The Immunoprotective Effect of ROP27 Protein of Eimeria tenella
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals and Parasite
2.3. Reagents
2.4. Plasmid Construction
2.5. Protein Expression and Purification
2.6. Preparation of Anti-E. tenella and Anti-rEtROP27 Positive Serum
2.7. Indirect ELISA Determination of Antibody Titer
2.8. Western Blotting Analysis
2.9. Primary Culture of Chicken Embryo Caecal Epithelial Cells
2.10. E. tenella Sporozoite Preparation
2.11. RT-PCR Analysis
2.12. Animal Experiment
2.13. Concentration of Serum Antibody
2.14. In Vivo Immunoprotective Parameters
2.15. Image and Statistical Analyses
3. Results
3.1. Gene Clone and Plasmid Construction
3.2. Expression and Purification of rEtROP27 Protein
3.3. Determination of rEtROP27 Polyclonal Antibody Titer
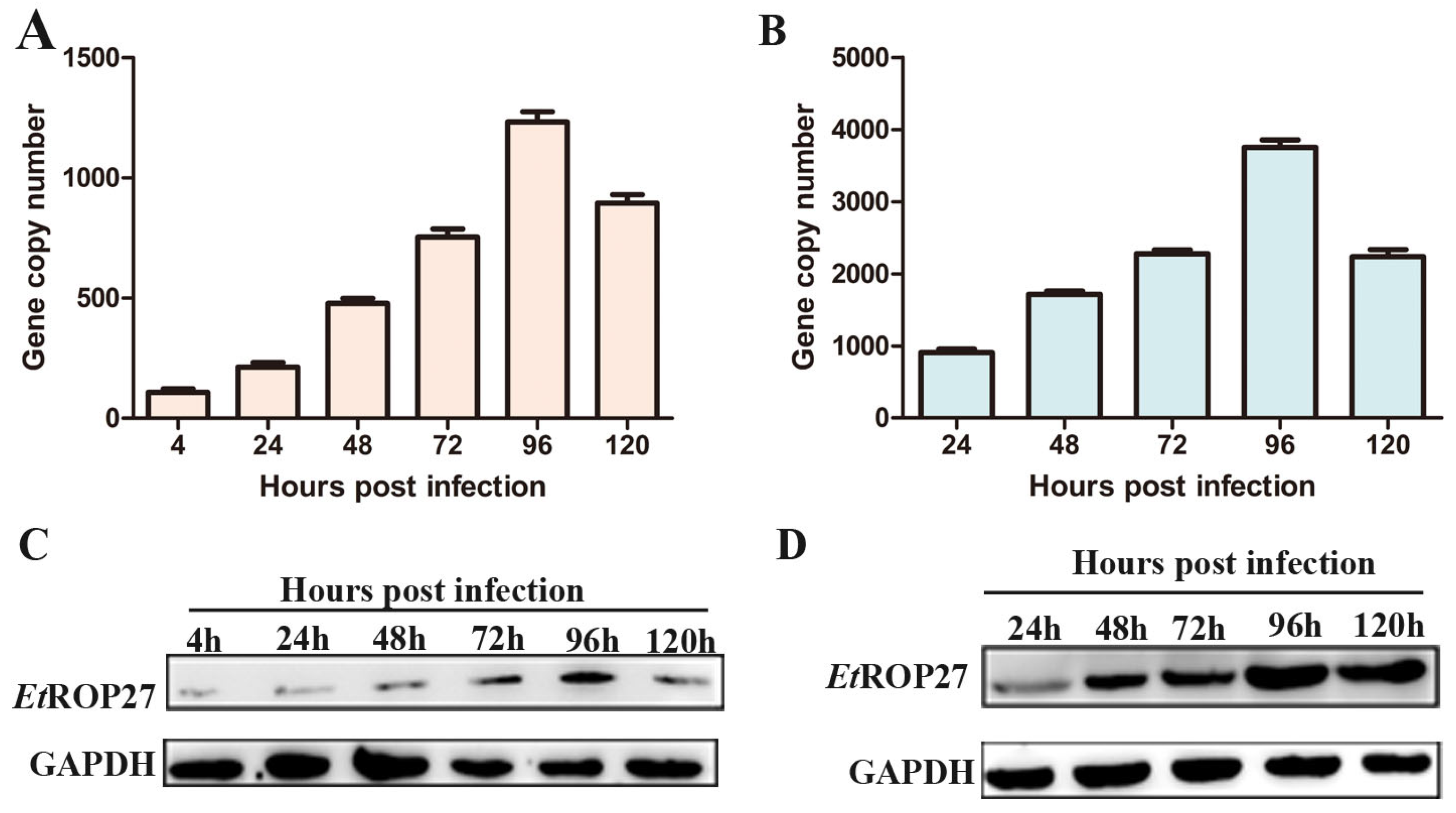
3.4. Changes in EtROP27 Expression
3.5. In Vivo Immunoprotective Effect of rEtROP27 Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, X.-L.; Wang, Y.-Y.; Zheng, M.-X.; Bai, R.; Zhang, L.; Duan, B.-T.; Lei, X.; Zhang, X.-S.; Zhao, Y.-J.; Cui, K.-L.; et al. The role of Ca2+ in the injury of host cells during the schizogenic stage of E. tenella. Poult. Sci. 2022, 101, 101916. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chen, Z.; Zheng, M.; Bai, R.; Zhang, L.; Zhang, X.; Duan, B.; Zhao, Y.; Yin, L.; Fan, B.; et al. The interaction between free Ca2+ in host cells and invasion of E. tenella. Parasitol. Res. 2022, 121, 965–972. [Google Scholar] [CrossRef]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Li, C.; Gu, X.L.; Lu, C.X.; Liao, Q.; Liu, X.Y.; Suo, X. Progress in epidemiological research on chicken coccidiosis in China. Acta Parasitol. Med. Entomol. Sin. 2018, 25, 230–241. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Zheng, M.; Zhang, L.; Bai, R.; Li, R.; Hao, S.; Bai, B.; Kang, H. Effects of the PI3K/Akt signaling pathway on the apoptosis of early host cells infected with Eimeria tenella. Parasitol. Res. 2020, 119, 2549–2561. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, B.-T.; Zhao, Y.-J.; Cui, K.-L.; Xu, T.; Zhang, X.-S.; Lv, X.-L.; Guo, L.-L.; Zheng, M.-X.; Bai, R. Pathogenic mechanism of Eimeria tenella autophagy activation of chicken embryo cecal epithelial cells induced by Eimeria tenella. Poult. Sci. 2023, 102, 102535. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, M.; Zhang, L.; Zhang, X.; Zhang, Y.; Cui, X.; Gong, X.; Xi, R.; Bai, R. Effect of ATP and Bax on the apoptosis of Eimeria tenella host cells. BMC Vet. Res. 2017, 13, 399. [Google Scholar] [CrossRef]
- Xu, Z.-Y.; Zheng, M.-X.; Zhang, L.; Gong, X.; Xi, R.; Cui, X.-Z.; Bai, R. Dynamic expression of death receptor adapter proteins tradd and fadd in Eimeria tenella-induced host cell apoptosis. Poult. Sci. 2017, 96, 1438–1444. [Google Scholar] [CrossRef]
- Li, S.; Zheng, M.-X.; Xu, H.-C.; Cui, X.-Z.; Zhang, Y.; Zhang, L.; Yang, S.-S.; Xu, Z.-Y.; Bai, R.; Sun, X.-G. Mitochondrial pathways are involved in Eimeria tenella-induced apoptosis of chick embryo cecal epithelial cells. Parasitol. Res. 2017, 116, 225–235. [Google Scholar] [CrossRef]
- Jenkins, M.C.; Parker, C.; Ritter, D. Eimeria Oocyst Concentrations and Species Composition in Litter from Commercial Broiler Farms During Anticoccidial Drug or Live Eimeria Oocyst Vaccine Control Programs. Avian Dis. 2017, 61, 214–220. [Google Scholar] [CrossRef]
- Lee, Y.; Lu, M.; Lillehoj, H.S. Coccidiosis: Recent Progress in Host Immunity and Alternatives to Antibiotic Strategies. Vaccines 2022, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Possenti, A.; Di Cristina, M.; Nicastro, C.; Lunghi, M.; Messina, V.; Piro, F.; Tramontana, L.; Cherchi, S.; Falchi, M.; Bertuccini, L.; et al. Functional Characterization of the Thrombospondin-Related Paralogous Proteins Rhoptry Discharge Factors 1 and 2 Unveils Phenotypic Plasticity in Toxoplasma gondii Rhoptry Exocytosis. Front. Microbiol. 2022, 13, 899243. [Google Scholar] [CrossRef] [PubMed]
- Sparvoli, D.; Delabre, J.; Penarete-Vargas, D.M.; Mageswaran, S.K.; Tsypin, L.M.; Heckendorn, J.; Theveny, L.; Maynadier, M.; Cova, M.M.; Berry-Sterkers, L.; et al. An apical membrane complex for triggering rhoptry exocytosis and invasion in Toxoplasma. EMBO J. 2022, 41, e111158. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.; Arévalo-Pinzón, G.; Rubio, L.; Chaloin, O.; Muller, S.; Curtidor, H.; Patarroyo, M.A. Receptor-ligand and parasite protein-protein interactions in Plasmodium vivax: Analysing rhoptry neck proteins 2 and 4. Cell. Microbiol. 2018, 20, e12835. [Google Scholar] [CrossRef]
- Oakes, R.D.; Kurian, D.; Bromley, E.; Ward, C.; Lal, K.; Blake, D.P.; Reid, A.J.; Pain, A.; Sinden, R.E.; Wastling, J.M.; et al. The rhoptry proteome of Eimeria tenella sporozoites. Int. J. Parasitol. 2013, 43, 181–188. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, M.-X.; Xi, R.; Xu, Z.-Y.; Zhang, X.-S.; Zheng, L.-L.; Bai, R.; Mi, C.-L.; Hao, F.-F.; Feng, Y.-P. Comparison of the host cells apoptosis induced by precocious strains and virulent strains of Eimeria tenella. Poult. Sci. 2019, 98, 4384–4390. [Google Scholar] [CrossRef]
- Zhang, L. Study on Pathogenicity Differences and Pathogenic Genes of E. tenella Precocious Strains and Virulent Strains. 2020. Available online: https://doi.org/10.27285/d.cnki.gsxnu.2020.000042 (accessed on 16 March 2023).
- Zhang, D.; Jiang, N.; Chen, Q. Vaccination with recombinant adenoviruses expressing Toxoplasma gondii MIC3, ROP9, and SAG2 provide protective immunity against acute toxoplasmosis in mice. Vaccine 2019, 37, 1118–1125. [Google Scholar] [CrossRef]
- Morehouse, N.F.; Baron, R.R. Coccidiosis: Evaluation of coccidiostats by mortality, weight gains, and fecal scores. Exp. Parasitol. 1970, 28, 25–29. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Long, P.L.; Millard, B.J.; Joyner, L.P.; Norton, C.C. A guide to laboratory techniques used in the study and diagnosis of avian coccidia. Folia Vet. Lat. 1976, 6, 201–217. [Google Scholar]
- Chapman, H. Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl. Int. J. Parasitol. 1998, 28, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Flores, R.A.; Cammayo, P.L.T.; Kim, S.; Kim, W.H.; Min, W. Anticoccidial activity of berberine against Eimeria-infected chickens. Korean J. Parasitol. 2021, 59, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.E.; Nolan, M.J.; Harman, K.; Boulton, K.; Hume, D.A.; Tomley, F.M.; Stabler, R.A.; Blake, D.P. Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. PLoS ONE 2017, 12, e0184890. [Google Scholar] [CrossRef] [PubMed]
- Bafundo, K.; Gomez, L.; Lumpkins, B.; Mathis, G.; McNaughton, J.; Duerr, I. Concurrent use of saponins and live coccidiosis vaccines: The influence of a quillaja and yucca combination on anticoccidial effects and performance results of coccidia-vaccinated broilers. Poult. Sci. 2021, 100, 100905. [Google Scholar] [CrossRef] [PubMed]
- Fatoba, A.J.; Adeleke, M.A. Transgenic Eimeria parasite: A potential control strategy for chicken coccidiosis. Acta Trop. 2020, 205, 105417. [Google Scholar] [CrossRef]
- Huang, J.; Chen, S.; Zhou, Z.; Sun, X.; Haseeb, M.; Lakho, S.A.; Zhang, Y.; Liu, J.; Shah, M.A.A.; Song, X.; et al. Poly (D, L-lactide-co-glycolide) delivery system improve the protective efficacy of recombinant antigen TA4 against Eimeria tenella infection. Poult. Sci. 2021, 100, 101083. [Google Scholar] [CrossRef]
- Patarroyo, M.A.; Molina-Franky, J.; Gómez, M.; Arévalo-Pinzón, G.; Patarroyo, M.E. Hotspots in Plasmodium and RBC Receptor-Ligand Interactions: Key Pieces for Inhibiting Malarial Parasite Invasion. Int. J. Mol. Sci. 2020, 21, 4729. [Google Scholar] [CrossRef]
- Lim, D.C.; Cooke, B.M.; Doerig, C.; Saeij, J.P. Toxoplasma and Plasmodium protein kinases: Roles in invasion and host cell remodelling. Int. J. Parasitol. 2012, 42, 21–32. [Google Scholar] [CrossRef]
- Pan, M.; Zhou, Y.; Wang, Y.; Li, L.; Song, Y.; Hou, L.; Zhao, J. Screening and Identification of the Host Proteins Interacting with Toxoplasma gondii Rhoptry Protein ROP16. Front. Microbiol. 2017, 8, 2408. [Google Scholar] [CrossRef]
- Ihara, F.; Nishikawa, Y. Toxoplasma gondii manipulates host cell signaling pathways via its secreted effector molecules. Parasitol. Int. 2021, 83, 102368. [Google Scholar] [CrossRef]
- Meng, Y.-J.; Mu, B.-J.; Liu, X.-X.; Yu, L.-M.; Zheng, W.-B.; Xie, S.-C.; Gao, W.-W.; Zhu, X.-Q.; Liu, Q. Transcriptional changes in LMH cells induced by Eimeria tenella rhoptry kinase family protein 17. Front. Vet. Sci. 2022, 9, 956040. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mu, B.; Zheng, W.; Meng, Y.; Yu, L.; Gao, W.; Zhu, X.; Liu, Q. Identification and Protective Efficacy of Eimeria tenella Rhoptry Kinase Family Protein 17. Animals 2022, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, N.; Sun, J.; Sun, L.; Li, H.; Wu, Z.; Li, H.; Zhang, X.; Zhao, X. Identification and Characterization of Eimeria tenella Rhoptry Protein 35 (EtROP35). Vet. Sci. 2022, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Bingxiang, W.; Ningning, Z.; Yakun, W.; Lingyu, S.; Hongmei, L.; Zhang, X.; Zhao, X. Characterization of the Eimeria tenella rhoptry protein with a nuclear localization sequence (EtROP30). Parasitol. Res. 2022, 121, 1507–1516. [Google Scholar] [CrossRef]
- Fernandez-Becerra, C.; Bernabeu, M.; Castellanos, A.; Correa, B.R.; Obadia, T.; Ramirez, M.; Rui, E.; Hentzschel, F.; López-Montañés, M.; Ayllon-Hermida, A.; et al. Plasmodium vivax spleen-dependent genes encode antigens associated with cytoadhesion and clinical protection. Proc. Natl. Acad. Sci. USA 2020, 117, 13056–13065. [Google Scholar] [CrossRef]
- Rezaei, F.; Sarvi, S.; Sharif, M.; Hejazi, S.H.; Pagheh, A.S.; Aghayan, S.A.; Daryani, A. A systematic review of Toxoplasma gondii antigens to find the best vaccine candidates for immunization. Microb. Pathog. 2019, 126, 172–184. [Google Scholar] [CrossRef]




| Groups | Immunization | Dose | Challenge |
|---|---|---|---|
| Unchallenged control | PBS + adjuvant | / | / |
| Challenged control | PBS + adjuvant | / | E. tenella sporulated oocysts (5 × 104) |
| EtROP27 (50 μg) | rEtROP27 protein + adjuvant | 50 μg | E. tenella sporulated oocysts (5 × 104) |
| EtROP27 (100 μg) | rEtROP27 protein + adjuvant | 100 μg | E. tenella sporulated oocysts (5 × 104) |
| EtROP27 (150 μg) | rEtROP27 protein + adjuvant | 150 μg | E. tenella sporulated oocysts (5 × 104) |
| Antibody Dilution Ratio | Negative Serum | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:100 | 1:200 | 1:400 | 1:800 | 1:1600 | 1:3200 | 1:6400 | 1:12,800 | 1:25,600 | |
| * | * | * | 3.442 | 2.532 | 2.015 | 1.503 | 1.022 | 0.516 | 0.36 |
| Group | Relative Body Weight Gain (%) | Oocyst Index | Cecum Mean Lesion Score | Anticoccidial Index |
|---|---|---|---|---|
| Unchallenged control | 100 | 0 | 0 | 200.00 |
| Challenged control | 60.25 | 5 | 2.9 | 87.25 |
| EtROP27 (50 μg) | 80.45 | 1 | 2.0 | 159.45 |
| EtROP27 (100 μg) | 89.47 | 1 | 1.7 | 171.47 |
| EtROP27 (150 μg) | 86.75 | 1 | 1.9 | 166.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Lv, X.; Zheng, M.; Wei, Y. The Immunoprotective Effect of ROP27 Protein of Eimeria tenella. Animals 2023, 13, 3500. https://doi.org/10.3390/ani13223500
Li M, Lv X, Zheng M, Wei Y. The Immunoprotective Effect of ROP27 Protein of Eimeria tenella. Animals. 2023; 13(22):3500. https://doi.org/10.3390/ani13223500
Chicago/Turabian StyleLi, Menggang, Xiaoling Lv, Mingxue Zheng, and Yingyi Wei. 2023. "The Immunoprotective Effect of ROP27 Protein of Eimeria tenella" Animals 13, no. 22: 3500. https://doi.org/10.3390/ani13223500
APA StyleLi, M., Lv, X., Zheng, M., & Wei, Y. (2023). The Immunoprotective Effect of ROP27 Protein of Eimeria tenella. Animals, 13(22), 3500. https://doi.org/10.3390/ani13223500






