Simple Summary
Coccidiosis is caused by an intracellular parasite that damages the intestinal integrity, negatively affecting the digestion and absorption of nutrients and consequently worsening weight gain, feed efficiency, and pigmentation of birds, even causing mortality. Therefore, it has a negative impact on the economy of the poultry industry. Currently, the disease is mainly treated by using anticoccidials drugs added to the diet. The drug resistance, as well as the residue of drugs in the meat, has prompted the development of natural alternatives to combat coccidiosis. The purpose of this research was to determine whether an Alliaceae encapsulated extract added to the broiler chickens diet decreased the number of oocysts excreted in feces and the harm caused to the intestinal mucosa, consequently improving the productive performance of broiler chickens challenged with Eimeria spp. Under our experimental conditions, both the inclusion of Alliaceae extract, as well as the use of conventional anticoccidials (nicarbazin/narasin/salinomycin), diminished the detrimental effect of Eimeria spp. Moreover, the Alliaceae extract favored the abundance of acid butyric bacteria (Ruminococcus spp. and Intestinimonas spp.) in the cecum, related to intestinal health. Based on the current findings, the Alliaceae extract could be a natural additive used to lessen the effects of coccidiosis infections.
Abstract
This study analyzed the effects of an Alliaceae encapsulated extract (AE-e) on daily gain (ADG), feed intake (ADFI), feed conversion ratio (FCR), oocysts per gram of feces (OPG), intestinal lesion (LS), and microbiota composition in broilers challenged with Eimeria spp. A total of 4800 one day Cobb-500 were allotted into 10 treatment groups with 12 replicates of 40 birds in a 2 × 4 + 2 factorial arrangement. The first factor was non-challenged (NC) or challenged (C), the second was four levels of AE-e added in the basal diet, 0 (AE0), 250 (AE250), 500 (AE500), and 750 mg·kg−1 (AE750), plus two ionophore controls, non-challenged (NC-Ion) and challenged (C-Ion). No interactions were observed between factors (NC0, NC250, NC500, NC750, C0, C250, C500, and C750), while C-Ion improved FCR at 21 d. The challenge affected negatively ADG and FCR and promoted enteropathogens in cecum. AE750 improved FCR in the finisher and cumulative phases, while C-Ion had fewer total OPG than C0 and C250. Likewise, at 21d, C250, C500, and C-Ion had fewer LS than C0, while at 28 d, C750 showed lower than C-Ion. In the cecum microbiota, C500 had more Ruminococcus, Firmicutes b, and Intestinimonas than C-Ion. In summary, AE-e showed beneficial results in broilers infected with Eimeria spp.
1. Introduction
Currently, coccidiosis disease continues to be one of the most serious problems in the commercial broiler poultry industry, resulting in great economic loss all over the world [1]. Eimeria acervulina, Eimeria maxima, and Eimeria tenella are the main species that cause disease in broiler chickens, impairing both intestinal function and growth performance [2]. To date, coccidiosis prevention has been through the addition of synthetic anticoccidials such as nicarbazin, decoquinate, and zoalene, as well as ionophores [3] such as monensin, lasalocid, salinomycin, narasin, etc., to either the poultry diet or drinking water, or the use of vaccines [4]. However, Eimeria spp. has developed drug resistance, causing a loss of effectiveness in the anticoccidials [5]. In addition, there is a global concern about drug resistance and the presence of residual drugs in meat [6] that has prompted the study and development of natural alternatives to prevent or control coccidiosis [7], such as phytochemicals, which are a suitable alternative due to their favorable effects against Eimeria spp. [8].
Several works have demonstrated that extracts and the essential oil of Allium cepa and Allium sativum improved the average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR), as well as intestinal health and carcass quality in broiler chickens [9,10,11]. Likewise, blends of the genus Allium with oregano essential oil and eugenol [12], as well as organic acids [8,13], have shown benefits in broiler performance and health. The genus Allium has different sulfur compounds that have been studied as additives in animal nutrition [14]. These compounds are conformed by sulfur atoms attached to a cyanate group in cyclic or non-cyclic forms [15]. The most studied are the sulfoxydes s-methyl-L-cysteine (methionine), s-allil-L-cysteine (alliin), s-propenyl-L-cysteine (isoalliin), and s-propyl-L-cysteine (propiin). Propyl propane thiosulfinate (PTS) and propyl propane thiosulfonate (PTSO) are derived from the natural degradation of propiin [16,17]. Unlike other sulfur compounds from the genus Allium, PTSO is chemically stable but is insoluble in water. For this reason, it should be provided with a water-soluble carrier to increase its availability and absorption [18,19]. In addition, PTS and PTSO have shown to reduce the viability of E. acervulina sporozoites, improving the innate immune response [20], and inhibit the sporulation of E. tenella [21], and they are able to promote the growth of beneficial bacteria and decrease intestinal pathobionts [22] in broiler chickens. Recently, Aguinaga-Casanas, et al. [19] conducted a study in vitro that showed that PTSO inhibit the capability of E. acervulina sporozoites to penetrate Madin-Darby bovine kidney cells (MDBK cells), concluding that PTSO is a promising alternative to coccidiosis treatment. The Allium spp. could be used as an alternative to anticoccidials in broiler production due to its proven benefits. Nevertheless, it is necessary to carry out more studies to understand the mechanism by which they exert their favorable effects, as well as determinate the appropriate inclusion level in the poultry diet and the period of use to enhance broiler performance [23].
Based on the above information, we hypothesize that an Alliaceae (A. cepa and A. sativum) encapsulated extract (AE-e) used as a feed additive decreases the oocysts per gram in feces (OPG), reduces the intestinal lesion score (LS), and improves productive performance, as well as modulates positively the intestinal microbiota in Eimeria challenged birds. Therefore, the aim of this study was to examine the effects of increasing levels of AE-e on ADG, ADFI, FCR, OPG, LS, and intestinal microbiota composition in broiler chickens challenged with a mixture of Eimeria spp.
2. Materials and Methods
2.1. Ethical Standard
The present study was approved on 5 October 2020, by the Animal Welfare and Experimentation Ethics Committee of the National Autonomous University of México SICUAE-DC-2020/3-6 in compliance with the Mexican Official Norm NOM-062-ZOO-1999.
2.2. Housing, Animals and Experimental Design
A total of 4800 one-day-old Cobb-500 broiler chickens were housed in 120 4 m2 pens separated by wire mesh partitions and new wood shaving litter throughout the period of study of 49 d. The facility temperature was set as 30 °C during the first week using thermostatically controlled propane gas heaters, reducing 2.5 °C each week. After the fourth week, the temperature was controlled through curtains and kept between 18–21 °C. The first 4 d after reception, the chickens had access to 23 h of light; after that, a natural photoperiod was maintained throughout the study. The birds were assigned in a completely randomized experimental design with a 2 × 4 + 2 factorial arrangement. One factor was the challenge level, formed by a non-challenged group (NC) and a challenged group (C) with Eimeria spp. The second was 4 levels of AE-e (0, 250, 500, and 750 mg AE-e per each kg of feed). In addition, two ionophore controls, C-Ion and NC-Ion, were used to contrast the challenged and non-challenged AE-e treatments, respectively. The experimental unit was the pen. To prevent cross contamination, the C and NC birds were housed in separate but identical buildings.
2.3. Alliaceae Encapsulated Extract Supplementation
We used a concentrated liquid commercial Alliaceae extract (GarliconTM; DOMCA S.A.U., Granada, Spain), which has shown positive effects on bird productivity [20,24,25]. It was encapsulated into a dextrin–lecithin matrix and validated by the presence of PTSO, which has a concentration of 12 g·kg−1, as determined by gas chromatography–mass spectrometry (Gas chromatograph model 7890A Agilent Technologies Inc., coupled to a simple quadrupole mass detector model 5975C Agilent Technologies Inc., Santa Clara, CA, USA). The retention time of the chromatography peak was indicated for the PTSO according to the databases of the NIST/EPA/NIH Mass Spectra Library, version 1.7 (Gaithersburg, MD, USA). The analysis was carried out in the laboratory of the Center for Research in Applied Sciences and Advanced Technology of the National Polytechnic Institute (IPN, Querétaro, Mexico).
2.4. Diets and Experimental Groups
A corn–soybean meal basal diet was formulated to meet the nutritional specifications for the Cobb-500 lineage™ (Table 1). The basal diet was split into five portions to be mixed with the experimental doses of AE-e or ionophore and then were pelleted at 80 °C for 30 s. The broiler chickens were on two phases of feeding, a starter phase (1–21 day of age) and a finisher phase (22–49 day of age). The feed was restricted from 15:00 h to 08:00 h to avoid ascites syndrome, and the water was provided ad libitum.

Table 1.
Ingredients (kg·t−1 of feed) and calculated chemical composition (% as fed) and metabolizable energy (EM, kcal·kg−1) in the basal diets.
The birds were assigned to either a NC or C group and feed with the basal diet containing 4 different levels of AE-e as follows: basal diet without AE-e (AE0); basal diet with added 250 mg·kg−1 of AE-e (AE250); basal diet with added 500 mg·kg−1 of AE-e (AE500); and basal diet with added 750 mg·kg−1 of AE-e (AE750). Two positive control treatments, non-challenged ionophore control (NC-Ion) and a challenged ionophore control (C-Ion), both formed by a basal diet with 50 ppm of nicarbazin and 50 ppm of narasin added for the starter phase and 60 ppm of salinomycin for the finisher phase, summarizing a total of 10 treatments (NC0, NC250, NC500, NC750, C0, C250, C500, C750, NC-Ion, C-Ion) with 12 replicates of 40 birds for each one.
2.5. Productive Performance
Each pen was monitored for body weight (BW), weight gain (WG), and feed intake (FI) at 0, 21, and 49 d of age. On the same day as the event took place, we recorded the age and weight of dead birds to determine (a) ADG: [(mean final BW of live birds in the pen) − (mean initial BW of all birds in that pen)]/days of testing. (b) ADFI: (total feed consumed in a pen)/(birds alive × days on test in the pen + days dead birds on test in that pen). (c) FCR: (total feed consumption in a pen)/(WG of birds alive + WG of dead birds in the same pen) [26].
2.6. Eimeria Challenge
At 12 d of age, the broiler chickens from the challenged group (C) were inoculated directly into the crop with 0.5 mL of a mixture of sporulated oocysts of E. acervulina 1 × 105, E. maxima 2 × 104, and E. tenella 2 × 104 using sterile plastic syringes, while the birds from the non-challenged group (NC) received a sham 0.5 mL of distilled water.
The Eimeria mixture was obtained from a non-governmental laboratory of parasitology, Morelos, México, and it was assessed by counting the oocysts sporulated from the different species of Eimeria at the National Autonomous University of México (UNAM) and by PCR Sanger sequencing at the Faculty of Chemistry, Querétaro University, México (UAQ).
2.7. Eimeria Oocysts Count
On d 9, 16, and 23 post-inoculation (p.i.), approximately 10 g of fresh fecal material was collected from each pen and mixed thoroughly in a plastic bag and kept at 4 °C until the total count of oocysts. Five grams of each sample was homogenized in a saturated NaCl solution (400 g·L−1) and filtered through a 300-mesh sieve. The filtrate was centrifuged at 800× g for 2 min and an aliquot of the supernatant was poured into a Mc Master Chamber and counted at 10× magnification on a compound microscope following the technique described by Long, et al. [27]. The morphological characteristics of the sporulated oocysts were used to identify E. acervulina, E. maxima, and E. tenella; the number of oocysts was expressed as OPG. The total OPG is the sum of OPG for all three species.
2.8. Intestinal Lesion Score (LS)
The LS in the duodenum, jejunum, and cecum were evaluated at 9- and 16-day p.i., twenty-four birds from each treatment were randomly selected and humanely killed by cervical dislocation [28,29]. The gastrointestinal tract was removed and opened; the scores for macroscopic lesions for E. acervulina, E. maxima, and E. tenella were determined according to the scale of Johnson and Reid [30]. A score of “0” represented no visual lesions, “1” was minimal lesions, “2” was moderate lesions, “3” was severe lesions, and “4” was extremely severe lesions.
2.9. Anticoccidial Index (ACI)
The relative ratio weight gain (rBWG), survival rate (SR), total mean lesion score (TMLS), and OPG value are necessary to calculate the anticoccidial index (ACI) and are recognizes as good indicators of the efficacy of the anticoccidial compounds. The ACI was calculated for each group according to the following equation proposed by Merk, et al. [31]:
ACI = (rBWG + SR) × 100 − (TMLS × 10 + OPG value)
The variables were calculated as follows:
- rBWG: BWG rate of the challenged unmedicated control or drug treated group/BWG rate of unchallenged unmedicated control group × 100.
- BWG rate: (Final BW − initial BW)/initial BW × 100.
- SR: Number of final birds alive/ number of total initial birds × 100.
- TMLS: Sum of the LS caused by all the Eimeria spp.
- OPG value: OPG in unchallenged unmedicated control or challenged drug-treated group/OPG in infected/unmedicated control group × 100 [32].
2.10. Intestinal Microbiota Samples
At 21 d of age (9 d p.i.), 6 chickens from 5 treatments, NC0, C0, NC500, C500, and C-Ion, were randomly selected and sacrificed by the manual cervical dislocation method [29,33]. The gastrointestinal tract was dissected, the ileum and cecum contents were scraped carefully and collected in cryogenic vials, snap frozen in liquid nitrogen, and stored at −80 °C until the microbiota composition analysis [34,35].
2.11. DNA Extraction, 16s rRNA Gene Amplification, and Library Preparation for Sequencing
The bacterial DNA from the ileal and cecal contents was extracted using the ZymoBIOMICS™ DNA Miniprep kit (D4300 Zymo Research, Irvine, CA, USA), according to the manufacturer recommendations, it was quantified by fluorometry using Qubit chemistry (Invitrogen, Waltham, MA, USA), while its integrity was assessed by spectrometry (NanoDrop, Thermo Fisher Scientific, Whaltam, MA, USA). The libraries were made following the two-step polymerase chain reaction (PCR) protocol suggested by Illumina (Illumina Part# 15044223 Rev.B, San Diego, CA, USA) to sequence a single segment comprising the 16S rRNA V3-V4 region [36]. The libraries were quantified by fluorometry, pooled at 4nM with 10% PhiX sequencing control, and sequenced using the Illumina MiSeq platform to obtain 300 paired-end reads following the manufacturer´s instructions (Illumina, San Diego, CA, USA).
2.12. Bioinformatic Analysis
The paired-end raw reads were analyzed with Cutadapt v1.15 to eliminate any traces of 16S-rRNA amplification primers or Illumina adapter sequences and then scanned with Trimmomatic v36 [37] to filter out the lower quality reads. The forward and reverse reads of each pair were then overlapped into single fragments using FLASH v1.2.11 software, employing an expected fragment length of 409 ± 20 bp and an expected read length of 279 bp and were further filtered with DADA2 (included in the QIIME2 v2020.8 suite) [38]; to eliminate reads where 2 or more sequencing errors were expected, groups of reads were produced by experimental errors (noise removal) and chimeric fragments.
To assign a taxonomic classification to the pre-processed sequences, we used the naive Bayes classifier [39], as implemented in the QIIME2 suite [38]. The classifier was trained with the annotations of the SILVA 138 ribosomal reference database [40] using the sequences grouped to a similarity of 99%.
The Shannon and Simpson indexes, as well as the total OTU counts, were obtained for each sample to study the intestinal microbial α-diversity. β-diversity was assessed by measuring the Bray–Curtis dissimilarity of each pair of samples, followed by nonmetric multi-dimensional scaling (NMDS) to observe the clustering of the different sample groups. The α and β-diversity profiles were visualized through box-plots and NMDS scaling plots [41]. To obtain the relative abundance of the OTUs, the number of reads per OTU was normalized by library size. Only the abundance changes in genus with a significance p ≤ 0.05 were represented in a heatmap.
2.13. Statistical Analyses
The statistical analyses were performed using the JMP statistical software v 17.0.0 (SAS Institute Inc., Cary, NC, USA) and R version 4.0.2. The data normality and variance homogeneity among groups were tested using the Shapiro–Wilk and Levene´s tests, respectively. The variables with non-normal distributions were analyzed by nonparametric statistics. The significance level was set at p ≤ 0.05, and a trend was set between p > 0.05 and ≤0.10.
For the analysis of productive performance, the initial BW was included as a covariate. The ADFI, ADG, and FCR were analyzed by 2-way ANOVA. The ACI was analyzed by one-way ANOVA. Post hoc Tukey tests were performed. The experimental design considered two controls with ionophores to contrast the treatments. Contrast A was NC-Ion vs. NC0, NC250, NC500, and NC750; contrast B was C-Ion vs. C0, C250, C500, and C750; and contrast C was NC-Ion vs. C-Ion. Additionally, the AE-e factor was analyzed to determine if the effects of different doses of AE-e had a linear trend. Furthermore, AE-e treatments included an analysis of the polynomial orthogonal contrast trend in the variable ACI.
Since there were no detected oocyst or coccidia lesions in nonchallenged birds, the OPG and LS were analyzed only in the challenged birds using the Kruskal–Wallis test and, as post hoc, the Steel–Dwass test, the medians, and quantiles q25 and q75 were reported. Correlations were carried out between ADG, FCR, OPG, and LS using Spearman Rho analysis.
The α-diversity changes among groups were assessed with the Kruskal–Wallis test, while the β-diversity was assessed by the NMDS and PerMANOVA tests to identify significant differences in the clustering position of the groups. To obtain the diversity measures and the corresponding statistical tests, we used the R phyloseq v1.38 package [41]. We used the DESeq2 package to estimate the differential abundance of specific clades between group sample pairs, using the Wald test and adjusting the p-values through the Benjamini–Hochberg multiple sampling correction.
3. Results
3.1. Productive Performance
The following tables provide comprehensive data on the interactions between the Eimeria challenge and AE-e supplementation (Table 2) and subsequently present independently the effects of the Eimeria challenge (Table 3) and the effects of AE-e supplementation (Table 4) on ADG, ADFI, and FCR. No significant differences (p > 0.05) were found in the interaction of factors on ADG, ADFI, and FCR in starter and finisher phases or in the cumulative period of study (Table 2). As well as the orthogonal contrast (contrast A), NC-Ion vs. NC0, NC250, NC500, and NC750 did not show differences in ADFI, ADG, and FCR (p > 0.05) in both growing phases. On the other hand, the comparison of contrast B, C-Ion vs. C0, C250, C500 and C750, did not show differences in ADFI and ADG throughout the study period (p > 0.05). However, at 21 d, C-Ion showed lower FCR, 1.30 vs. 1.39, respectively, (p < 0.01) and a trend (p = 0.07) to improve ADG. There was no effect on FCR in the finisher or cumulative period (p > 0.05). Finally, in contrast C, NC-Ion vs. C-Ion, there was a trend in the starter phase in which the challenged group (C-Ion) had lower ADG (p = 0.06) and higher FCR (p = 0.07) than NC-Ion.

Table 2.
Initial body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) of broilers chickens fed a corn–soybean diet supplemented with different doses of Alliaceae encapsulated extract (AE-e) 1 or anticoccidial drugs (Ion) 2 under challenge with Eimeria spp. 3.

Table 3.
Effects of the challenge with Eimeria spp. 1 on average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) of broiler chickens fed a corn–soybean diet.

Table 4.
Initial body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) of broiler chickens fed a corn–soybean diet supplemented with different doses of Alliaceae encapsulated extract (AE-e) 1.
At the starter phase, the challenge affected the productive performance. Broiler chickens from the challenged group (C) had a reduction of 12.4% in ADG compared to those from the non-challenged (NC) group (p < 0.0001); moreover, the FCR was also deteriorated by 12.8%, 1.40 vs. 1.22 (p < 0.0001), respectively. Despite not finding changes in the finisher phase (p > 0.05), the negative effect observed in the starter phase continued in the cumulative period, ADG (p < 0.001) and FCR (p = 0.01) in broiler chickens from C group compared to NC group. However, the ADFI was not affected by the challenge (p > 0.05; Table 3).
Table 4 shows that during the starter phase, the inclusion of AE-e did not influence performance (p > 0.05). Nevertheless, in the finisher phase, the FCR was better in AE750, 1.97 compared to AE0, 2.20 (p = 0.03). Moreover, in the cumulative period, AE750 continued to show better FCR, 1.73 regarding 1.84 from AE0 (p = 0.01); however, the AE250 and AE500 inclusion did not show a difference in FCR (p > 0.05). In addition, no significant differences in ADG and ADFI were observed between treatments during the study (p > 0.05); however, AE500 displayed a trend to improve ADG in the cumulative study (p = 0.10). On the other hand, a positive linear trend was observed in ADG (p = 0.05) and FCR (p = 0.006) in the finisher phase, as well as FCR in the cumulative period (p = 0.004).
3.2. Oocysts Shedding
Table 5 describes the effect of feed supplementation with AE-e or ionophores on the OPG of Eimeria-challenged broiler chickens (C0, C250, C500, C750, and C-Ion), because of the absence of OPG in non-challenged treatments, NC0, NC250, NC500, NC750, and NC-Ion were omitted from analysis. The differences between treatments were observed at 9 d p.i., corresponding to 21 d of age. The higher OPG values of E. acervulina and E. tenella were observed in C0, 7.75 and 22.26-fold changes, respectively, regarding C-Ion (p < 0.05).

Table 5.
Medians and ranges (Q25–Q75) of oocysts per gram of feces (OPG) in broiler chickens supplemented with different doses of Alliaceae encapsulated extract (AE-e) 1 or anticoccidial drugs (Ion) 2, under challenge with Eimeria spp. 3.
While C250, C500, and C750 had a similar OPG shedding in both species compared to C-Ion and C0 (p > 0.05), in addition, E. maxima did not show differences between treatments (p > 0.05). Moreover, the total OPG values were significantly higher (+5-fold) in C0 and C250 than C-Ion (p < 0.01), whereas C500 and C750 had a similar OPG shedding compared to C-Ion, C0, and C250 (p > 0.05). The decrease in OPG excretion from 21 to 28 d of age was more evident in the groups C250 (−69.66-fold), C500 (−69.85-fold), and C750 (−30.73-fold) than C-Ion (−1.94-fold) and C0 (−27.11-fold). At 28 and 35 day of age, E. acervulina, E. maxima, and E. tenella OPG differences between treatments were not detected (p > 0.05).
3.3. Intestinal Lesion Score
Due to the absence of injuries in the NC groups, NC0, NC250, NC500, NC750, and NC-Ion, we only show the analysis of the challenged groups, C0, C250, C500, C750, and C-Ion. The LS of duodenum, jejunum, and cecum, as well as TMLS, are described in Table 6. At 21 days of age (9 d p.i.), the LS observed in the duodenum reveled that the birds given the C0 diet showed a more severe LS of 1.5, which was significantly (p < 0.0001) higher than the 0.5, 1, and 0 scores from birds given the C250, C500, and C-Ion diets, respectively. Remarkably, the LS observed in broilers from the C250 diet were similar to those observed in the broilers on the C-Ion diet. In addition, no significant differences (p > 0.05) were detected among the C250, C500, and C750 diets. However, in the jejunum, significant differences were identified (p = 0.03) between the C0 and C750 vs. the C250 and C-Ion diets; despite the LS medians being equal in the groups, the range q25–q75 was higher in C0 and C750, (0-1), than in C250 and C-Ion, (0-0). In addition, in this intestinal section, the C500 diet had similar effects to the others on LS (p > 0.05). In the cecum, the LS in the broilers given the C-Ion diet were lower than those observed in birds fed the C0, C250, and C750 diets (p < 0.001) but not compared to C500 (p > 0.05). Furthermore, on day 21, the total mean lesion score (TMLS) was significantly higher (p < 0.001) in birds from diet C0 compared to C250, C500, and C-Ion. Moreover, the chicken on the C-Ion diet had lower TMLS compared to other treatments at 21 d of age (p < 0.0001). On the other hand, on day 28, the LS caused by E. acervuline, E. maxima, and E. tenella were not different between all treatments (p > 0.05). However, C-Ion showed higher TMLS compared to the C750 diet (p = 0.02), while the C0, C250, and C500 diets did not show significant differences (p > 0.05).

Table 6.
Medians and ranges (Q25–Q75) in intestinal lesion scores (LS) in broiler chickens supplemented with different doses of Alliaceae encapsulated extract (AE-e) 1 or anticoccidial drugs (Ion) 2, under challenge with Eimeria spp.3.
3.4. Anticoccidial Index (ACI)
Table 7 describes the ACI of the different treatments, NC0 had superior ACI compared to C0, C250, C500, and C750 (p < 0.0001). C-Ion performed better than C0 and C250 (p < 0.0001). The groups C500 and C750 had similar results compared to C-Ion, C0, and C250 (p > 0.05). There were linear (p < 0.04) and quadratic (p < 0.03) positive trends in ACI when comparing the responses to C0, C250, C500 and C750.

Table 7.
Calculated anticoccidial index (ACI) in 21 d broiler chickens supplemented with different doses of Alliaceae encapsulated extract (AE-e) 1 or anticoccidial drugs (Ion) 2, under challenge with Eimeria spp. 3, as well as orthogonal polynomial contrast comparison of AE-e 4.
Spearman correlations (rho) were calculated between OPG, TMLS, ADG, and FCR; the coefficient showed that the presence of OPG at 21 d of age had a positive correlation of 0.80 and 0.71 with TMLS at 21 and 28 d of age, respectively (p < 0.0001). In addition, this OPG had also a positive correlation of 0.73 (p < 0.0001) with the FCR at 21 d. On the other hand, there was a negative correlation (−0.54) with ADG at 21 d of age and −0.29 at 49 d of age (p < 0.01). At 28 d of age, OPG and TMLS showed a positive correlation of 0.42 (p < 0.001). The TMLS at 21 and 28 d were positively correlated (0.67). TMLS at 21d showed a positive correlation of 0.72 with the FCR in the starter phase (p < 0.001), as well as a positive correlation of 0.22 with the cumulative period (p < 0.05), while showing a negative correlation (−0.51) with the ADG at the same age (p < 0.001) and of −0.18 with ADG at 49 d (p < 0.05). Therefore, TMLS at 28 d was negatively correlated (−0.18) with ADG at 49 d of age (p < 0.05).
3.5. Analysis of Bacterial Composition, 16s rRNA
To evaluate the effects of 5 treatments, NC0, NC500, C0, C500, and C-Ion, on the intestinal microbiota composition in broiler chickens, we studied the ileum and cecum microbial community using 16S rRNA sequencing. We obtained a total of 2,683,053 high-quality sequences of the V3–V4 region of the 16S rRNA gene; a total of 10,710 operational taxonomic units (OTUs) at the 99% sequence similarity level were identified in all samples.
3.6. Alpha- and Beta-Diversity
We detected no differences in the α-diversity indexes (Shannon and Simpson) or in the OTU count of different treatment pairs in the ileum and cecum (p > 0.05). In addition, the β-diversity assessed by NMDS from Bray–Curtis distance matrices and a permutational analysis of variance in the ileum section showed no differences in the microbiome between the treatments (p > 0.05). On the other hand, in the cecum, there were significant differences in the clustering position in the groups at the taxonomic levels of family, Figure 1a (p < 0.01), and genus, Figure 1b (p = 0.01). The results showed that NC0 and NC500 were different from C0 and C500, while C-Ion showed no differences against any treatment, except in the taxonomic category family, which was different from NC0, Figure 1.
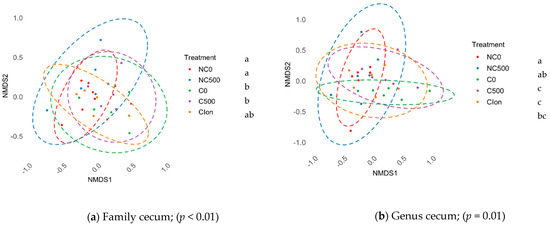
Figure 1.
β-diversity comparison between treatments 1. NMDS of Bray-Curtis distances in (a) Family cecum and (b) Genus cecum. abc The treatments not sharing superscript are different (p < 0.05). NC0, Non-challenged + basal diet; NC500, Non-challenged + basal diet + 500 mg·kg−1 AE-e; C0, Challenged + basal diet; C500, Challenged + basal diet + 500 mg·kg−1 AE-e; C-Ion, Challenged + basal diet + 50 ppm nicarbazin–50 ppm narasin. Challenged with E. acervulina 1 × 105, E. maxima 2 × 104, and E. tenella 2 × 104.
3.7. Relative Intestinal Microbiota Abundance
Figure 2 depicts the analysis of the relative abundance of different bacterial clades in the cecum at the phylum and genus level for each group of samples. In the cecum microbiota, Firmicutes was the dominant phylum (Figure 2a), with a relative abundance ranging from 57.8% (C0 treatment) to 79.4% (NC0 treatment). At the genus level (Figure 2b), Bacteroides was the taxon with the highest relative abundance (9.7–22.1%), highlighting their presence in the groups challenged, C0, C500, and C-Ion.
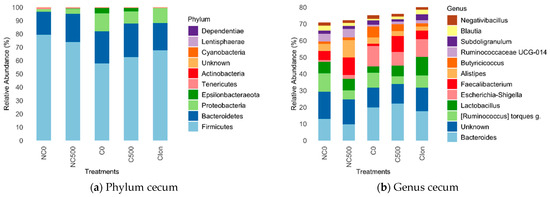
Figure 2.
Relative abundance in the intestinal microbiota in broiler chickens at 21 d in (a) Phylum cecum, (b) Genus cecum. Treatments: NC0, Non-challenged + basal diet; NC500, Non-challenged + basal diet + 500 mg·kg−1 AE-e; C0, Challenged + basal diet; C500, Challenged + basal diet + 500 mg·kg−1 AE-e; C-Ion, Challenged + basal diet+50 ppm nicarbazin–50 ppm narasin. Challenged with E. acervulina 1 × 105, E. maxima 2 × 104, and E. tenella 2 × 104. The relative abundances were obtained after normalizing the reads per clade counts by sequencing the library size and obtaining the average for each clade across treatments. The 12 most abundant clades are shown.
To further explore the effect of the treatments on the cecum bacterial communities, we searched for bacterial genus abundance changes among pairs of treatments, C0 vs. NC0; C500 vs. NC500; C-Ion vs. C0; NC500 vs. NC0; C500 vs. C0; and C500 vs. C-Ion (Figure 3). The results are presented in a heat map from a hierarchical clustering analysis based only on significant changes (p < 0.05). The results from this study showed that the pair comparisons C0 vs. NC0 and C500 vs. NC500 had more bacterial change-in-abundance differences than the other comparisons studied: C-Ion vs. C0; NC500 vs. NC0; C500 vs. C0; and C500 vs. C-Ion. There was a significantly higher abundance (p < 0.05) of genus Ruminococcus 2 in birds from C500 compared to NC500, C0, and C-Ion (+23.36 log2-fc, +8.0 log2-fc, and +23.08 log2-fc, respectively). Moreover, the abundance change in Intestinimonas in the C500 group was +1.78 log2-fc higher than the C-Ion group. However, Escherichia-Shigella, another predominant bacterium in broiler chicken intestines, was also found to be abundant (+4.2 log2fc) in C0 with respect to the NC0 (p < 0.05). Other bacteria, such as Tyzzerella, Eggerthella, Clostridium innocuum g., Ruminococcaceae UCG-009, u. Clostridia b., and Ruminococcus 1., show a higher abundance under the Eimeria challenge conditions. On the other hand, NC0 has higher numbers (p < 0.05) of Bacillus, Hydrogenoanaerobacterium, and Ruminococus 1 than its challenged counterparts.
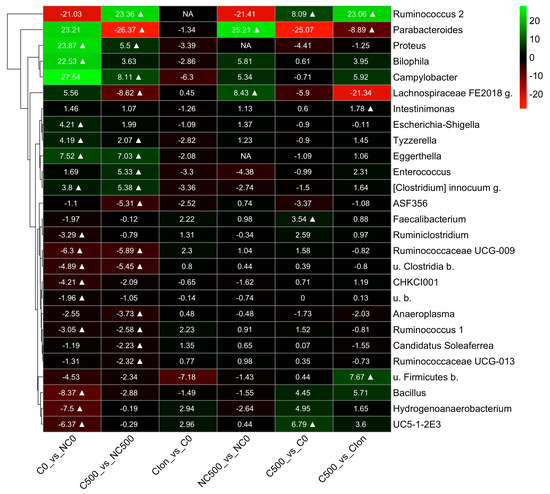
Figure 3.
Heat map of the bacterial genus from the cecum of broiler chickens at 21d (9d p.i.). The color block in the heatmap indicates the normalized abundance log2 fold change (log2-fc) of each pair of groups at the bacterial genus: C0 vs. NC0; C500 vs. NC500; C-Ion vs. C0; NC500 vs. NC0; C500 vs. C0; and C500 vs. C-Ion. The positive and negative changes are indicated by the intensity of the green and red color, respectively. Significant changes (with an adjusted p ≤ 0.05) are marked with a symbol ∆. The non-available (NA) label indicates that the corresponding comparison was not performed as there were no counts for most of the samples of the group pair. A hierarchical clustering tree based on the log2-fc signals of the clades is shown to the left of the heatmap. The abbreviations u. b. and g. in the genus names stand for “unknown”, “bacterium”, and “genus”, respectively.
4. Discussion
In our study, the Eimeria challenge caused a negative impact on growth performance parameters, being more evident at 9 d p.i. (21 days of age). The decrease in the ADG in infected birds, as well as the increases in the FCR, has been reported in other works [8,26,42,43]. In our study, the inclusion of AE750 improved the FCR of broilers in the finisher phase, and in the cumulative study regarding AE0, these results are in agreement with Peinado, et al. [44], who also include two levels of PTSO in the broiler chicken diet and did not find any effect on ADFI, whereas ADG improved using 45 mg per PTSO kg−1 of the diet. Moreover, the FCR was enhanced with both doses tested, 45 and 90 mg per PTSO kg−1 of diet. Similar results were obtained by Kim, et al. [20], who reported that broilers challenged with E. acervuline and fed with 10 ppm of PTSO (67%) and PTS (33%) showed a better growth than the birds that were not supplemented (p < 0.05). Furthermore, Kairalla, et al. [11] reported that some feed additives, particularly garlic (A. sativum), have shown to improve FCR, as was also previously demonstrated by Aarti and Khusro [45]. This result is important since Eimeria infection destroys epithelial cells and affects intestinal villi, causing poor nutrient digestion and severe damage to the host intestinal mucosa, resulting in clinical or subclinical symptoms [46]. In this regard, in a previous study, we demonstrated that organosulfur compounds from garlic, particularly PTSO in 250 g·t−1 of feed, improved the amino acid and energy digestibility in broiler chickens fed with a soybean meal–yellow corn diet [47].
ADG or FCR are not the only good indicators for measuring the effectiveness of anticoccidial drug, but LS and OPG are also considered complementary indicators [48] to the performance. In our study, the higher OPG excretion detected was 9 d p.i., then OPG gradually decreased over time; at 16 d p.i., it is barely noticeable and almost disappears at 23 d p.i. This trend after infection was reported by You [49]. In addition to the treatment effect, the reduction in OPG may be due in part to self-limitation of parasitosis and the immune response developed by the host [50]. We found that OPG shedding was decreased in the challenged birds supplemented with anticoccidial treatment (C-Ion), while 500 and 750 mg·kg−1 of AE-e tended to reduce OPG shedding. A similar effect was observed on the OPG of broiler chickens challenged with Eimeria and supplemented with garlic extracts (A. sativum) [51,52], their active derivative compounds [20], or a premix of garlic and oregano essential oils [53]. The ACI results were better for C-Ion than for C0 and C250. A positive linear and quadratic trend in ACI suggests that generally, birds challenged with Eimeria spp. would perform better when 500 or 750 mg·kg−1 of AE-e is included in their diet.
The correlation analysis in our study indicates that the reduction in the LS was supported by the decreasing OPG. Similar results were found by Elkhtam, et al. [51], where OPG, the clinical symptoms of the disease, and the LS decreased with the addition of garlic extract (A. sativum) to the diet of the broiler chickens challenged with Eimeria spp. The LS could explain the negative effect on ADG and FCR at 21 and 28 d of age, which was previously noticed by Reid and Johnson [54], who contrasted the LS of birds infected with E. acervulina with their weight reached, finding that at higher LS, a lower ADG was obtained, mostly by one week after the challenge; however, LS are not always correlated in coccidia infections, as reported by Ringenier, et al. [55], who did not find a relationship between LS and FCR in broilers at 28 d of age, concluding that broiler chickens are able to cope with a certain level of gut damage before it influences the overall performance. Other researchers such as Conway, et al. [56] concluded that the correlation between OPG, LS, ADG, and FCR depends on the type of Eimeria and the use or not of anticoccidials.
On the other hand, it has been reported that diet composition [57,58] and phytochemicals modulate intestinal microbiota [44,53]. It is well known that a healthy and functional intestinal microbiome is related to a positive productive performance of the chickens [59,60]. It was reported that when growing broiler chickens that feed on diets supplemented with Allium derivatives, PTSO or PTS are able to influence intestinal microbiota composition [22,61], decreasing enteropathogens and increasing the nutrient absorption in the intestine [22,62,63,64]. In the current study, neither AE-e nor anticoccidials modified the α- or β-diversity in the ileum or the α-diversity in the cecum. Similar findings were noted by Abdelli, et al. [13], who found that dietary supplementation with natural compounds such as organic acids and essential oils does not always result in changes in diversity in microbial populations within the gastrointestinal tract. Nevertheless, in the cecum, we found changes in β-diversity between clades at the taxonomic levels of family and genus, where the challenged groups showed a different spatial distribution to those not challenged, concluding that the infection with Eimeria spp. influences β-diversity, as reported by other researchers [33,65,66]. The analysis of the microbiota clearly showed that the groups under challenge of Eimeria spp., regardless of the presence of anticoccidial drugs or AE-e in the diet, had a higher number of enteropathogens belonging to the Enterobacteriaceae family such as Proteus, Escherichia, and Shigella, as well as other opportunistic pathogens, including the genera Tyzerella, Eggertella, and Biophila.
In our study, we found that in the cecum, at the genus level, C500 had a higher abundance of Ruminococcus, Firmicutes b., and Intestinimonas than C-Ion, considering that Ruminococcus bacteria synthesize digestive enzymes such as cellulases, xylanases, and cellobioses [67,68,69], which contributes to the hydrolysis and fermentation of non-structural carbohydrates, producing butyric acid. It has also been reported that some species of Ruminococcus produce bacteriocins that contribute to controlling undesirable bacterial populations, enhancing the growth of Lactobacillus and promoting intestinal health. All this could explain the improvement in nutrient digestibility and the productive behavior of birds when Ruminococcus is present in abundance. On the other hand, species of the Intestinimonas genus are producers of short-chain fatty acids increasing butyric fatty acid from simple sugars and amino acids such as lysine [70,71]. It connects two important metabolic characteristics, butyric acid production and amino acid fermentation in the intestinal tract. Thus, in this study, the beneficial effects of AE-e on the modulation of the intestinal microbiota were consistent with the results of Vezza, et al. [57], who demonstrated in a murine model of metabolic syndrome that PTSO supplementation at doses of 0.1, 0.5, and 1 mg·kg−1·day−1 counteracts intestinal dysbiosis.
5. Conclusions
In summary, the dose of 750 mg·kg−1 of an Alliaceae encapsulated extract added to the diet of broiler chickens improved the feed conversion ratio in the finisher phase compared to broiler chickens fed a diet without additives. Furthermore, during the finisher phase and cumulative study, its addition to the diet resulted in a positive linear trend in average daily gain and feed conversion ratio.
The anticoccidial index showed a quadratic trend in which the dose of 500 mg·kg−1 of the Alliaceae encapsulated extract displayed the best response. Moreover, it promoted the abundance of some butyrate-producing bacteria such as Intestinimonas and Ruminococcus in the cecum.
However, the best anticoccidial index, feed conversion ratio, oocyst shedding, and intestinal lesion score was observed in the group fed with the anticoccidial ionophore program.
Further research is required to explain the mode of action, as well as determine the optimal dose of the Alliaceae encapsulated extract in the diet of broiler chickens to lessen or control the detrimental effects of coccidiosis under industrial conditions. It is necessary to verify whether using an Alliaceae encapsulated extract in dual programs combined with ionophores or vaccines is feasible.
Author Contributions
Conceptualization, G.V.-P. and M.E.O.-G.; methodology, G.V.-P., M.E.O.-G. and J.C.B.-V.; validation, M.E.O.-G. and J.C.B.-V.; formal analysis, G.V.-P., M.d.C.C.-R., M.E.O.-G. and A.L.; investigation, G.V.-P. and M.E.O.-G.; resources, G.V.-P.; data curation, G.V.-P. and A.L.; writing—original draft preparation, G.V.-P.; writing—review and editing A.H.R.-P. and M.d.C.C.-R.; visualization, A.H.R.-P. and M.d.C.C.-R.; supervision, G.G.-V., G.T. and A.H.R.-P.; project administration, G.V.-P., M.E.O.-G. and J.C.B.-V.; funding acquisition, G.V.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grupo Nutec (Avenida del Marqués 32, Parque Industrial Bernardo Quintana, El Marqués, Querétaro, México, 76246). “https://www.gponutec.com/ (accessed on 16 October 2023)”, funding number iiia 2-19 L-A.
Institutional Review Board Statement
This experiment was approved on 5 October 2020 by the Institutional Subcommittee for the Care and Use of Experimental Animals (protocol SICUAE. DC-2020/3-6) of the School of Veterinary Medicine and Zootechnics of the National Autonomous University of México (UNAM), in compliance with the Mexican Official Norm NOM-062-ZOO-1999.
Informed Consent Statement
The study was conducted at the facilities of the SANUREN (Specialized Animal Nutrition Research Network) experimental farm owned by the Grupo Nutec company. Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available due to privacy.
Acknowledgments
The authors wish to thank all staff members of the Specialized Animal Nutrition Research Network (SANUREN, Querétaro, México) and Carlos Vázquez-Hernández staff member of OMICs who contributed to this study.
Conflicts of Interest
At the time of submitting this manuscript, G. Villar-Patiño, M.E. Olvera-García, and J.C. Baltazar-Vázquez declare a potential direct conflict of interest as they work at Grupo NUTEC. https://www.gponutec.com/ (accessed on 24 October 2023). M.C. Camacho-Rea, G. Gómez-Verduzco, G. Téllez, A. Labastida, and A.H. Ramírez-Pérez have no conflicts of interest.
References
- Britez, J.D.; Rodriguez, A.E.; Di Ciaccio, L.; Marugán-Hernandez, V.; Tomazic, M.L. What do we know about surface proteins of chicken parasites Eimeria? Life 2023, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, L.; Li, L.; Tian, D.; Li, W.; Xu, L.; Yan, R.; Li, X.; Song, X. Protective immunity induced by Eimeria common antigen 14–3-3 against Eimeria tenella, Eimeria acervulina and Eimeria maxima. BMC Vet. Res. 2018, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, T.; Abbas, R.Z.; Imran, M.; Abbas, A.; Butt, A.; Aslam, S.; Ahmad, J. Vaccines against chicken coccidiosis with particular reference to previous decade: Progress, challenges, and opportunities. Parasitol. Res. 2022, 121, 2749–2763. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Pineda, C.; Navarro-Ruíz, J.L.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Chicken coccidiosis: From the parasite lifecycle to control of the disease. Front. Vet. Sci. 2021, 8, 787653. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.R.; Silva, L.J.G.; Pereira, A.M.P.T.; Esteves, A.; Duarte, S.C.; Pena, A. Coccidiostats and poultry: A comprehensive review and current legislation. Foods 2022, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, F.; Al-Quraishy, S.; Steinbrenner, H.; Sies, H.; Dkhil, M.A. Towards identifying novel anti-Eimeria agents: Trace elements, vitamins, and plant-based natural products. Parasitol. Res. 2014, 113, 3547–3556. [Google Scholar] [CrossRef]
- Stefanello, C.; Rosa, D.P.; Dalmoro, Y.K.; Segatto, A.L.; Vieira, M.S.; Moraes, M.L.; Santin, E. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci. 2019, 6, 491. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Khan, S.; Chand, N.; Sadique, U.; Khan, R.U. In vivo effects of Allium cepa L. on the selected gut microflora and intestinal histomorphology in broiler. Acta Histochem. 2017, 119, 446–450. [Google Scholar] [CrossRef]
- Malematja, E.; Manyelo, T.G.; Ng’ambi, J.W.; Nemauluma, M.F.D.; Kolobe, S.D. Effects of onion extracts (Allium cepa) inclusion in diets on growth performance, carcass characteristics, and bone morphometric of broiler chickens. Anim. Biosci. 2023, 36, 1075–1082. [Google Scholar] [CrossRef]
- Kairalla, M.A.; Alshelmani, M.I.; Aburas, A.A. Effect of diet supplemented with graded levels of garlic (Allium sativum L.) powder on growth performance, carcass characteristics, blood hematology, and biochemistry of broilers. Open Vet. J. 2022, 12, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, N.K.; Kheravii, S.K.; Keerqin, C.; Ionescu, C.; Blanchard, A.; Wu, S.B. Potential of a mixture of eugenol and garlic tincture to improve performance and intestinal health in broilers under necrotic enteritis challenge. Anim. Nutr. 2022, 8, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, N.; Perez, J.F.; Vilarrasa, E.; Cabeza Luna, I.; Melo-Duran, D.; D’Angelo, M.; Sola-Oriol, D. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals 2020, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Lee, W.D.; Niu, K.M.; Kim, S.K. The genus Allium as poultry feed additive: A review. Animals 2019, 9, 1032. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Gabric, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursac Kovacevic, D. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.S.; Nandagopal, M.G.; Amin Nordin, S.; Thilakavathy, K.; Joseph, N. Prevailing knowledge on the bioavailability and biological activities of sulphur compounds from Alliums: A potential drug candidate. Molecules 2020, 25, 4111. [Google Scholar] [CrossRef] [PubMed]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.J.; Banos-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, J. In Vitro antibacterial activity of propyl-propane-thiosulfinate and Propyl-propane-thiosulfonate derived from Allium spp. against gram-negative and gram-positive multidrug-resistant bacteria isolated from human samples. Biomed Res. Int. 2018, 2018, 7861207. [Google Scholar] [CrossRef]
- Abad, P.; Arroyo-Manzanares, N.; Rivas-Montoya, E.; Ochando-Pulido, J.M.; Guillamon, E.; García-Campaña, A.M.; Martinez-Ferez, A.; Plaizier, J. Effects of different vehiculization strategies for the allium derivative propyl propane thiosulfonate during dynamic simulation of the pig gastrointestinal tract. Can. J. Anim. Sci. 2019, 99, 244–253. [Google Scholar] [CrossRef]
- Aguinaga-Casanas, M.A.; Mut-Salud, N.; Falcon-Pineiro, A.; Alcaraz-Martinez, A.; Guillamon, E.; Banos, A. In vitro antiparasitic activity of propyl-propane-thiosulfinate (PTS) and propyl-propane-thiosulfonate (PTSO) from Allium cepa against Eimeria acervulina sporozoites. Microorganisms 2022, 10, 2040. [Google Scholar] [CrossRef]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Lillehoj, E.P.; Bravo, D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 2013, 109, 76–88. [Google Scholar] [CrossRef]
- Pourali, M.; Kermanshahi, H.; Golian, A.; Razmi, G.; Soukhtanloo, M. Antioxidant and anticoccidial effects of garlic powder and sulfur amino acids on Eimeria-infected and uninfected broiler chickens. Iran. J. Vet. Res. 2014, 15, 227–232. [Google Scholar]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Aranda-Olmedo, I.; Rubio, L.A. Garlic derivative PTS-O modulates intestinal microbiota composition and improves digestibility in growing broiler chickens. Anim. Feed. Sci. Technol. 2013, 181, 87–92. [Google Scholar] [CrossRef]
- Khan, R.; Nikousefat, Z.; Tufarelli, V.; Naz, S.; Javdani, M.; Laudadio, V. Garlic (Allium sativum) supplementation in poultry diets, effects on production and physiology. World’s Poult. Sci. J. 2012, 68, 417–424. [Google Scholar] [CrossRef]
- Rabelo-Ruiz, M.; Ariza-Romero, J.J.; Zurita-Gonzalez, M.J.; Martin-Platero, A.M.; Banos, A.; Maqueda, M.; Valdivia, E.; Martinez-Bueno, M.; Peralta-Sanchez, J.M. Allium-based phytobiotic enhances egg production in laying hens through microbial composition changes in ileum and cecum. Animals 2021, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Arroyo-Manzanares, N.; Ariza, J.J.; Baños, A.; García-Campaña, A.M. Effect of Allium extract supplementation on egg quality, productivity, and intestinal microbiota of laying hens. Animals 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, P.A.; Conway, D.P.; McKenzie, M.E.; Dayton, A.D.; Chapman, H.D.; Mathis, G.F.; Skinner, J.T.; Mundt, H.C.; Williams, R.B.; World Association for the Advancement of Veterinary, P. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet. Parasitol. 2004, 121, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Long, P.L.; Millard, B.J.; Joyner, L.P.; Norton, C.C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 1976, 6, 201–217. [Google Scholar]
- Kraieski, A.L.; Hayashi, R.M.; Sanches, A.; Almeida, G.C.; Santin, E. Effect of aflatoxin experimental ingestion and Eimeria vaccine challenges on intestinal histopathology and immune cellular dynamic of broilers: Applying an Intestinal Health Index. Poult. Sci. 2017, 96, 1078–1087. [Google Scholar] [CrossRef]
- Moraes, P.O.; Cardinal, K.M.; Gouvêa, F.L.; Schroeder, B.; Ceron, M.S.; Lunedo, R.; Frazzon, A.P.G.; Frazzon, J.; Ribeiro, A.M.L. Comparison between a commercial blend of functional oils and monensin on the performance and microbiota of coccidiosis-challenged broilers. Poult. Sci. 2019, 98, 5456–5464. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Merk Sharp; Dohome Laboratory. Anticoccidial Index; Merk Company: Kenilworth, NJ, USA, 1976. [Google Scholar]
- Wang, L.; Guo, W.; Haq, S.U.; Guo, Z.; Cui, D.; Yang, F.; Cheng, F.; Wei, X.; Lv, J. Anticoccidial activity of Qinghao powder against Eimeria tenella in broiler chickens. Front. Vet. Sci. 2021, 8, 709046. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, W. Interactions of Microbiota and Mucosal Immunity in the Ceca of Broiler Chickens Infected with Eimeria tenella. Vaccines 2022, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Koo, B.-S.; Lee, S.; Mo, J.; Oh, K.; Mo, I. Bacterial diversity and its relationship to growth performance of broilers. Korean J. Vet. Res. 2017, 57, 159–167. [Google Scholar] [CrossRef]
- Yang, C.; Kennes, Y.; Lepp, D.; Yin, X.; Wang, Q.; Yu, H.; Yang, C.; Gong, J.; Diarra, M. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 2020, 99, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. AEM 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Glockner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Ritzi, M.M.; Abdelrahman, W.; Mohnl, M.; Dalloul, R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014, 93, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Osho, S.O.; Xiao, W.W.; Adeola, O. Response of broiler chickens to dietary soybean bioactive peptide and coccidia challenge. Poult. Sci. 2019, 98, 5669–5678. [Google Scholar] [CrossRef] [PubMed]
- Peinado, M.J.; Ruiz, R.; Echavarri, A.; Rubio, L.A. Garlic derivative propyl propane thiosulfonate is effective against broiler enteropathogens in vivo. Poult. Sci. 2012, 91, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Aarti, C.; Khusro, A. Role of garlic (Allium sativum) as feed supplements in poultry industries: An overview. WNOFNS 2020, 29, 151–161. [Google Scholar]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Villar-Patiño, G.; Camacho-Rea, M.d.C.; Olvera-García, M.E.; Soria-Soria, A.; Baltazar-Vázquez, J.C.; Gómez-Verduzco, G.; Solano, L.; Téllez, G.; Ramírez-Pérez, A.H. The effect of encapsulated Propyl propane thiosulfonate (PTSO) on apparent ileal digestibility and productive performance in broiler chickens. Animals 2023, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Chasser, K.M.; Duff, A.F.; Wilson, K.M.; Briggs, W.N.; Latorre, J.D.; Barta, J.R.; Bielke, L.R. Research Note: Evaluating fecal shedding of oocysts in relation to body weight gain and lesion scores during Eimeria infection. Poult. Sci. 2020, 99, 886–892. [Google Scholar] [CrossRef]
- You, M.-J. Detection of four important Eimeria species by multiplex PCR in a single assay. Parasitol. Int. 2014, 63, 527–532. [Google Scholar] [CrossRef]
- Kim, W.H.; Chaudhari, A.A.; Lillehoj, H.S. Involvement of T cell immunity in avian coccidiosis. Front. Immunol. 2019, 10, 2732. [Google Scholar] [CrossRef]
- Elkhtam, A.; Shata, A.; El-Hewaity, M.H. Efficacy of turmeric (Curcuma longa) and garlic (Allium sativum) on Eimeria species in broilers. IJBAS 2014, 3, 349. [Google Scholar] [CrossRef][Green Version]
- Ali, M.; Chand, N.; Khan, R. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019, 1, 79–84. [Google Scholar] [CrossRef]
- Sidiropoulou, E.; Skoufos, I.; Marugan-Hernandez, V.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Blake, D.P.; Tzora, A. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Vet. Sci. 2020, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.M.; Johnson, J. Pathogenicity of Eimeria acervulina in light and heavy coccidial infections. Avian Dis. 1970, 14, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Ringenier, M.; Caekebeke, N.; De Meyer, F.; Van Limbergen, T.; Eeckhaut, V.; Ducatelle, R.; Van Immerseel, F.; Dewulf, J. A field study on correlations between macroscopic gut health scoring, histological measurements and performance parameters in broilers. Avian Pathol. 2021, 50, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.P.; McKenzie, M.E.; Dayton, A.D. Relationship of coccidial lesion scores and weight gain in infections of Eimeria acervulina, E. maxima and E. tenella in broilers. Avian Pathol. 1990, 19, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Garrido-Mesa, J.; Diez-Echave, P.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; García, F.; Sánchez, M.; Toral, M.; Romero, M.; Duarte, J.; et al. Allium-derived compound Propyl propane thiosulfonate (PTSO) attenuates metabolic alterations in mice fed a high-fat diet through its anti-inflammatory and prebiotic properties. Nutrients 2021, 13, 2595. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.; Clinger, E.; Hao, L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Pillay, C.; Nyaga, M.M.; Sabiu, S. Poultry gut health-microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. J. Anim. Sci. Biotechnol. 2021, 12, 119. [Google Scholar] [CrossRef]
- Mohammadi, R.; Hosseini-Safa, A.; Ehsani Ardakani, M.J.; Rostami-Nejad, M. The relationship between intestinal parasites and some immune-mediated intestinal conditions. Gastroenterol. Hepatol. Bed Bench 2015, 8, 123–131. [Google Scholar]
- Kumar, S.; Sharadamma, K.C.; Radhakrish, P.M. Effects of a garlic active based growth promoter on growth performance and specific pathogenic intestinal microbial counts of broiler chicks. Int. J. Poult. Sci. 2010, 9, 244–246. [Google Scholar] [CrossRef]
- Pelicano, E.R.; Souza, P.A.; Souza, H.B.A.; Figueiredo, D.F.; Boiago, M.M.; Carvalho, S.R.; Bordon, V.F. Intestinal mucosa development in broiler chickens fed natural growth promoters. Braz. J. Poult. Sci. 2005, 7, 221–229. [Google Scholar] [CrossRef]
- Kim, G.B.; Seo, Y.M.; Kim, C.H.; Paik, I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011, 90, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Frick, J.S.; Autenrieth, I.B. The gut microflora and its variety of roles in health and disease. Curr. Top. Microbiol. Immunol. 2013, 358, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.M.; Miska, K.B.; Jenkins, M.C.; Yan, X.; Proszkowiec-Weglarz, M. Effects of Eimeria acervulina infection on the luminal and mucosal microbiota of the duodenum and jejunum in broiler chickens. Front. Microbiol. 2023, 14, 1147579. [Google Scholar] [CrossRef] [PubMed]
- Jebessa, E.; Guo, L.; Chen, X.; Bello, S.F.; Cai, B.; Girma, M.; Hanotte, O.; Nie, Q. Influence of Eimeria maxima coccidia infection on gut microbiome diversity and composition of the jejunum and cecum of indigenous chicken. Front. Immunol. 2022, 13, 994224. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; McPherson, C.A.; Martin, J. Expression of two xylanase genes from the rumen cellulolytic bacterium Ruminococcus flavefaciens 17 cloned in pUC13. Microbiology 1991, 137, 123–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pettipher, G.L.; Latham, M.J. Characteristics of enzymes produced by Ruminococcus flavefaciens which degrade plant cell walls. Microbiology 1979, 110, 21–27. [Google Scholar] [CrossRef]
- Saburi, W.; Yamamoto, T.; Taguchi, H.; Hamada, S.; Matsui, H. Practical preparation of epilactose produced with cellobiose 2-epimerase from Ruminococcus albus NE1. Biosci. Biotechnol. Biochem. 2010, 74, 1736–1737. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; de Waard, P.; Plugge, C.M.; de Vos, W.M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6, 10062. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Wu, S.; Madsen, M.H.; Shi, S. Supplementing the early diet of broilers with soy protein concentrate can improve intestinal development and enhance short-chain fatty acid-producing microbes and short-chain fatty acids, especially butyric acid. J. Anim. Sci. Biotechnol. 2022, 13, 97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).