Enhancing Milk Production by Nutrient Supplements: Strategies and Regulatory Pathways
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Enhancement of Milk Production by Amino Acids and Peptides
2.1. Amino Acid
2.1.1. Methionine
2.1.2. Leucine
2.1.3. Valine
2.1.4. Lysine
2.1.5. Other Amino Acids
| Items | Treatments | Functions | Potential Signaling Pathways | References |
|---|---|---|---|---|
| Amino acid (+) | Dairy cow mammary epithelial cells | Cell growth ↑ Casein synthesis ↑ | Septin6-mTOR signaling pathway | [20] |
| Essential amino acids (+) | Bovine mammary epithelial cells | Cell proliferation ↑ β-casein production ↑ | SARS- mTOR signaling pathway | [22] |
| Amino acid (+) | Bovine mammary epithelial cells | Milk protein ↑ Fat synthesis ↑ | GlyRS-mTOR signaling pathway | [21] |
| Essential amino acids (+) | Bovine mammary epithelial cell line, mammary tissue explants | β-casein expression ↑ | mTOR signaling | [23] |
| Methionine (+) | Cow mammary gland tissue | Milk protein synthesis ↑ | AKT phosphorylation | [24] |
| Methionine (+) | Bovine mammary epithelial cells (0.6 mmol/L) | Milk protein ↑ Fat synthesis ↑ Cell proliferation ↑ | SNAT2-PI3K signaling pathway | [27] |
| Methionine (+) | Bovine mammary epithelial cells (0.6 mmol/L) | Cell growth ↑ β-casein synthesis ↑ ASCT2 expression ↑ | ASCT2/SARS/mTOR signaling pathway | [28] |
| Methionine (+) | Bovine mammary epithelial cells | Lipid droplet formation ↑ β-casein ↑ | FABP5-SREBP-1c signaling pathway | [29] |
| Methionine (+) | Lactating Saanen goats | Milk production ↑ Milk protein ↑ | —— | [32] |
| Methionine (+) | Holstein cows | Milk yield ↑ | —— | [33] |
| Methionine (+) | Holstein cows | Milk production ↑ Milk protein ↑ | —— | [34] |
| Methionine (+) | Holstein cows | Milk protein production ↑ | —— | [35] |
| Methionine (+) | Holstein cows | Milk production ↑ | —— | [36] |
| Leucine (+) | Bovine mammary epithelial cells (0.75 mmol/L) | Milk protein ↑ Milk fat synthesis ↑ | PI3K-DDX59 signaling pathway | [40] |
| Leucine (+) | Cow mammary epithelial cells | Cell growth ↑ Casein synthesis ↑ | GNG12-mTORC1 signaling pathway | [41] |
| Leucine and methionine (+) | Bovine mammary epithelial cells | Milk Fat ↑ | mTOR-CRTC2-SREBP-1c signaling pathway | [69] |
| Leucine and methionine (+) | Bovine mammary epithelial cells | mTOR phosphorylation ↑ β-casein synthesis ↑ | NCOA5- PI3K-mTOR signaling pathway | [31] |
| Leucine and methionine (+) | Bovine mammary epithelial cells | Milk synthesis ↑ Cell proliferation ↑ | AnxA2 PI3K-mTOR-SREBP-1c/Cyclin D1 signaling pathway | [70] |
| Leucine and methionine (+) | Bovine mammary epithelial cells | β-casein, triglycerides, Lactose synthesis ↑ Cell viability ↑ Cell proliferation ↑ | mTOR-SREBP-1c signaling pathway | [71] |
| Leucine and methionine (+) | Bovine mammary epithelial cells | Milk protein ↑ Milk fat ↑ Cell proliferation ↑ | GRP78-mTOR signaling pathway | [30] |
| Leucine, acetate, and their interaction (+) | Bovine mammary epithelial cells (1.8 mmol/L Leucine or 8–10 mmol/L acetate) | Milk protein ↑ | JACK2/STAT5, mTOR, and AMPK pathway | [42] |
| Valine | Primiparous gilts (total lysine: lysine = 0.93:1) | Milk fat synthesis ↑ | —— | [45] |
| L-Valine (+) | Porcine mammary epithelial cells (0.5 mmol/L) | Cell numbers ↑ Protein synthesis ↑ | mTOR and Ras/ERK signaling pathways | [46] |
| Valine (+) | Porcine mammary epithelial cells | Fatty acids synthesis ↑ Intracellular triacylglycerol content ↑ | AKT-mTOR-SREBP1 signaling pathway | [47] |
| Valine (+) | Holstein cows | Milk production ↑ | —— | [49] |
| Branched-chain amino acids (+) | Multiparous sows (Yorkshire × Landrace) | Milk production ↑ | —— | [50] |
| Lysine (+) | Bovine mammary epithelial cells (0.70 mmol/L) | Cells numbers ↑ Milk fat synthesis ↑ | GPRC6A-PI3K-FABP5 signaling pathway | [51] |
| Lysine (+) | Bovine mammary epithelial cells (1.0 mmol/L) | Protein synthesis ↑ | ATB0,+,mTOR and JAK2-STAT5 pathways | [52] |
| Lysine (+) | Bovine mammary epithelial cells (0.70 mmol/L) | β-casein synthesis ↑ | SLC6A14-ERK-CDK1-mTOR signaling pathway | [53] |
| Lysine (+) | Mouse mammary epithelial cells | Cell proliferation ↑ | PI3K/AKT/mTOR signal axis | [54] |
| Lysine, methionine (+) | Bovine mammary epithelial cells (Lys/Met ratio = 3:1, 1.2 mmol/L Lys, 0.4 mmol/L Met) | Casein biosynthesis ↑ | JAK2/ELF5, mTOR, and its downstream RPS6KB1 and EIF4EBP1 signaling | [55] |
| Methionine and arginine (+) | Bovine mammary epithelial cells | α-s1-casein abundance ↑ | mTOR signaling; AMPK pathways | [25] |
| Leucine or arginine (-) | Mid-lactation Holstein cows (5 day continuous Leucine-163 g/d; arginine-158 g/d) | Milk yield ↓ Milk protein yield ↓ | —— | [72] |
| N-carbamoylglutamate (+) | Holstein cows (20 g/d per cow, n = 15) | Milk production ↑ | —— | [56] |
| 5-aminolevulinic acid (+) | Holstein cows (10 mg/kg per cow) | Milk protein ↑; Milk casein contents ↑ | —— | [65] |
| Glutamine (+) | Lactating sows (Large White) (1%) | Milk yield ↑ | —— | [66] |
| Taurine (+) | Bovine mammary epithelial cells | Milk protein ↑ Fat synthesis ↑ mTOR phosphorylation ↑ SREBP-1c expression ↑ | GPR87-PI3K-SETD1A signaling pathway | [62] |
| Leucine and histidine (+) | Immortalized bovine mammary epithelial cell (0.45–10.80 mmol/L Leucine 0.15–9.60 mmol/L histidine | Milk protein ↑ | mTOR signaling pathway | [59] |
| Histidine, lysine, methionine, leucine | Immortalized bovine mammary epithelial cell line (His: Lys: Met: Leu = 5:6:1:7) | β-casein expression ↑ | mTOR signaling pathway | [57] |
| Threonine, isoleucine, valine, leucine | Immortalized bovine mammary epithelial cell line (Lysine: valine = 1.12:1 when Lysine: methionine is ideal) | β-casein expression ↑ | mTOR signaling pathway | [58] |
| Rumen-protected-methionine, lysine, histidine (+) | Holstein cows | Milk production ↑ | —— | [68] |
| Rumen-protected gamma-aminobutyric acid | Holstein dairy cows | Milk protein yield ↑ | —— | [67] |
2.2. Peptides
3. The Enhancement of Milk Production by Lipids
| Items | Treatments | Functions | Potential Signaling Pathways | References |
|---|---|---|---|---|
| Acetate (+) | Bovine mammary epithelial cells (6 mmol/L) | Milk fat synthesis ↑ Cell proliferation ↑ | mTOR/eIF4E signaling pathway | [97] |
| Acetate, β-hydroxybutyrate and their interaction (+) | Dairy cow mammary epithelial cells | Triglyceride contents ↑ Lipid droplet formation ↑ | SREBP1 signaling | [98] |
| Long Chain Fatty Acids (+) | Goat mammary epithelial cells | PPARG expression ↑ | —— | [99] |
| Branched-chain volatile fatty acids (+) | Chinese Holstein cows (60 g BCVFA per cow per day) | Milk fat synthesis ↑ | —— | [102] |
| β-sitosterol (+) | Bovine mammary epithelial cells (0.1 to 10 μmol/L) | β-casein synthesis ↑ | JAK2/STAT5 and mTOR signaling pathways | [103] |
| Oleic acid, stearic acid, and palmitic acid (+) | Dairy cow mammary epithelial cells | Triglyceride contents ↑ | FABP3-SREBP1/PPARG signaling | [100] |
| Oleic acid, linoleic acid, and linolenic acid | Bovine mammary epithelial cells (The ratio is 2:13.3:1) | Fat and protein synthesis ↑ | —— | [101] |
| Palmitic acid (+) | Holstein cows | Milk production ↑ Milk fat ↑ | —— | [102] |
| Palmitic acid, n-6/n-3 fatty acids (+) | Holstein cows | Milk production ↑ | —— | [103] |
| Linseed (+) | Cilentana dairy goats (20%) | Milk production ↑ | —— | [108] |
| Linseed (+) | Italian Friesian dairy cows (700 g/head/d) | Milk production ↑ | —— | [107] |
| Soybean, flaxseed oils (+) | Anglo-Nubian goats | Milk production ↑ | —— | [109] |
| Linseed, verbascoside, vitamin E (+) | Lacaune ewes | Milk production ↑ | —— | [106] |
| Rapeseed oil (+) | Holstein cows | Milk production ↑ | —— | [110] |
| Fish oil (+) | Polish holstein-friesian cows | Milk production ↑ | —— | [112] |
| Fish oil (+) | Holstein cows | Milk production ↑ | —— | [111] |
| Microalgae Schizochytrium spp. (+) | Crossbred dairy ewes [Lacaune x Local (Greek) breed] | Improves milks’ fatty acid profile | —— | [113] |
| Schizochytrium limacinum marine algae (+) | Multiparous Alpine goats | DHA and rumenic acid concentration↑ | —— | [114] |
| Docosahexaenoic acid-rich microalgae (+) | Holstein cows | Improves milks’ fatty acid profile | —— | [115] |
4. The Enhancement of Milk Production by Carbohydrates
5. The Enhancement of Milk Production by Other Chemicals
| Items | Treatments | Functions | Potential Signaling Pathways | References |
|---|---|---|---|---|
| Daidzein (+) | Primary bovine mammary epithelial cells (20 µmol/L) | α- and β-casein ↑ Lipid synthesis ↑ Cell amount ↑ | ERα-dependent NFƘB1 signaling | [125] |
| Polyphenols from lentisk ethanolic extract (+) | Bovine mammary epithelial cells | Lactose synthesis ↑ Secretion of whey proteins ↑ Casein contents ↑ | —— | [126] |
| Prolactin (+) | Bovine mammary epithelial cells | Milk protein synthesis ↑ | LAT1 signaling | [129] |
| Prolactin (+) | Bovine mammary epithelial cells | Milk protein synthesis ↑ Tudor-SN expression ↑ | —— | [131] |
| Estrogen (+) | Bovine mammary epithelial cells | Milk fat synthesis ↑ | FABP5/SREBP-1c signaling | [29] |
| Estrogen or prolactin (+) | Bovine mammary epithelial cells | Milk synthesis ↑ Cell proliferation ↑ | PI3K-mTOR-SREBP-1c/Cyclin D1 signaling pathway | [70] |
| Estrogen and prolactin (+) | Bovine mammary epithelial cells | Milk protein synthesis ↑ Milk fat synthesis ↑ Cell proliferation ↑ | GRP78/mTOR signaling pathway | [30] |
| Prolactin and β-estradiol (+) | Bovine mammary epithelial cells | β-casein synthesis ↑ Triglyceride synthesis ↑, Lactose synthesis ↑; Cell proliferation ↑ | U2AF65/mTOR-SREBP-1c signaling pathway | [71] |
| Prolactin and epidermal growth factor (+) | Mouse mammary epithelial cell line HC11 | β-casein ↑ | PI3K/Akt/mTOR signaling pathways | [133] |
| Sodium butyrate (+) | Bovine mammary epithelial cells | Milk fat synthesis ↑ | GPR41/AMPK/mTOR/S6K- SREBP1signaling pathway | [134] |
| Camellia seed oil (+) | Differentiated bovine mammary epithelial cells | β-casein ↑ | PI3K-mTOR-S6K1 and JAK2-STAT5 signaling pathways | [137] |
| All-trans retinoic acid (+) | Bovine mammary epithelial cells | Casein synthesis ↑ Fatty acid composition ↑ | JAK2/STAT5 pathway and downstream mTOR signaling pathway | [138] |
| Folic acid (+) | Lactating cows (120 mg/500 kg per cow) | Milk production ↑ | —— | [139] |
| Grape seed proanthocyanidin extract (+) | Holstein dairy cattle (20 mg GSPE/kg of body weight/day) | Milk yield ↑ | —— | [140] |
| Lutein (+) | Lactating Holstein cows | Milk lactose synthesis ↑ | —— | [141] |
6. STRING Database Analysis
7. Conclusion and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| PI3K | Phosphatidylinositol-3 Kinase |
| PKB | Protein kinase B |
| mTOR | Mammalian target of rapamycin |
| S6K1 | Ribosomal protein S6 kinase 1 |
| 4EBP1 | 4e-binding protein 1 |
| mTORC1 | mTOR complex 1 |
| SARS | Seryl-tRNA synthetase |
| GlyRS | Glycyl-tRNA synthetase |
| BMECs | Bovine mammary epithelial cells |
| SNAT2 | Sodium-coupled neutral amino acid transporter 2 |
| ASCT2 | Amino-acid transporter 2 |
| SREBP1 | Sterol response element-binding protein 1 |
| FABP5 | Fatty acid binding protein 5 |
| NCOA5 | Nuclear receptor co-activator 5 |
| GRP78 | Glucose-regulated protein 78 |
| LAT1 | L-type amino acid transporter 1 |
| GATOR1 | GAP activity towards Rags 1 |
| GATOR2 | GAP activity towards Rags 2 |
| DDX59 | DEAD-Box Helicase 59 |
| GNG12 | Guanine nucleotide-binding protein subunit gamma-12 |
| JAK2 | Janus kinase 2 |
| STAT5 | Signal transducers and activators of transcription 5 |
| ERK | Extracellular signal-regulated kinase |
| MAPK | Mitogen-activated protein kinase |
| GPRC6A | G protein-coupled receptor family C group 6 member A |
| ELF5 | E74 Like ETS Transcription Factor 5) |
| RPS6KB1 | Ribosomal protein S6 kinase B1 |
| EIF4EBP1 | Eukaryotic translation initiation factor 4E binding protein 1 |
| NCG | N-carbamoylglutamate |
| 5-ALA | 5-aminolevulinic acid |
| FOXO1 | Forkhead Box O1 |
| GPCRs | G protein-coupled receptors |
| SETD1A | SET domain containing 1A |
| SLC15 | Proton-coupled oligopeptide transporter family |
| AG | Acylated ghrelin |
| UAG | Unacylated ghrelin |
| MECs | Mammary epithelial cells |
| PPARG | Peroxisome proliferators-activated receptor gamma |
| FABP | Fatty acid binding protein |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| AMPK | Adenosine 5‘-monophosphate (AMP)-activated protein kinase |
| Tudor - SN | Tudor staphylococcal nuclease |
| U2AF65 | U2 snRNP auxiliary factor 65 kDa |
| AnxA2 | Annexin A2 |
| EGF | Epidermal growth factor |
| GPR41 | G protein-coupled receptor41 |
| GSPE | Grape seed proanthocyanidin extract |
| FFAR3 | Free fatty acid receptor 3 |
References
- FAO. Dairy Market Review: Emerging Trends and Outlook. Available online: https://www.fao.org/3/cb7982en/cb7982en.pdf (accessed on 16 December 2021).
- Smith, N.W.; Fletcher, A.J.; Hill, J.P.; McNabb, W.C. Modeling the Contribution of Milk to Global Nutrition. Front. Nutr. 2021, 8, 716100. [Google Scholar] [CrossRef] [PubMed]
- FAO. OECD-FAO Agricultural Outlook 2021–2030. Available online: https://www.fao.org/3/cb5332en/Dairy.pdf (accessed on 2 September 2021).
- Hegde, N.G. Livestock Development for Sustainable Livelihood of Small Farmers. Asian J. Res. Anim. Vet. Sci. 2019, 3, 1–17. [Google Scholar]
- Miglior, F.; Fleming, A.; Malchiodi, F.; Brito, L.F.; Martin, P.; Baes, C.F. A 100-Year Review: Identification and genetic selection of economically important traits in dairy cattle. J. Dairy Sci. 2017, 100, 10251–10271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, A.; Bhattacharyya, S. Artificial insemination for milk production in India: A statistical insight. Indian J. Anim. Sci. 2020, 90, 1186–1190. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Dahl, G.E. MILK Symposium Introduction: Dairy production in developing countries. J. Dairy Sci. 2020, 103, 9677–9680. [Google Scholar] [CrossRef]
- Jones, G.M.; Pearson, R.E.; Clabaugh, G.A. Relationships between somatic cell counts and milk production. J. Dairy Sci. 1984, 67, 1823–1831. [Google Scholar] [CrossRef]
- Otto, P.I.; Guimaraes, S.E.F.; Calus, M.P.L.; Vandenplas, J.; Machado, M.A.; Panetto, J.C.C.; da Silva, M. Single-step genome-wide association studies (GWAS) and post-GWAS analyses to identify genomic regions and candidate genes for milk yield in Brazilian Girolando cattle. J. Dairy Sci. 2020, 103, 10347–10360. [Google Scholar] [CrossRef]
- Teissier, M.; Sanchez, M.P.; Boussaha, M.; Barbat, A.; Hoze, C.; Robert-Granie, C.; Croiseau, P. Use of meta-analyses and joint analyses to select variants in whole genome sequences for genomic evaluation: An application in milk production of French dairy cattle breeds. J. Dairy Sci. 2018, 101, 3126–3139. [Google Scholar] [CrossRef] [Green Version]
- Sexton, F. Average Milk Production of a Holstein Cow Per Day in India. Available online: https://fatinsl.info (accessed on 4 January 2020).
- Tricarico, J.M.; Kebreab, E.; Wattiaux, M.A. MILK Symposium review: Sustainability of dairy production and consumption in low-income countries with emphasis on productivity and environmental impact. J. Dairy Sci. 2020, 103, 9791–9802. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. New functions for amino acids: Effects on gene transcription and translation. Am. J. Clin. Nutr. 2006, 83, 500S–507S. [Google Scholar] [CrossRef] [Green Version]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Kandel, E.S.; Hay, N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 2000, 253, 210–229. [Google Scholar] [CrossRef]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [Green Version]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Basang, Z.; Hu, L.; Liu, L.; Jiang, N. Septin6 regulates cell growth and casein synthesis in dairy cow mammary epithelial cells via mTORC1 pathway. J. Dairy Res. 2019, 86, 181–187. [Google Scholar] [CrossRef]
- Luo, C.; Qi, H.; Huang, X.; Li, M.; Zhang, L.; Lin, Y.; Gao, X. GlyRS is a new mediator of amino acid-induced milk synthesis in bovine mammary epithelial cells. J. Cell. Physiol. 2019, 234, 2973–2983. [Google Scholar] [CrossRef]
- Dai, W.T.; White, R.R.; Liu, J.X.; Liu, H.Y. Seryl-tRNA synthetase-mediated essential amino acids regulate beta-casein synthesis via cell proliferation and mammalian target of rapamycin (mTOR) signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2018, 101, 10456–10468. [Google Scholar] [CrossRef] [Green Version]
- Li, S.S.; Loor, J.J.; Liu, H.Y.; Liu, L.; Hosseini, A.; Zhao, W.S.; Liu, J.X. Optimal ratios of essential amino acids stimulate beta-casein synthesis via activation of the mammalian target of rapamycin signaling pathway in MAC-T cells and bovine mammary tissue explants. J. Dairy Sci. 2017, 100, 6676–6688. [Google Scholar] [CrossRef]
- Ma, Y.F.; Batistel, F.; Xu, T.L.; Han, L.Q.; Bucktrout, R.; Liang, Y.; Coleman, D.N.; Parys, C.; Loor, J.J. Phosphorylation of AKT serine/threonine kinase and abundance of milk protein synthesis gene networks in mammary tissue in response to supply of methionine in periparturient Holstein cows. J. Dairy Sci. 2019, 102, 4264–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Chen, Y.; Cortes, I.M.; Coleman, D.N.; Dai, H.; Liang, Y.; Parys, C.; Fernandez, C.; Wang, M.; Loor, J.J. Supply of methionine and arginine alters phosphorylation of mechanistic target of rapamycin (mTOR), circadian clock proteins, and α-s1-casein abundance in bovine mammary epithelial cells. Food Funct. 2020, 11, 883–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.T.; Zhao, F.Q.; Liu, J.X.; Liu, H.Y. ASCT2 Is Involved in SARS-Mediated beta-Casein Synthesis of Bovine Mammary Epithelial Cells with Methionine Supply. J. Agric. Food Chem. 2020, 68, 13038–13045. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Meng, C.; Jin, X.; Li, X.; Li, P.; Gao, X. Methionine Promotes Milk Protein and Fat Synthesis and Cell Proliferation via the SNAT2-PI3K Signaling Pathway in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2018, 66, 11027–11033. [Google Scholar] [CrossRef]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yu, M.; Zhou, C.; Qi, H.; Wen, X.; Hou, X.; Li, M.; Gao, X. FABP5 is a critical regulator of methionine- and estrogen-induced SREBP-1c gene expression in bovine mammary epithelial cells. J. Cell. Physiol. 2018, 234, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, X.; Zhen, Z.; Yu, Y.; Qiu, Y.; Xiang, W. GRP78 regulates milk biosynthesis and the proliferation of bovinemammaryepithelial cells through the mTOR signaling pathway. Cell. Mol. Biol. Lett. 2019, 24, 57. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Zhang, L.; Cui, Y.; Yu, Y.; Gao, X.; Ao, J. NCOA5 is a master regulator of amino acid-induced mTOR activation and beta-casein synthesis in bovine mammary epithelial cells. Biochem. Biophys. Res. Commun. 2020, 529, 569–574. [Google Scholar] [CrossRef]
- Flores, A.; Mendoza, G.; Pinos-Rodriguez, J.M.; Plata, F.; Vega, S.; Bárcena, R. Effects of rumen-protected methionine on milk production of dairy goats. Ital. J. Anim. Sci. 2016, 8, 271–275. [Google Scholar] [CrossRef]
- Sen, E.; Orman, A.; Kara, C.; Turkmen, I.I.; Cetin, I. Improved Lactational Performance in Dairy Cows Supplemented with Methionine or Rumen-Protected Choline During the Transition Period. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 2. [Google Scholar]
- King, L.; Wickramasinghe, J.; Dooley, B.; McCarthy, C.; Branstad, E.; Grilli, E.; Baumgard, L.; Appuhamy, R. Effects of Microencapsulated Methionine on Milk Production and Manure Nitrogen Excretions of Lactating Dairy Cows. Animals 2021, 11, 3545. [Google Scholar] [CrossRef]
- Toledo, M.Z.; Stangaferro, M.L.; Gennari, R.S.; Barletta, R.V.; Perez, M.M.; Wijma, R.; Sitko, E.M.; Granados, G.; Masello, M.; Van Amburgh, M.E.; et al. Effects of feeding rumen-protected methionine pre- and postpartum in multiparous Holstein cows: Lactation performance and plasma amino acid concentrations. J. Dairy Sci. 2021, 104, 7583–7603. [Google Scholar] [CrossRef]
- Park, J.K.; Yeo, J.M.; Bae, G.S.; Kim, E.J.; Kim, C.H. Effects of supplementing limiting amino acids on milk production in dairy cows consuming a corn grain and soybean meal-based diet. J. Anim. Sci. Technol. 2020, 62, 485–494. [Google Scholar] [CrossRef]
- Dodd, K.M.; Tee, A.R. Leucine and mTORC1: A complex relationship. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1329–E1342. [Google Scholar] [CrossRef]
- Saxton, R.A.; Knockenhauer, K.E.; Wolfson, R.L.; Chantranupong, L.; Pacold, M.E.; Wang, T.; Schwartz, T.U.; Sabatini, D.M. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 2016, 351, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, X.; Liu, Y.; Su, Z.; Dawar, F.U.; Dan, H.; He, Y.; Gui, J.F.; Mei, J. Leucine mediates autophagosome-lysosome fusion and improves sperm motility by activating the PI3K/Akt pathway. Oncotarget 2017, 8, 111807–111818. [Google Scholar] [CrossRef]
- Qiu, Y.; Qu, B.; Zhen, Z.; Yuan, X.; Zhang, L.; Zhang, M. Leucine Promotes Milk Synthesis in Bovine Mammary Epithelial Cells via the PI3K-DDX59 Signaling. J. Agric. Food Chem. 2019, 67, 8884–8895. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, S.; Dai, W.; Zheng, N.; Wang, J. Proteomic analyses reveal GNG12 regulates cell growth and casein synthesis by activating the Leu-mediated mTORC1 signaling pathway. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 1092–1101. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Yan, S.M.; Chen, L.; Shi, B.L.; Guo, X.Y. Effect of interaction between leucine and acetate on the milk protein synthesis in bovine mammary epithelial cells. Anim. Sci. J. 2019, 90, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Qi, Y.G.; Zhang, X.L.; Wu, Z.H.; Chen, J.M.; Chen, F.; Guan, W.T.; Zhang, S.H. Regulation of the JAK2-STAT5 Pathway by Signaling Molecules in the Mammary Gland. Front. Cell Dev. Biol. 2020, 8, 604896. [Google Scholar] [CrossRef]
- Wagner, K.U.; Rui, H. Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland. Biol. 2008, 13, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Xu, M.; Gao, K.; Wang, L.; Yang, X.; Wen, X.; Xiao, H.; Li, M.; Jiang, Z. Mammary tissue proteomics in a pig model indicates that dietary valine supplementation increases milk fat content via increased de novo synthesis of fatty acid. Food Sci. Nutr. 2021, 9, 6213–6223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, W.; Yi, D.; Zhao, D.; Song, Z.; Hou, Y.; Wu, G. Regulation of protein synthesis in porcine mammary epithelial cells by L-valine. Amino. Acids 2019, 51, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Xu, M.; Gao, K.; Zhu, C.; Wang, L.; Yang, X.; Wen, X.; Xiao, H.; Jiang, Z.; Wu, D. Valine increases milk fat synthesis in mammary gland of gilts through stimulating AKT/MTOR/SREBP1 pathwaydagger. Biol. Reprod. 2019, 101, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Shahbazian, D.; Vu, H.; Holz, M.K.; Cohen, M.S.; Taunton, J.; Sonenberg, N.; Blenis, J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J. Biol. Chem. 2007, 282, 14056–14064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hultquist, K.M.; Casper, D.P. Effects of feeding rumen-degradable valine on milk production in late-lactating dairy cows. J. Dairy Sci. 2016, 99, 1201–1215. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, R.; Gabriel, A.S.; Wu, G. Dietary supplementation with branched-chain amino acids enhances milk production by lactating sows and the growth of suckling piglets. J Anim. Sci. Biotechnol. 2022, 13, 65. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Wang, L.; Zhang, M.; Gao, X. Lysine Enhances the Stimulation of Fatty Acids on Milk Fat Synthesis via the GPRC6A-PI3K-FABP5 Signaling in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2019, 67, 7005–7015. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Zou, Y.; Zhao, F.Q.; Liu, J.; Liu, H. Lysine Stimulates Protein Synthesis by Promoting the Expression of ATB0,+ and Activating the mTOR Pathway in Bovine Mammary Epithelial Cells. J. Nutr. 2018, 148, 1426–1433. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Hu, G.; Li, W.; Wang, J.; Ge, Y.; Li, F.; Guo, W.; Kan, X.; Fu, S.; Liu, J. Lysine promotes proliferation and beta-casein synthesis through the SLC6A14-ERK1/2-CDK1-mTOR signaling pathway in bovine primary mammary epithelial cells. J. Therm. Biol. 2022, 110, 103375. [Google Scholar] [CrossRef]
- Li, W.; Long, X.Y.; Li, F.; Cao, Y.; Liu, J.X.; Fu, S.P.; Guo, W.J.; Hu, G.Q. Lysine stimulates the development of the murine mammary gland at puberty via PI3K/AKT/mTOR signalling axis. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1420–1430. [Google Scholar] [CrossRef]
- Wang, F.; van Baal, J.; Ma, L.; Loor, J.J.; Wu, Z.L.; Dijkstra, J.; Bu, D.P. Short communication: Relationship between lysine/methionine ratios and glucose levels and their effects on casein synthesis via activation of the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2019, 102, 8127–8133. [Google Scholar] [CrossRef]
- Gu, F.; Miao, C.; Jiang, L.; Wang, D.; Liu, H.; Liu, J. Dietary supplementation with N-carbamoylglutamate initiated from the prepartum stage improves lactation performance of postpartum dairy cows. Anim. Nutr. 2021, 7, 232–238. [Google Scholar] [CrossRef]
- Gao, H.N.; Zhao, S.G.; Zheng, N.; Zhang, Y.D.; Wang, S.S.; Zhou, X.Q.; Wang, J.Q. Combination of histidine, lysine, methionine, and leucine promotes beta-casein synthesis via the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2017, 100, 7696–7709. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, Z.; Wang, L.; Saremi, B.; Helmbrecht, A.; Wang, Z.; Loor, J.J. Increasing the availability of threonine, isoleucine, valine, and leucine relative to lysine while maintaining an ideal ratio of lysine:methionine alters mammary cellular metabolites, mammalian target of rapamycin signaling, and gene transcription. J. Dairy Sci. 2018, 101, 5502–5514. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.N.; Hu, H.; Zheng, N.; Wang, J.Q. Leucine and histidine independently regulate milk protein synthesis in bovine mammary epithelial cells via mTOR signaling pathway. J. Zhejiang Univ. Sci. B 2015, 16, 560–572. [Google Scholar] [CrossRef] [Green Version]
- Inam, U.L.; Piao, F.; Aadil, R.M.; Suleman, R.; Li, K.; Zhang, M.; Wu, P.; Shahbaz, M.; Ahmed, Z. Ameliorative effects of taurine against diabetes: A review. Amino Acids 2018, 50, 487–502. [Google Scholar] [CrossRef]
- Solon, C.S.; Franci, D.; Ignacio-Souza, L.M.; Romanatto, T.; Roman, E.A.; Arruda, A.P.; Morari, J.; Torsoni, A.S.; Carneiro, E.M.; Velloso, L.A. Taurine enhances the anorexigenic effects of insulin in the hypothalamus of rats. Amino Acids 2012, 42, 2403–2410. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Y.; Wang, Z.; Liu, Y.; Yu, Y.; Gao, X. Taurine promotes milk Synthesis via the GPR87-PI3K-SETD1A Signaling in BMECs. J. Agric. Food Chem. 2019, 67, 1927–1936. [Google Scholar] [CrossRef]
- Wauson, E.M.; Zaganjor, E.; Lee, A.Y.; Guerra, M.L.; Ghosh, A.B.; Bookout, A.L.; Chambers, C.P.; Jivan, A.; McGlynn, K.; Hutchison, M.R.; et al. The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol. Cell 2012, 47, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Melick, C.H.; Lama-Sherpa, T.D.; Curukovic, A.; Jewell, J.L. G-Protein Coupled Receptor Signaling and Mammalian Target of Rapamycin Complex 1 Regulation. Mol. Pharmacol. 2022, 101, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, A.O.; Shirai, M.; Takeya, H.; Sugimura, S.; Miyanari, S.; Taniguchi, S.; Sato, K. Effects of 5-aminolevulinic acid supplementation on milk production, iron status, and immune response of dairy cows. J. Dairy Sci. 2019, 102, 11009–11015. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Qin, J.F.; Wang, L.; Gao, K.G.; Zheng, C.T.; Huang, L.; Jiang, Z.Y. Improved Milk Glutamine Level and Growth Performance of Suckling Piglets by Glutamine Supplementation in Maternal Diet. Ann. Anim. Sci. 2018, 18, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.M.; Liu, H.Y.; Wang, C.; Liu, J.X.; Ferguson, J.D. Effects of rumen-protected gamma-aminobutyric acid on feed intake, performance and antioxidative status in transition cows. Livest. Sci. 2013, 153, 66–72. [Google Scholar] [CrossRef]
- Giallongo, F.; Harper, M.T.; Oh, J.; Lopes, J.C.; Lapierre, H.; Patton, R.A.; Parys, C.; Shinzato, I.; Hristov, A.N. Effects of rumen-protected methionine, lysine, and histidine on lactation performance of dairy cows. J. Dairy Sci. 2016, 99, 4437–4452. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhou, C.J.; Li, X.Y.; Yu, M.M.; Li, M.; Gao, X.J. CRTC2 Is a Key Mediator of Amino Acid-Induced Milk Fat Synthesis in Mammary Epithelial Cells. J. Agric. Food Chem. 2019, 67, 10513–10520. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, D.; Zhen, Z.; Ao, J.; Yuan, X.; Gao, X. Annexin A2 positively regulates milk synthesis and proliferation of bovine mammary epithelial cells through the mTOR signaling pathway. J. Cell. Physiol. 2018, 233, 2464–2475. [Google Scholar] [CrossRef]
- Yu, Y.; Zhen, Z.; Qi, H.; Yuan, X.; Gao, X.; Zhang, M. U2AF65 enhances milk synthesis and growth of bovine mammary epithelial cells by positively regulating the mTOR-SREBP-1c signalling pathway. Cell Biochem. Funct. 2019, 37, 93–101. [Google Scholar] [CrossRef]
- Tian, W.; Wu, T.; Zhao, R.; Xu, J.; He, Y.; Wang, H. Responses of milk production of dairy cows to jugular infusions of a mixture of essential amino acids with or without exclusion leucine or arginine. Anim. Nutr. 2017, 3, 271–275. [Google Scholar] [CrossRef]
- Dicou, E. Biologically active, non membrane-anchored precursors: An overview. FEBS J. 2008, 275, 1960–1975. [Google Scholar] [CrossRef]
- Smith, D.E.; Clemencon, B.; Hediger, M.A. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol. Asp. Med. 2013, 34, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Shennan, D.B.; Boyd, C.A. The functional and molecular entities underlying amino acid and peptide transport by the mammary gland under different physiological and pathological conditions. J. Mammary Gland. Biol. 2014, 19, 19–33. [Google Scholar] [CrossRef]
- Nishi, Y.; Yoh, J.; Hiejima, H.; Kojima, M. Structures and molecular forms of the ghrelin-family peptides. Peptides 2011, 32, 2175–2182. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, G.; Huang, Y.; Xu, D.; Ren, J.; Jiang, L.; Wu, C.; Tong, D. Expression of ghrelin and GHSR-1a in mammary glands of dairy goat during the lactation and the effects of gherlin on regulation of mammary function in vitro. Mol. Cell. Endocrinol. 2013, 370, 20–31. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Lv, Q.; Zhang, C.; Xu, S.; Yang, D.; Huang, B.; Zeng, Y.; Gao, Y.; Wang, W. AG and UAG induce β-casein expression via activation of ERK1/2 and AKT pathways. J. Mol. Endocrinol. 2016, 56, 213–225. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006, 58, 621–631. [Google Scholar] [CrossRef]
- Ulasov, I.V.; Borovjagin, A.V.; Timashev, P.; Cristofanili, M.; Welch, D.R. KISS1 in breast cancer progression and autophagy. Cancer Metastasis Rev. 2019, 38, 493–506. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.; Huang, B.; Kan, X.; Chen, G.; Wang, W.; Fu, S. Kisspeptin-10 Induces beta-Casein Synthesis via GPR54 and Its Downstream Signaling Pathways in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2017, 18, 2621. [Google Scholar] [CrossRef] [Green Version]
- Papaoiconomou, E.; Lymperi, M.; Petraki, C.; Philippou, A.; Msaouel, P.; Michalopoulou, P.; Kafiri, G.; Vassilakos, G.; Zografos, G.; Koutsilieris, M. Kiss-1/GPR54 Protein Expression in Breast Cancer. Anticancer Res. 2014, 34, 1401–1407. [Google Scholar]
- Goertzen, C.G.; Dragan, M.; Turley, E.; Babwah, A.V.; Bhattacharya, M. KISS1R signaling promotes invadopodia formation in human breast cancer cell via beta-arrestin2/ERK. Cell. Signal. 2016, 28, 165–176. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, F.; Liu, J.; Liu, H. Dipeptide (Methionyl-Methionine) Transport and Its Effect on beta-Casein Synthesis in Bovine Mammary Epithelial Cells. Cell. Physiol. Biochem. 2018, 49, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.M.; Wu, Y.M.; Liu, H.Y.; Zhao, K.; Liu, J.X. Effects of tripeptides and lactogenic hormones on oligopeptide transporter 2 in bovine mammary gland. J. Anim. Physiol. Anim. Nutr. 2011, 95, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, X.; Guo, C.; Du, R.; Ailun, G.; Ao, C.; Gao, M. Identification and characterization of bovine mammary peptide transporters in response to tripeptide and lactogenic hormone treatment. Czech J. Food Sci. 2017, 62, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.N.; Wan, P.; Chen, H.; Chen, X.; Sun, H.L.; Pan, J.Y. Identification of octopus peptide and its promotion of beta-casein synthesis in a mouse mammary epithelial cell line. J. Food Biochem. 2020, 44, e13467. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enteral Nutr. 2015, 39, 18s–32s. [Google Scholar] [CrossRef]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Li, N.; Zhao, F.; Wei, C.; Liang, M.; Zhang, N.; Wang, C.; Li, Q.Z.; Gao, X.J. Function of SREBP1 in the milk fat synthesis of dairy cow mammary epithelial cells. Int. J. Mol. Sci. 2014, 15, 16998–17013. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.F.; Luo, J.; Zhao, W.S.; Yang, Y.C.; Tian, H.B.; Shi, H.B.; Bionaz, M. Overexpression of SREBP1 (sterol regulatory element binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J. Dairy Sci. 2016, 99, 783–795. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Jiang, Q.; Ding, W.; Yue, P.; Wang, J.; Zhao, K.; Zhang, H. Interleukin 4 inhibits high mobility group box-1 protein-mediated NLRP3 inflammasome formation by activating peroxisome proliferator-activated receptor-gamma in astrocytes. Biochem. Biophys. Res. Commun. 2019, 509, 624–631. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Orekhov, A.N. Peroxisome Proliferator-Activated Receptor (PPAR) Gamma Agonists as Therapeutic Agents for Cardiovascular Disorders: Focus on Atherosclerosis. Curr. Pharm. Des. 2017, 23, 1119–1124. [Google Scholar] [CrossRef]
- Skat-Rordam, J.; Hojland Ipsen, D.; Lykkesfeldt, J.; Tveden-Nyborg, P. A role of peroxisome proliferator-activated receptor gamma in non-alcoholic fatty liver disease. Basic Clin. Pharmacol. Toxicol. 2019, 124, 528–537. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef] [Green Version]
- Chmurzynska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, X.; Yan, S.; Shi, B.; Sheng, R. Acetate regulates milk fat synthesis through the mammalian target of rapamycin/eukaryotic initiation factor 4E signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2021, 104, 337–345. [Google Scholar] [CrossRef]
- Ali, I.; Li, C.; Li, L.; Kuang, M.; Shafiq, M.; Wang, Y.; Yang, M.; Wang, G. Effect of acetate, beta-hydroxybutyrate and their interaction on lipogenic gene expression, triglyceride contents and lipid droplet formation in dairy cow mammary epithelial cells. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 66–75. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Zhao, W.; Bionaz, M.; Luo, J.; Loor, J.J. Nutrigenomic effect of saturated and unsaturated long chain fatty acids on lipid-related genes in goat mammary epithelial cells: What is the role of PPARγ? J. Vet. Sci. 2019, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.Y.; Hou, X.M.; Qu, B.; Zhang, N.; Li, N.; Cui, Y.J.; Li, Q.Z.; Gao, X.J. Functional analysis of FABP3 in the milk fat synthesis signaling pathway of dairy cow mammary epithelial cells. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 865–873. [Google Scholar] [CrossRef]
- Sheng, R.; Yan, S.M.; Qi, L.Z.; Zhao, Y.L. Effect of the ratios of unsaturated fatty acids on the expressions of genes related to fat and protein in the bovine mammary epithelial cells. Vitr. Cell. Dev. Biol. Anim. 2015, 51, 381–389. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Guo, G.; Huo, W.J.; Zhang, S.L.; Pei, C.X.; Zhang, Y.L.; Wang, H. Effects of branched-chain volatile fatty acids on lactation performance and mRNA expression of genes related to fatty acid synthesis in mammary gland of dairy cows. Animal 2018, 12, 2071–2079. [Google Scholar] [CrossRef]
- Liu, X.; Shen, J.; Zong, J.; Liu, J.; Jin, Y. Beta-Sitosterol Promotes Milk Protein and Fat Syntheses-Related Genes in Bovine Mammary Epithelial Cells. Animals 2021, 11, 3238. [Google Scholar] [CrossRef]
- Rico, J.E.; Allen, M.S.; Lock, A.L. Compared with stearic acid, palmitic acid increased the yield of milk fat and improved feed efficiency across production level of cows. J. Dairy Sci. 2014, 97, 1057–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, E.; Golabadi, D.; Piadeh, A. Effect of supplementing palmitic acid and altering the dietary ratio of n-6:n-3 fatty acids in low-fibre diets on production responses of dairy cows. Br. J. Nutr. 2021, 126, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Casamassima, D.; Nardoia, M.; Palazzo, M.; Vizzarri, F.; D’Alessandro, A.G.; Corino, C. Effect of dietary extruded linseed, verbascoside and vitamin E supplements on yield and quality of milk in Lacaune ewes. J. Dairy Res. 2014, 81, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Pezzi, P.; Giammarco, M.; Vignola, G.; Brogna, N. Effects of extruded linseed dietary supplementation on milk yield, milk quality and lipid metabolism of dairy cows. Ital. J. Anim. Sci. 2016, 6, 333–335. [Google Scholar] [CrossRef]
- Musco, N.; Tudisco, R.; Esposito, G.; Iommelli, P.; Totakul, P.; D’Aniello, B.; Lombardi, P.; Amato, R.; Wanapat, M.; Infascelli, F. Effects of Linseed Supplementation on Milk Production, Composition, Odd- and Branched-Chain Fatty Acids, and on Serum Biochemistry in Cilentana Grazing Goats. Animals 2022, 12, 783. [Google Scholar] [CrossRef]
- Kholif, A.E.; Morsy, T.A.; Abd El Tawab, A.M.; Anele, U.Y.; Galyean, M.L. Effect of Supplementing Diets of Anglo-Nubian Goats with Soybean and Flaxseed Oils on Lactational Performance. J. Agric. Food Chem. 2016, 64, 6163–6170. [Google Scholar] [CrossRef]
- Dai, X.J.; Wang, C.; Zhu, Q. Milk performance of dairy cows supplemented with rapeseed oil, peanut oil and sunflower seed oil. Czech J. Anim. Sci. 2011, 56, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Barfourooshi, H.J.; Towhidi, A.; Sadeghipanah, H.; Zhandi, M.; Zeinoaldini, S.; Dirandeh, E.; Akers, R.M. Effect of Dietary Fish Oil on Mammary Gland Development and Milk Production of Holstein Cow. Ann. Anim. Sci. 2018, 18, 973–990. [Google Scholar] [CrossRef] [Green Version]
- Kupczynski, R.; Szoltysik, M.; Janeczek, W.; Chrzanowska, J.; Kinal, S.; Kroliczewska, B. Effect of dietary fish oil on milk yield, fatty acids content and serum metabolic profile in dairy cows. J. Anim. Physiol. Anim. Nutr. 2011, 95, 512–522. [Google Scholar] [CrossRef]
- Zisis, F.; Kyriakaki, P.; Satolias, F.F.; Mavrommatis, A.; Simitzis, P.E.; Pappas, A.C.; Surai, P.F.; Tsiplakou, E. The Effect of Dietary Inclusion of Microalgae Schizochytrium spp. on Ewes’ Milk Quality and Oxidative Status. Foods 2022, 11, 2950. [Google Scholar] [CrossRef]
- Pajor, F.; Egerszegi, I.; Szucs, A.; Poti, P.; Bodnar, A. Effect of Marine Algae Supplementation on Somatic Cell Count, Prevalence of Udder Pathogens, and Fatty Acid Profile of Dairy Goats’ Milk. Animals 2021, 11, 1097. [Google Scholar] [CrossRef]
- Marques, J.A.; Del Valle, T.A.; Ghizzi, L.G.; Zilio, E.M.C.; Gheller, L.S.; Nunes, A.T.; Silva, T.B.P.; Dias, M.; Grigoletto, N.T.S.; Koontz, A.F.; et al. Increasing dietary levels of docosahexaenoic acid-rich microalgae: Ruminal fermentation, animal performance, and milk fatty acid profile of mid-lactating dairy cows. J. Dairy Sci. 2019, 102, 5054–5065. [Google Scholar] [CrossRef]
- Habel, J.; Sundrum, A. Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows. Animals 2020, 10, 1028. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Keating, A.F. Expression and regulation of glucose transporters in the bovine mammary gland. J. Dairy Sci. 2007, 90 (Suppl. 1), E76–E86. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Ji, J.; Yan, X.H. Cross-talk between AMPK and mTOR in regulating energy balance. Crit. Rev. Food Sci. Nutr. 2012, 52, 373–381. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, 224. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, C.; Yoshino, K.; Yonezawa, K. mTOR integrates amino acid- and energy-sensing pathways. Biochem. Biophys. Res. Commun. 2004, 313, 443–446. [Google Scholar] [CrossRef]
- Cai, J.; Wang, D.; Zhao, F.Q.; Liang, S.; Liu, J. AMPK-mTOR pathway is involved in glucose-modulated amino acid sensing and utilization in the mammary glands of lactating goats. J. Anim. Sci. Biotechnol. 2020, 11, 32. [Google Scholar] [CrossRef]
- Gomes de Paiva, P.; Ferreira de Jesus, E.; Del Valle, T.A.; Ferreira de Almeida, G.; Costa, A.G.B.V.B.; Consentini, C.E.C.; Zanferari, F.; Takiya, C.S.; Bueno, I.C.d.S.; Rennó, F.P. Effects of chitosan on ruminal fermentation, nutrient digestibility, and milk yield and composition of dairy cows. Anim. Prod. Sci. 2017, 57, 10939–10952. [Google Scholar] [CrossRef] [Green Version]
- Akers, R.M. Major advances associated with hormone and growth factor regulation of mammary growth and lactation in dairy cows. J. Dairy Sci. 2006, 89, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qi, H.; Gao, X. Daidzein promotes milk synthesis and proliferation of mammary epithelial cells via the estrogen receptor α-dependent NFκB1 activation. Anim. Biotechnol. 2022, 33, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hadaya, O.; Bransi-Nicola, R.; Shalev, Y.; Azaizeh, H.; Roth, Z.; Muklada, H.; Deutch, T.; Landau, S.Y.; Argov-Argaman, N. Pistacia lentiscus extract enhances mammary epithelial cells’ productivity by modulating their oxidative status. Sci. Rep. 2020, 10, 20985. [Google Scholar] [CrossRef] [PubMed]
- Shiu, R.P.; Friesen, H.G. Mechanism of action of prolactin in the control of mammary gland function. Annu. Rev. Physiol. 1980, 42, 83–96. [Google Scholar] [CrossRef]
- Fendrick, J.L.; Raafat, A.M.; Haslam, S.Z. Mammary gland growth and development from the postnatal period to postmenopause: Ovarian steroid receptor ontogeny and regulation in the mouse. J. Mammary Gland. Biol. 1998, 3, 7–22. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, M.; Shi, Y.; Song, S.; Hou, X.; Lin, Y. Prolactin regulates LAT1 expression via STAT5 (signal transducer and activator of transcription 5) signaling in mammary epithelial cells of dairy cows. J. Dairy Sci. 2020, 103, 6627–6634. [Google Scholar] [CrossRef]
- Sokolov, A.M.; Holmberg, J.C.; Feliciano, D.M. The amino acid transporter Slc7a5 regulates the mTOR pathway and is required for granule cell development. Hum. Mol. Genet. 2020, 29, 3003–3013. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, H.; Wen, X.P.; Li, P.; Gao, X.J.; Ao, J.X. The phosphorylation of Tudor-SN mediated by JNK is involved in the regulation of milk protein synthesis induced by prolactin in BMECs. J. Cell. Physiol. 2019, 234, 6077–6090. [Google Scholar] [CrossRef]
- Raynal, P.; Pollard, H.B. Annexins: The problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta 1994, 1197, 63–93. [Google Scholar] [CrossRef]
- Pauloin, A.; Chanat, E. Prolactin and epidermal growth factor stimulate adipophilin synthesis in HC11 mouse mammary epithelial cells via the PI3-kinase/Akt/mTOR pathway. Biochim. Biophys. Acta 2012, 1823, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Zhang, Y.; Ge, Y.; Li, W.; Cao, Y.; Qu, Y.; Liu, S.; Guo, Y.; Fu, S.; Liu, J. Sodium butyrate promotes milk fat synthesis in bovine mammary epithelial cells via GPR41 and its downstream signalling pathways. Life Sci. 2020, 259, 118375. [Google Scholar] [CrossRef]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Shen, J.; Liao, X.; Liu, X.; Zhang, J.; Zhou, C.; Jin, Y. Camellia (Camellia oleifera Abel.) seed oil promotes milk fat and protein synthesis-related gene expression in bovine mammary epithelial cells. Food Sci. Nutr. 2020, 8, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.D.; Zhou, C.H.; Zhang, J.; Shen, J.L.; Wang, Y.J.; Jin, Y.C.; Li, S.L. Effect of all-trans retinoic acid on casein and fatty acid synthesis in MAC-T cells. Asian-Australas. J. Anim. Sci. 2020, 33, 1012–1022. [Google Scholar] [CrossRef]
- Khan, M.Z.; Liu, L.; Zhang, Z.; Khan, A.; Wang, D.; Mi, S.; Usman, T.; Liu, G.; Guo, G.; Li, X.; et al. Folic acid supplementation regulates milk production variables, metabolic associated genes and pathways in perinatal Holsteins. J. Anim. Physiol. Anim. Nutr. 2020, 104, 483–492. [Google Scholar] [CrossRef]
- Huang, Y.; Oikonomou, G.; Hu, J.; Li, Y.; Du, X.; Du, Y.; Liu, Y.; Zhang, P.; Wang, P.; Yu, H.; et al. Effect of feeding grape seed Proanthocyanidin extract on production performance, metabolic and anti-oxidative status of dairy cattle. Arq. Bras. Med. Vet. Zootec. 2019, 71, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, C.; Liu, J.; Liu, H. Proteomic analysis of the effects of lutein on mammary gland metabolism in dairy cows. J. Dairy Res. 2018, 85, 152–156. [Google Scholar] [CrossRef]
- de Morentin, P.B.M.; Martinez-Sanchez, N.; Roa, J.; Ferno, J.; Nogueiras, R.; Tena-Sempere, M.; Dieguez, C.; Lopez, M. Hypothalamic mTOR: The Rookie Energy Sensor. Curr. Mol. Med. 2014, 14, 3–21. [Google Scholar] [CrossRef]
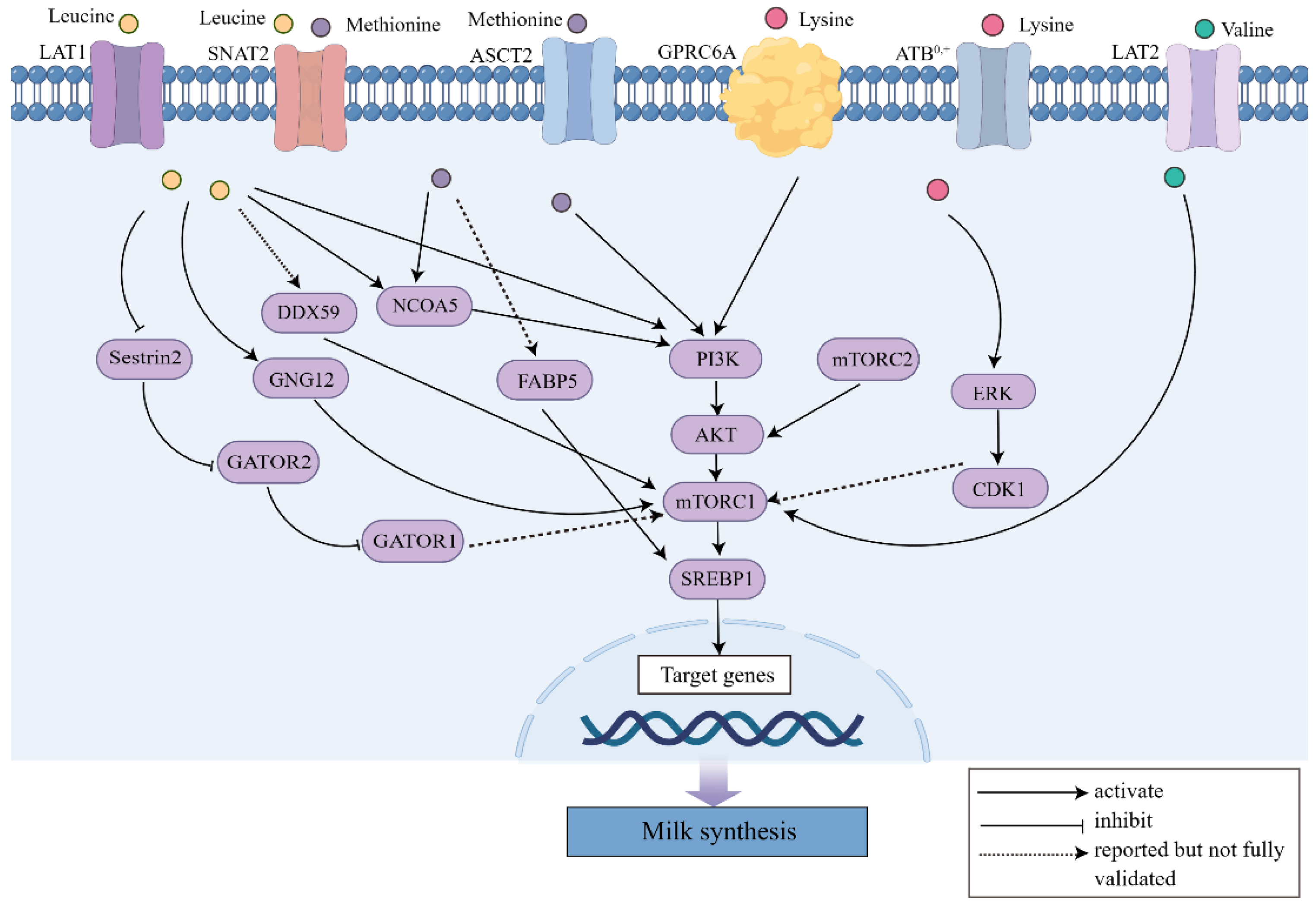
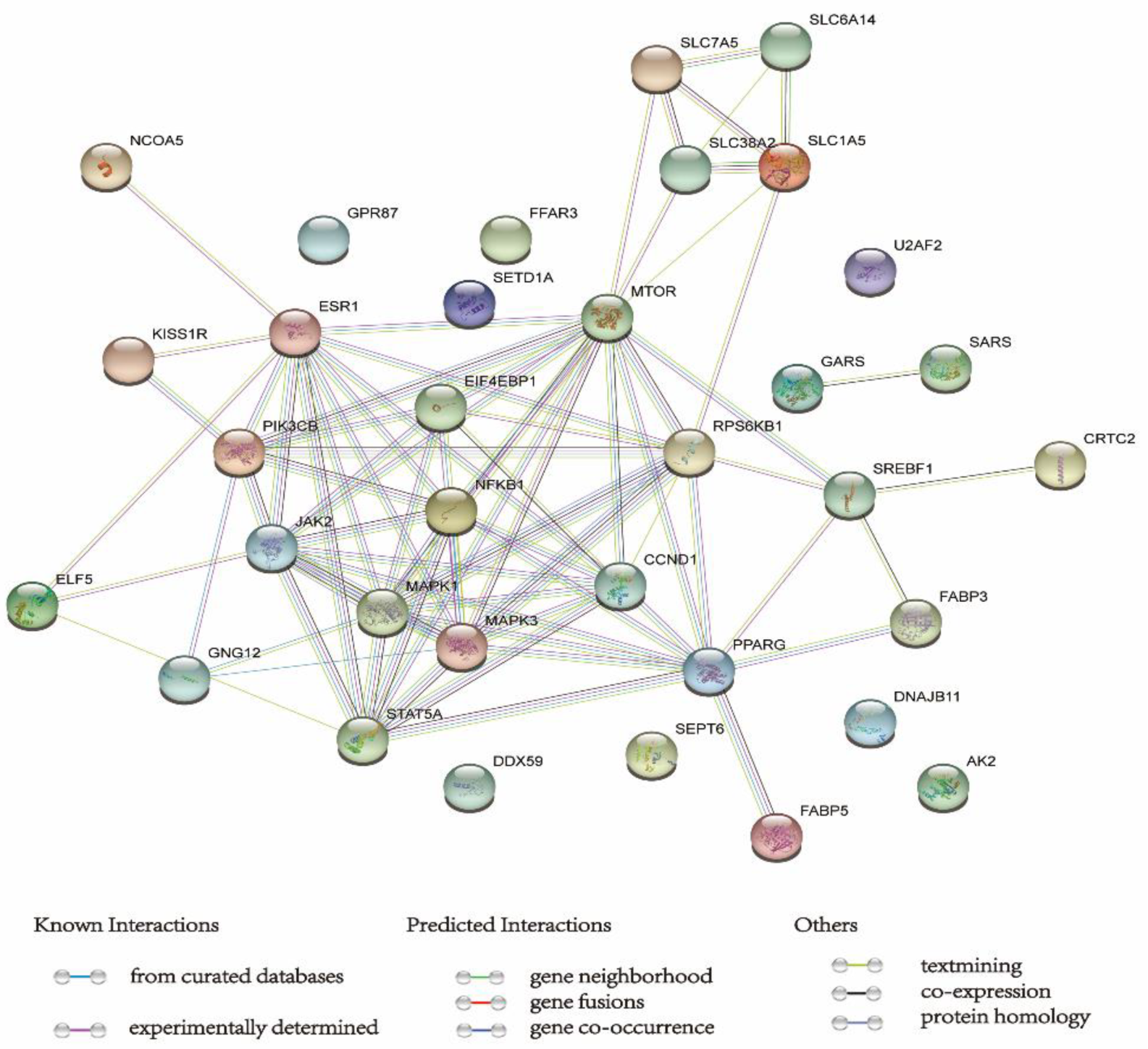
| Items | Treatments | Functions | Potential Signaling Pathways | References |
|---|---|---|---|---|
| Ghrelin (+) | Mature Guanzhong Saanen dairy goats and mammary epithelial cells | β-Casein synthesis ↑ | —— | [77] |
| Ghrelin (+) | Primary bovine mammary epithelial cells | β-Casein synthesis ↑ | ERK1/2 and AKT signaling pathways | [78] |
| Kisspeptin-10 (+) | Bovine mammary epithelial cells (100 nmol/L) | β-Casein Synthesis ↑ | CSN2 via GPR54 and its downstream signaling pathways mTOR, ERK1/2, STAT5 and AKT | [81] |
| Methionyl-methionine dipeptide (+) | Bovine mammary epithelial cells (80 µg/mL) | PepT2 expression ↑ β- Casein synthesis ↑ | AK2-STAT5 and mTOR signaling pathways | [84] |
| Threonyl-phenylalanyl-phenylalanine (+) | Bovine mammary epithelial cells (5, 10 and 15%) | PepT2 mRNA abundance↑ | —— | [85] |
| Threonyl-phenylalanyl-phenylalanine (+) | Bovine mammary epithelial cells (add lactogenic hormone treatment) | PepT mRNA abundance↑ | —— | [86] |
| Octopus peptide (+) | Mouse mammary epithelial cell line | β- Casein synthesis ↑ Cell proliferation ↑ | —— | [87] |
| Items | Treatments | Functions | Potential Signaling Pathways | References |
|---|---|---|---|---|
| Glucose (+) | lactating dairy goats (60 g/d) | Amino acid ↑ | AMPK-mTOR signaling pathways | [122] |
| Glucose (+) | Bovine mammary epithelial cells | β-casein ↑ Cell proliferation ↑ | AMPK/mTOR signaling pathways | [55] |
| Chitosan (+) | Holstein cows (225 mg/kg bodyweight) | Milk yield ↑ Fat-corrected milk, protein and lactose production ↑ | —— | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, F.; Li, P.; Hao, G.; Liu, Y.; Wang, T.; Liu, B. Enhancing Milk Production by Nutrient Supplements: Strategies and Regulatory Pathways. Animals 2023, 13, 419. https://doi.org/10.3390/ani13030419
Pan F, Li P, Hao G, Liu Y, Wang T, Liu B. Enhancing Milk Production by Nutrient Supplements: Strategies and Regulatory Pathways. Animals. 2023; 13(3):419. https://doi.org/10.3390/ani13030419
Chicago/Turabian StylePan, Fengguang, Peizhi Li, Guijie Hao, Yinuo Liu, Tian Wang, and Boqun Liu. 2023. "Enhancing Milk Production by Nutrient Supplements: Strategies and Regulatory Pathways" Animals 13, no. 3: 419. https://doi.org/10.3390/ani13030419
APA StylePan, F., Li, P., Hao, G., Liu, Y., Wang, T., & Liu, B. (2023). Enhancing Milk Production by Nutrient Supplements: Strategies and Regulatory Pathways. Animals, 13(3), 419. https://doi.org/10.3390/ani13030419







