinomas. PLoS ONE 2019, 14, e0224504. [Google Scholar] [CrossRef]
- Spoerri, M.; Guscetti, F.; Hartnack, S.; Boos, A.; Oei, C.; Balogh, O.; Nowaczyk, R.M.; Michel, E.; Reichlerand, I.M.; Kowalewski, M.P. Endocrine control of canine mammary neoplasms: Serum reproductive hormone levels and tissue expression of steroid hormone, prolactin and growth hormone receptors. BMC Vet. Res. 2015, 11, 235. [Google Scholar] [CrossRef]
- Brunetti, B.; Bacci, B.; Angeli, C.; Benazzi, C.; Muscatello, L.V. p53, ER and Ki67 Expression in Canine Mammary Carcinomas and Correlation with Pathological variables and prognosis. Vet. Pathol. 2021, 58, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef] [PubMed]
- Caceres, S.; Monsalve, B.; Peña, L.; de Andres, P.J.; Alonso-Diez, A.; Illera, M.J.; Woodward, W.A.; Reuben, J.M.; Silvan, G.; Illera, J.C. In vitro and in vivo effect of flutamide on steroid hormone secretion in canine and human inflammatory breast cancer cell lines. Vet. Comp. Oncol. 2018, 16, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Tsai, M.H.; Liao, J.W.; Chan, J.P.W.; Wong, M.L.; Chang, S.C. Evaluation of hormone receptor expression for use in predicting survival of female dogs with malignant mammary gland tumors. J. Am. Med. Assoc. 2009, 235, 391–396. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, C.C.; Chang, T.J.; Wong, M.L. Prognostic factors associated with survival and two years after surgery in dogs with malignant mammary tumors: 79 cases (1998–2002). J. Am. Vet. Med. Assoc. 2005, 227, 1625–1629. [Google Scholar] [CrossRef]
- de Las Mulas, J.M.; Millan, Y.; Dios, R. A prospective analysis of immunohistochemically determined estrogen receptor alpha and progesterone receptor expression and host and tumor factors as predictors of disease-free period in mammary tumors of the dog. Vet. Pathol. 2005, 42, 200–212. [Google Scholar] [CrossRef]
- de Oliveira Brandão, Y.; Busato Toledo, M.; Chequin, A.; Cristo, T.G.; Sousa, R.S.; Souza Ramos, E.A.; Klassen, G. DNA Methylation Status of the Estrogen Receptor a Gene in Canine Mammary Tumors. Vet. Pathol. 2018, 55, 510–516. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef]
- Kumar, R.; Jin, X.; Zhen, Y.; Hu, P. MicroRNA regulates Estrogen Receptor Alpha in Breast Cancer Metastasis. J. Cancer Res. Therap. Oncol. 2014, 2, 102. [Google Scholar] [CrossRef]
- Abbate, J.M.; Giannetto, A.; Arfuso, F.; Brunetti, B.; Lanteri, G. RT-qPCR Expression Profiles of selected oncogenic and oncosuppressor miRNAs in formalin-fixed, paraffin-embedded Canine Mammary Tumors. Animals 2022, 12, 2898. [Google Scholar] [CrossRef]
- Fish, E.J.; Irizarry, K.J.; Delnnocentes, P.; Ellis, C.J.; Prasad, N.; Moss, A.G.; Bird, R.C. Malignant canine mammary epithelial cells shed exosomes containing differentially expressed microRNA that regulate oncogenic networks. BMC Cancer 2018, 18, 832. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.J.; Martinez-Romero, E.S.; Delnnocentes, P.; Koehler, J.W.; Prasad, N.; Smith, A.N.; Curt Bird, R. Circulating microRNA as biomarkers of canine mammary carcinoma in dogs. J. Vet. Intern. Med. 2020, 34, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; Peña, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L. Surgical Pathology of Tumors of Domestic Animals Volume 2: Mammary Tumors; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Washington, DC, USA, 2019; Volume 2. [Google Scholar]
- Peña, L.; De Andres, P.J.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: Relationship with clinical and histological characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.; Gama, A.; Goldschmidt, M.H.; Abadie, J.; Benazzi, C.; Castagnaro, M.; Díez, L.; Gärtner, F.; Hellmén, E.; Kiupel, M.; et al. Canine Mammary Tumors: A Review and Consensus of Standard Guidelines on Epithelial and Myoepithelial Phenotype Markers, HER2, and Hormone Receptor Assessment using Immunohistochemistry. Vet. Pathol. 2014, 51, 127–145. [Google Scholar] [CrossRef]
- Ferreira, E.; Gobbi, H.; Saraiva, B.S.; Cassali, G.D. Histological and immunohistochemical identification of atypical ductal mammary hyperplasia as a preneoplastic marker in dogs. Vet. Pathol. 2012, 49, 322–329. [Google Scholar] [CrossRef]
- Nguyen, F.; Peña, L.; Ibisch, C.; Loussouarn, D.; Gama, A.; Rieder, N.; Belousov, A.; Campone, M.; Abadie, J. Canine invasive mammary carcinomas as models of human breast cancer. Part 1: Natural history and prognostic factors. Breast Cancer Res. Treat. 2018, 167, 635–648. [Google Scholar] [CrossRef]
- Bulkowska, M.; Rybicka, A.; Senses, K.M.; Ulewicz, K.; Witt, K.; Szymanska, J.; Taciak, B.; Klopfleisch, R.; Hellmén, E.; Dolka, I.; et al. MicroRNA expression patterns in canine mammary cancer show significant differences between metastatic and non-metastatic tumors. BMC Cancer 2017, 17, 728. [Google Scholar] [CrossRef]
- Brinkhof, B.; Spee, B.; Rothuizen, J.; Penning, L.C. Development and evaluation of canine reference genes for accurate quantification of gene expression. Anal. Biochem. 2006, 356, 36–43. [Google Scholar] [CrossRef]
- Etschmann, B.; Wilcken, B.; Stoevesand, K.; von der Schulenburg, A.; Sterner-Kock, A. Selection of Reference Genes for Quantitative Real-Time PCR Analysis in Canine Mammary Tumors using the GeNorm Algorithm. Vet. Pathol. 2006, 43, 934–942. [Google Scholar] [CrossRef]
- Lopez-Tarruella, S.; Schiff, R. The dynamics of estrogen receptor status in breast cancer: Re-shaping the paradigm. Clin. Cancer Res. 2007, 13, 6921–6925. [Google Scholar] [CrossRef]
- Borge, K.S.; Melin, M.; Rivera, P.; Thoresen, S.I.; Webster, M.T.; von Euler, H.; Lindblad-Toh, K.; Lingaas, F. The ESR1 gene is associated with risk for canine mammary tumors. BMC Vet. Res. 2013, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Lüder Ripoli, F.; Hammer, S.C.; Willenbrock, S.; Hewicker-Trautwein, M.; Kielbowicz, Z.; Murua Escobar, H.; Nolte, I. Hormone receptor expression analyses in neoplastic and nonneoplastic canine mammary tissue by a bead based multiplex branched DNA Assay: A gene expression study in fresh frozen and formalin-fixed, paraffin-embedded samples. PLoS ONE 2016, 11, e0163311. [Google Scholar] [CrossRef] [PubMed]
- Lamp, O.; Honscha, K.U.; Schweizer, S.; Heckmann, A.; Blaschzik, S.; Einspanier, A. The metastatic potential of canine mammary tumours can be assessed by mRNA expression analysis of connective tissue modulators. Vet. Comp. Oncol. 2013, 11, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Klose, P.; Gruber, A.D. The combined expression pattern of BMP2, LTBP4, and DERL1 discriminates malignant from benign canine mammary tumors. Vet. Pathol. 2010, 47, 446–454. [Google Scholar] [CrossRef]
- Nieto, A.; Peña, L.; Perez-Alenza, M.D.; Sanchez, M.A.; Flores, J.M.; Castano, M. Immunohistologic detection of estrogen receptor alpha in canine mammary tumors: Clinical and pathologic associations and prognostic significance. Vet. Pathol. 2000, 37, 239–247. [Google Scholar] [CrossRef]
- Ljepoja, B.; Garcia-Roman, J.; Sommer, A.K.; Wagner, E.; Roidl, A. MiRNA-27a sensitizes breast cancer cells to treatment with selective estrogen receptor modulators. Breast 2019, 43, 31–38. [Google Scholar] [CrossRef]
- Luengo-Gil, G.; Garcia-Martinez, E.; Chaves-Benito, A.; Conesa-Zamora, P.; Navarro-Manzano, E.; Gonzalez-Billalabeitia, E.; Garcia-Garre, E.; Martinez-Carrasco, A.; Vicente, V.; Ayala de la Peña, F. Clinical and biological impact of miR-18a expression in breast cancer after neoadjuvant chemotherapy. Cell. Oncol. 2019, 42, 627–644. [Google Scholar] [CrossRef]
- Kleivi Sahlberg, K.; Bottai, G.; Naume, B.; Burwinkel, B.; Calin, G.A.; Børresen-Dale, A.L.; Santarpia, L. A serum microRNA signature predicts tumor relapse and survival in triple-negative breast cancer patients. Clin. Cancer Res. 2015, 21, 1207–1214. [Google Scholar] [CrossRef]
- Leivonen, S.K.; Mäkelä, R.; Ostling, P.; Kohonen, P.; Haapa-Paananen, S.; Kleivi, K.; Enerly, E.; Aakula, A.; Hellström, K.; Sahlberg, N.; et al. Protein lysate microarray analysis to identify microRNAs regulating estrogen receptor signaling in breast cancer cell lines. Oncogene 2009, 28, 3926–3936. [Google Scholar] [CrossRef]
- Egeland, N.G.; Jonsdottir, K.; Aure, M.R.; Sahlberg, K.; Kristensen, V.N.; Cronin-Fenton, D.; Skaland, I.; Gudlaugsson, E.; Baak, J.P.A.; Janssen, E.A.M. MiR-18a and miR-18b are expressed in the stroma of oestrogen receptor alpha negative breast cancers. BMC Cancer 2020, 20, 377. [Google Scholar] [CrossRef]
- Haldosen, L.A.; Zhao, C.; Dahlman-Wright, K. Estrogen receptor beta in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, D.A.; Santana, A.E.; Cury, P.M.; Cordeiro, J.A. Immunohistochemical study of Ki-67 as a prognostic marker in canine mammary neoplasia. Vet. Clin. Pathol. 2004, 33, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Sarli, G.; Preziosi, R.; Benazzi, C.; Castellani, G.; Marcato, P.S. Prognostic value of histological stage and proliferative activity in canine malignant mammary tumors. J. Vet. Diagn. Investig. 2002, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
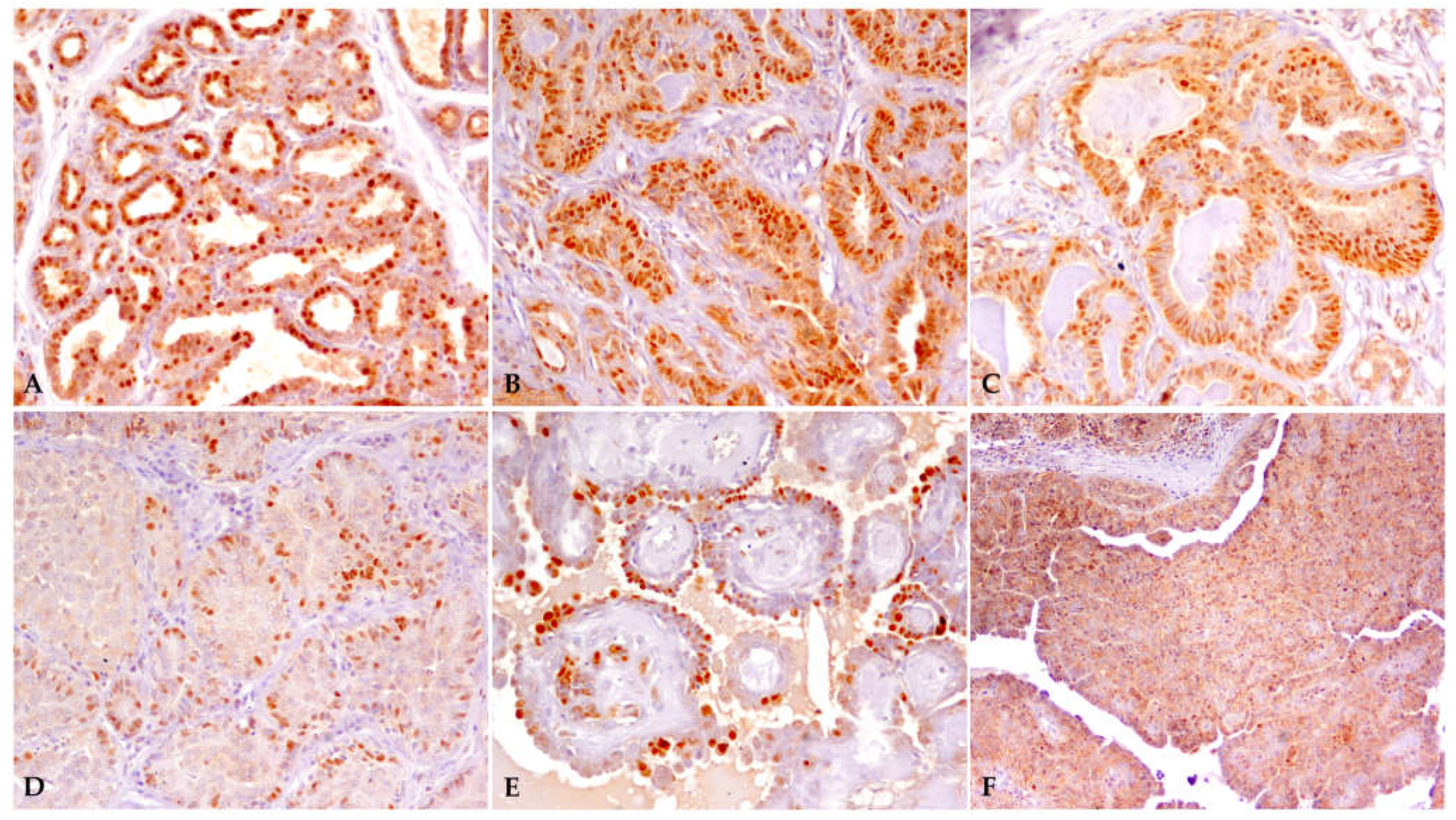
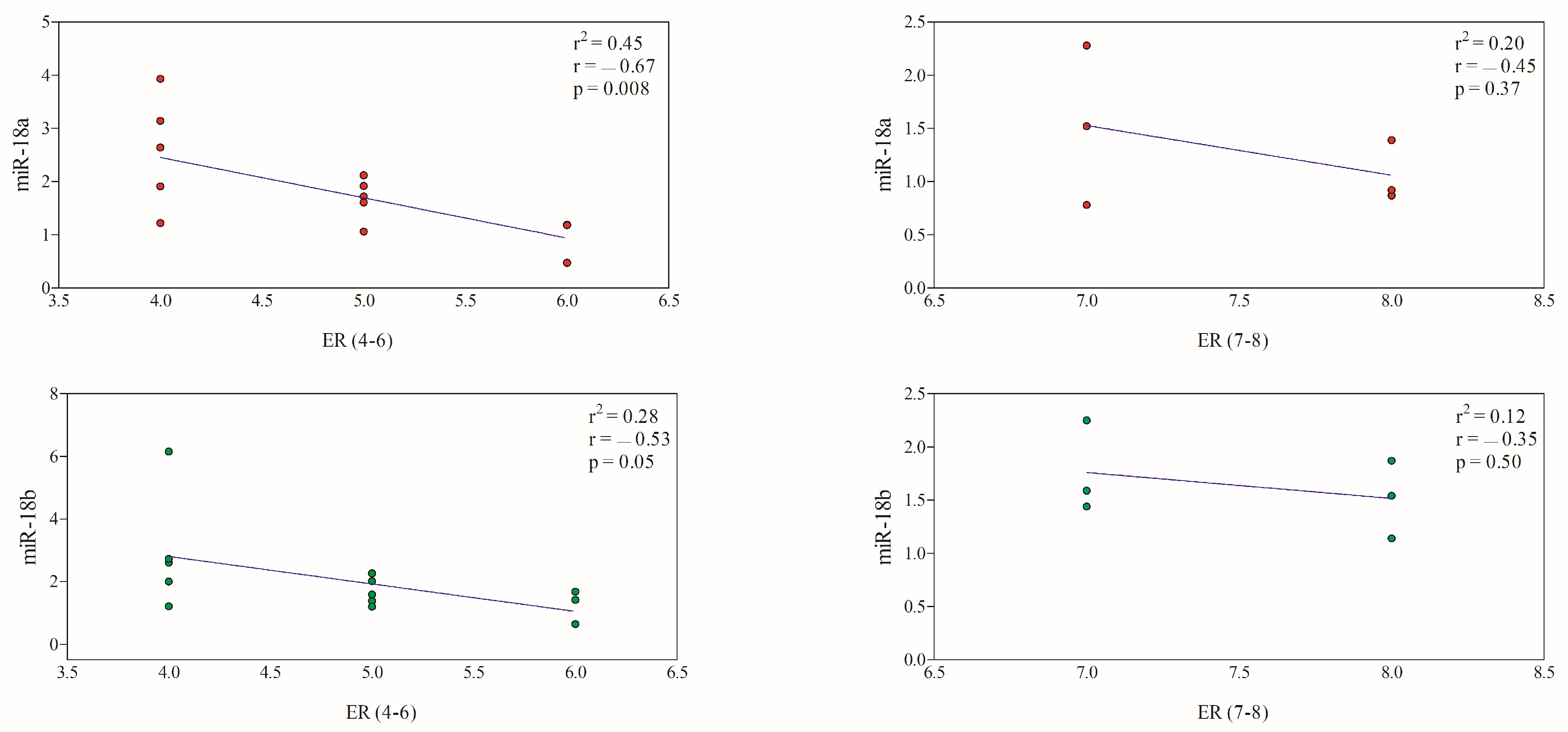

| Percentage of Cell Labeling | Score | Intensity of Labeling | Score |
|---|---|---|---|
| No labeling | 0 | Absent | 0 |
| <1% | 1 | Weak | 1 |
| 1–10% | 2 | Moderate | 2 |
| 10–33% | 3 | Strong | 3 |
| 33–66% | 4 | ||
| 66–100% | 5 |
| Gene | Forward Primer | Reverse Primer | Accession No. |
|---|---|---|---|
| Estrogen receptor 1 (ESR1) | AGCTCCTCCTCATCCTCTCC | AGGTCGTAGAGAGGCACCAC | |
| Ribosomal protein S19 (RPS19) | CCTTCCTCAAAAA/GTCTGGG | GTTCTCATCGTAGGGAGCAAG | XM_533657 |
| Hypoxanthine phosphoribosyltransferase 1 (HPRT) | TGCTCGAGATGATGAAGG | TCCCCTTGACTGGTCATT | NM_000194 |
| Samples | Histological Classification | n | Grade of Malignancy | Lymphatic Invasion | TS | Ki67 Index (%) |
|---|---|---|---|---|---|---|
| Controls (n = 4) | Normal Mammary Gland | 3 | 8 | - | ||
| Lobular Hyperplasia | 1 | 8 | 52.8 | |||
| Benign Tumors (n = 7) | Tubular Adenoma | 1 | 8 | 12 | ||
| Tubulopapillary Adenoma | 1 | 8 | 14.6 | |||
| Complex Adenoma | 1 1 | 8 7 | 32.4 36.8 | |||
| Intraductal Papillary Adenoma | 1 1 | 6 4 | 77.2 16.8 | |||
| Benign Mixed Tumor | 1 | 6 | 19.6 | |||
| Malignant Tumors (n = 15) | Tubular Carcinoma | 1 1 | I III | Yes | 5 5 | 60 30 |
| Tubulopapillary Carcinoma | 1 | I | 7 | 25.5 | ||
| Complex Carcinoma | 1 1 1 | I I I | 4 5 5 | 58.4 54.6 17.2 | ||
| Intraductal Papillary Carcinoma | 1 1 | I I | 2 * 5 | 47 19.2 | ||
| Mixed Carcinoma | 1 1 | I II | 4 7 | 7 75 | ||
| Solid Carcinoma | 1 1 1 1 | II III III III | 4 4 6 3 * | 54 52.6 61.8 60.2 | ||
| Inflammatory Carcinoma | 1 | III | Yes | 4 | 87.6 |
| ER Score | n | miR-18a | miR-18b |
|---|---|---|---|
| 4–6 | 14 | 1.86 ± 0.90 | 2.03 ± 1.41 |
| 7–8 | 6 | 1.29 ± 0.57 | 1.64 ± 0.38 |
| Ki-67 Index | |||
| <33.3% (19.39 ± 7.93) | 10 | 1.41 ± 0.78 | 1.62 ± 0.58 |
| >33.3% (60.43 ± 13.86) | 12 | 1.88 ± 0.82 | 2.31 ± 1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbate, J.M.; Arfuso, F.; Riolo, K.; Capparucci, F.; Brunetti, B.; Lanteri, G. Epigenetics in Canine Mammary Tumors: Upregulation of miR-18a and miR-18b Oncogenes Is Associated with Decreased ERS1 Target mRNA Expression and ERα Immunoexpression in Highly Proliferating Carcinomas. Animals 2023, 13, 1086. https://doi.org/10.3390/ani13061086
Abbate JM, Arfuso F, Riolo K, Capparucci F, Brunetti B, Lanteri G. Epigenetics in Canine Mammary Tumors: Upregulation of miR-18a and miR-18b Oncogenes Is Associated with Decreased ERS1 Target mRNA Expression and ERα Immunoexpression in Highly Proliferating Carcinomas. Animals. 2023; 13(6):1086. https://doi.org/10.3390/ani13061086
Chicago/Turabian StyleAbbate, Jessica Maria, Francesca Arfuso, Kristian Riolo, Fabiano Capparucci, Barbara Brunetti, and Giovanni Lanteri. 2023. "Epigenetics in Canine Mammary Tumors: Upregulation of miR-18a and miR-18b Oncogenes Is Associated with Decreased ERS1 Target mRNA Expression and ERα Immunoexpression in Highly Proliferating Carcinomas" Animals 13, no. 6: 1086. https://doi.org/10.3390/ani13061086
APA StyleAbbate, J. M., Arfuso, F., Riolo, K., Capparucci, F., Brunetti, B., & Lanteri, G. (2023). Epigenetics in Canine Mammary Tumors: Upregulation of miR-18a and miR-18b Oncogenes Is Associated with Decreased ERS1 Target mRNA Expression and ERα Immunoexpression in Highly Proliferating Carcinomas. Animals, 13(6), 1086. https://doi.org/10.3390/ani13061086







