Simple Summary
Anticoccidial agents, crucial for controlling parasitic infections in poultry, should ideally interfere with multiple stages of the parasite’s lifecycle. This study aimed to evaluate the influence of anticoccidial drugs, natural essential oils, and their pure bioactive compounds on the first round of schizogony of Eimeria tenella in vitro. The results revealed that both essential oils and conventional anticoccidial compounds were equally adept at preventing schizont formation. However, the pure bioactive compounds displayed only a slightly reduced level of development, indicating a potential decrease in pathogenicity. This investigation sheds light on the capacity of natural substances to disrupt the intracellular development of the E. tenella parasite, providing valuable insights into their mechanisms of action and their potential for safer alternatives in anticoccidial development.
Abstract
Coccidiosis poses a significant challenge in poultry production and is typically managed with ionophores and chemical anticoccidials. However, the emergence of drug resistance and limitations on their use have encouraged the exploration of alternative solutions, including botanical compounds and improvements in in vitro screening methods. Prior research focused only on the impact of these alternatives on Eimeria invasion, with intracellular development in cell cultures receiving limited attention. This study assessed the impact of thyme (Thymus vulgaris), oregano (Origanum vulgare), and garlic (Allium sativum) essential oils, as well as their bioactive compounds, on the initial phase of schizogony in Madin–Darby bovine kidney cells, comparing their effectiveness to two commercially used anticoccidial drugs. Using image analysis and quantitative PCR, the study confirmed the efficacy of commercial anticoccidials in reducing invasion and schizont formation, and it found that essential oils were equally effective. Notably, thymol and carvacrol exhibited mild inhibition of intracellular replication of the parasite but significantly reduced schizont numbers, implying a potential reduction in pathogenicity. In conclusion, this research highlights the promise of essential oils and their bioactive components as viable alternatives to traditional anticoccidial drugs for mitigating coccidiosis in poultry, particularly by disrupting the intracellular development of the parasites.
1. Introduction
Avian coccidiosis is one of the most concerning parasitic diseases as it poses a significant threat to the health of birds and the poultry industry, which is estimated to be up to USD 13 billion per year [1]. Eimeria spp. are recognized as the causative agent of the disease, and they are protozoan parasites that replicate inside the host’s enterocytes, causing inflammation, ulceration, and bleeding. This results in poor nutrient absorption and growth. High mortality rates are also common in severe cases, while, in sub-clinical cases, an infection with Eimeria spp. can predispose to secondary opportunistic pathogen infections, such as Clostridium perfringens, Campylobacter jejuni, and Salmonella infections [2,3,4].
The treatment and prevention of coccidiosis relies on the use of ionophores and chemical anticoccidials; however, spreading of resistance due to their extensive use has raised concerns in the poultry industry [5]. Significant research efforts have been directed towards exploring alternatives to anticoccidials. Particularly, attention has been focused on botanical compounds and natural remedies, which allegedly exhibit the ability to disrupt multiple stages of parasite development, offering promising avenues for combating this parasitic disease [6].
Among the various compounds that have been tested, essential oils (EOs) derived from Lamiaceae and Amaryllidaceae (i.e., Oregano and Allium spp.) have shown positive outcomes in alleviating the clinical signs of coccidiosis in vivo and in reducing oocyst shedding after infection. In addition, in some cases, an improvement in the Eimeria-induced lesions has been assessed [7,8,9]. The positive effects of these substances are believed to be due to the bioactive molecules they contain. Thymol and carvacrol are two of the main monoterpene phenols of Lamiaceae plants, like thyme and oregano. They have well-documented antibacterial and immunomodulatory proprieties, which make them good candidates to treat enteric infections [10,11]. Also, recent studies have claimed them as anti-protozoal agents against Lehismania spp. and Cryptosporidium parvum [12,13]. Allium spp. contain a wide pool of biologically active organosulfur compounds, including allin, ajoene, allicin, diallyl sulphide, and S-allylcysteine, showcasing antibacterial, anti-inflammatory, antiseptic, antiparasitic, and immunomodulatory properties [14,15].
However, due to their high variability, these botanical mixtures are far from being adequately characterized, especially on their anticoccidial proprieties and modes of action. To do so, an improvement of the available screening methodologies for novel anticoccidial candidates is needed.
The challenges associated with obtaining reliable and standardized in vitro models have led to a predominant reliance on in vivo research methods for studying these alternatives to ionophores and chemical anticoccidials. However, conducting research in vivo presents certain limitations, including the intricate and variable nature of these models and ethical concerns associated with animal experimentation, as well as the high cost and restricted availability of necessary resources [16].
Among the various species causing chicken coccidiosis, E. tenella holds particular significance due to its high pathogenicity. For this reason, it has been the most studied avian Eimeria species. Consequently, it serves as a valuable model for studying and conducting research on these parasites in vitro [16]. In addition, unlike other species, E. tenella is able to undergo the first round of schizogony in immortalized mammalian cell lines, like Madin–Darby bovine kidney (MDBK) cells [17]. This property has been used in previous studies to establish a robust in vitro assay for evaluating the first round of schizogony [18]. Moreover, researchers utilized a transgenic fluorescent strain of E. tenella, which expresses yellow fluorescent protein (YFP), to visually examine the developmental timeline and the impact of certain anticoccidial drugs [18].
In the present study, this methodology has been used to examine the anticoccidial proprieties of three essential oils derived from thyme (Thymus vulgaris), oregano (Oregano vulgaris), and garlic (Allium sativum), and their main bioactive compounds (‘nature-identical compounds’, NIC), on the development of E. tenella. Also, the effects of these natural compounds have been compared to two common anticoccidial drugs (AC): salinomycin, an ionophore drug that alters the membrane gradient, and robenidine, a guanidine derivative whose main action is the inhibition of maturation of 1st generation schizonts [19].
2. Material and Methods
2.1. Parasites and Birds
The strain E. tenella dYFP, derived from the Wisconsin strain [20], was propagated in 3-week-old White Leghorn chickens reared under specific pathogen-free conditions [21]. Oocyst harvest, excystation, and sporozoite purification were conducted following the methods described in a previous study [22]. Freshly purified sporozoites (0.1 × 106 per replicate) were used to infect a cell monolayer in a 96-well plate format.
2.2. Cell Culture
Madin–Darby bovine kidney cells (MDBK-NBL-1, ECACC-Sigma-Aldrich, Salisbury, UK) were maintained in cell culture flasks in Advanced DMEM (Cat. #11520436—Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 5% fetal bovine serum (Cat. #11573397—Thermo Fisher Scientific, Waltham, MA, USA), 1× penicillin/streptomycin solution (Cat. #11548876—Thermo Fisher Scientific, Waltham, MA, USA), and 1× Glutamax (Cat. #11574466—Thermo Fisher Scientific, Waltham, MA, USA). Four hours prior to invasion, the cells were seeded in 96-well plates (Cat. #10687551—Thermo Fisher Scientific, Waltham, MA, USA) at a concentration of 0.05 × 106 cells/well.
2.3. Chemicals and Reagents
Salinomycin from Streptomyces albus (Cat. # S4526, Sigma-Aldrich, St. Louis, MO, USA) and robenidine hydrochloride (Cat. # 33979, Sigma-Aldrich, St. Louis, MO, USA) were resuspended, respectively, with ethanol and dimethyl-sulfoxide (Cat. #D2650, Sigma-Aldrich, St. Louis, MO, USA) and used at a final concentration of 1 and 5 ppm, respectively.
All EOs in this study were analyzed at the same concentration (40 ppm), and the NIC were tested at 20 ppm. The choice of a single concentration was based on a previous study [23].
Oregano essential oil (OEO) was provided by Galen-N (Galen-N Ltd., Sofia, Bulgaria). Garlic oil (GAR) was purchased from Lluch Essence (Lluch Essence S.L.U, Barcelona, Spain) and thyme essential oil (TEO) from Grupo Indukern (Grupo Indukern, Madrid, Spain). All the stock solutions were prepared in ethanol and added to cells, with a final concentration of 40 ppm in supplemented Advanced DMEM. In all cases, the final concentration of ethanol was less than 0.5% (v/v).
Carvacrol (CAR—Cat. #W224511, analytical grade 99%, Sigma-Aldrich, St. Louis, MO, USA), diallyl-disulfide (DDS—Cat. #SMB00378, analytical grade ≥ 98%, Sigma-Aldrich, St. Louis, MO, USA), and thymol (THY—Cat. #T0501, analytical grade ≥ 98.5%, Sigma-Aldrich, St. Louis, MO, USA) were all diluted in ethanol to prepare stock solutions and used at a final concentration of 20 ppm in supplemented Advanced DMEM.
2.4. Development Assay
After purification, the sporozoites were added to the cells in the absence or presence of the described treatments. The cells were incubated at 41 °C with 5% CO2. After 4 h of incubation, the non-invading sporozoites were removed from the wells, and the medium with the treatments was replenished. After 48 h, the cells were provided with fresh medium along with the respective treatments.
After 24, 48, 72, and 96 h post-infection (hpi), the cells were either fixed in 4% paraformaldehyde or harvested with RTL buffer (Qiagen, Hilden, Germany), as described by the manufacturer’s protocol, for subsequent nucleic acid extraction. Each experiment was repeated at least twice.
2.5. Isolation of Nucleic Acids
Genomic DNA (gDNA) was isolated from the samples stored in RTL buffer. The AllPrep DNA/RNA 96 Kit (Cat. #80311—Qiagen, Hilden, Germany) was used for the isolation process, following the manufacturer’s instructions. A vacuum manifold designed for processing 96-well plates (Cat. #9014579—Qiagen, Hilden, Germany) was employed for this purpose.
2.6. Real Time Quantitative PCR (qPCR)
To perform qPCR, the CFX96 Touch R Real-Time PCR Detection System (C1000—Bio-Rad, Hercules, CA, USA) was used. The procedures were carried out according to previously described methods, utilizing the DNA-binding dye SsoFastTM EvaGreen Supermix (Cat. #172-5203—Bio-Rad, Hercules, CA, USA) [24].
For parasite quantification, the number of haploid genomes (equivalent to single sporozoites) per well (four wells per sample as technical replicates) was determined. This quantification was achieved using gDNA-specific primers targeting E. tenella 5S rDNA (Fw_5S: TCATCACCCAAAGGGATT, Rv_5S: TTCATACTGCGTCTAATGCAC) [20]. A standard curve of sporozoite gDNA, extracted using the same methods, was generated [25]. This standard curve encompassed gDNA equivalent to 107 genomes, followed by serial dilution until 102 genomes.
The inhibition of invasion at 24 hpi was calculated with the following equation developed by Thabet et al. [26]:
To compare the replication rates after treatments, excluding the effects of invasion, slopes of the replication lines were calculated between 24 and 72 hpi. The slopes were calculated with the formula below, as previously described by Arias-Maroto et al. [25]:
2.7. Image Analysis
The paraformaldehyde-fixed samples were observed using a fluorescence microscope (Nikon Eclipse Ti2—Nikon, Tokyo, Japan) at 20×, using Plan fluor Nikon lenses at a wavelength of 490 nm. Six random fields were captured and analyzed with a semi-automated procedure relying on a custom script designed on ImageJ. Each picture was converted into an 8-bit image before applying threshold masks separately and converting the picture into a binary image. On the binary images, two possible values (black or white) were given to each pixel, according to the intensity of the fluorescence. The final particle analysis involved identifying the edges of the black and white structures; this allowed the recording of particle numbers and sizes, both on average and individually. These data were collected and processed for statistical analysis. To assess general parasite growth, the average area, including any sort of stage (sporozoites and schizonts), was calculated for every field. Also, for specific cases where a certain level of growth was observed, the schizont area was calculated separately, assuming as schizonts the particles with an area higher than 50 μm2. The rate of schizont growth was calculated as the slope of the regression line between 24 and 72 hpi, as indicated by the formula reported in the previous section.
2.8. Statistical Analysis
GraphPad Prism 9.4.1 was utilized for statistical analysis. For the qPCR data, the number of genomes was normalized based on the mean value of the challenged control at 24 hpi of each independent experiment. For all data, the descriptive analysis was conducted, and normality was assessed with the Shapiro–Wilk test (p > 0.05). Normally distributed data were analyzed using either a one-way ANOVA (for schizont size) or a two-way ANOVA (for repeated measurements). Non-normally distributed data were analyzed using the Kruskal-Wallis test (for schizont number) or mixed-effects analysis (for repeated measurements). To define significant differences among treatments, post hoc multiple comparison analyses were performed: the mean of each treatment was compared with the mean of every other treatment using Tukey’s test for normally distributed data and mixed-effects analysis, while Dunn’s test was adopted for non-normally distributed data. Differences were considered significant when the p value was ≤0.05 and were denoted as letters.
3. Results
3.1. Characterization of E. tenella Intracellular Growth in MDBK Cells
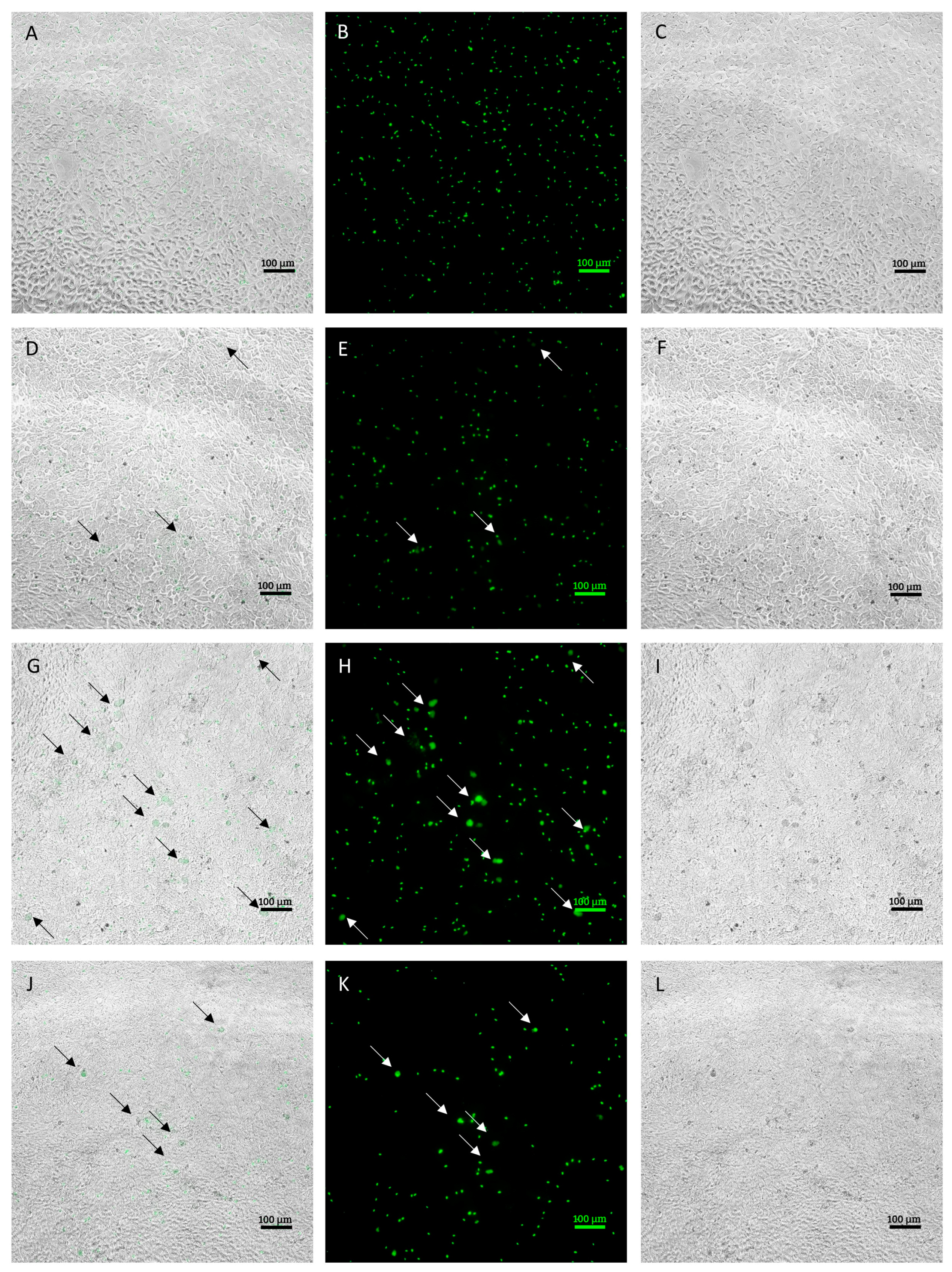
Infected MDBK cells were examined using a fluorescence microscope to evaluate the morphological characteristics of the different developmental stages obtained in culture on a 96-h time course. Figure 1 shows representative images of the development at each time point.
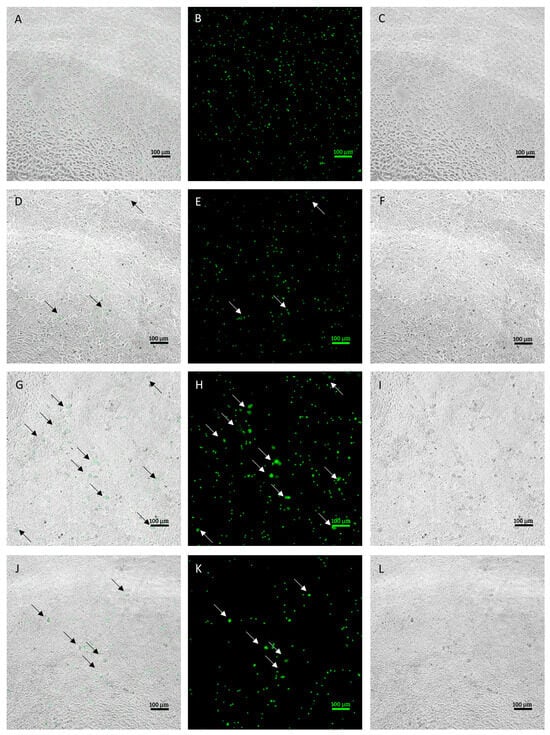
Figure 1.
Intracellular development time course (20× objective). Images (A–C) indicate the intracellular sporozoites at 24 h post infection (hpi) ((A) = merge, (B) = green fluorescence, (C) = brightfield). Images (D–F) indicate intracellular sporozoites at 48 hpi and early stages of development, indicated by black arrows in merge pictures and white arrows in green fluorescence pictures ((D) = merge, (E) = green fluorescence, (F) = brightfield). Images (G–I) indicate intracellular parasite at 72 hpi and late stages of development, indicated by white arrows ((G) = merge, (H) = green fluorescence, (I) = brightfield). Images J–L indicate intracellular parasites at 96 hpi. Residual developmental stages are indicated by white arrows ((J) = merge, (K) = green fluorescence, (L) = brightfield). Size bar ~100 μm.
During the first phases of infection, occurring within 24 hpi, sporozoites that successfully invaded the cells were observed. At 48 hpi, it was already possible to observe some early stages of development, corresponding to early schizonts. Those appeared as round objects, larger in size compared to intracellular sporozoites. At 72 hpi, larger schizonts were clearly observed in the infected monolayer. Compared to early schizonts, which are difficult to distinguish using bright field, late ones appeared more condensed and, in most cases, were clearly discernable in the monolayer. After 96 h, the number and size of schizonts dropped visibly due to schizont breakage.
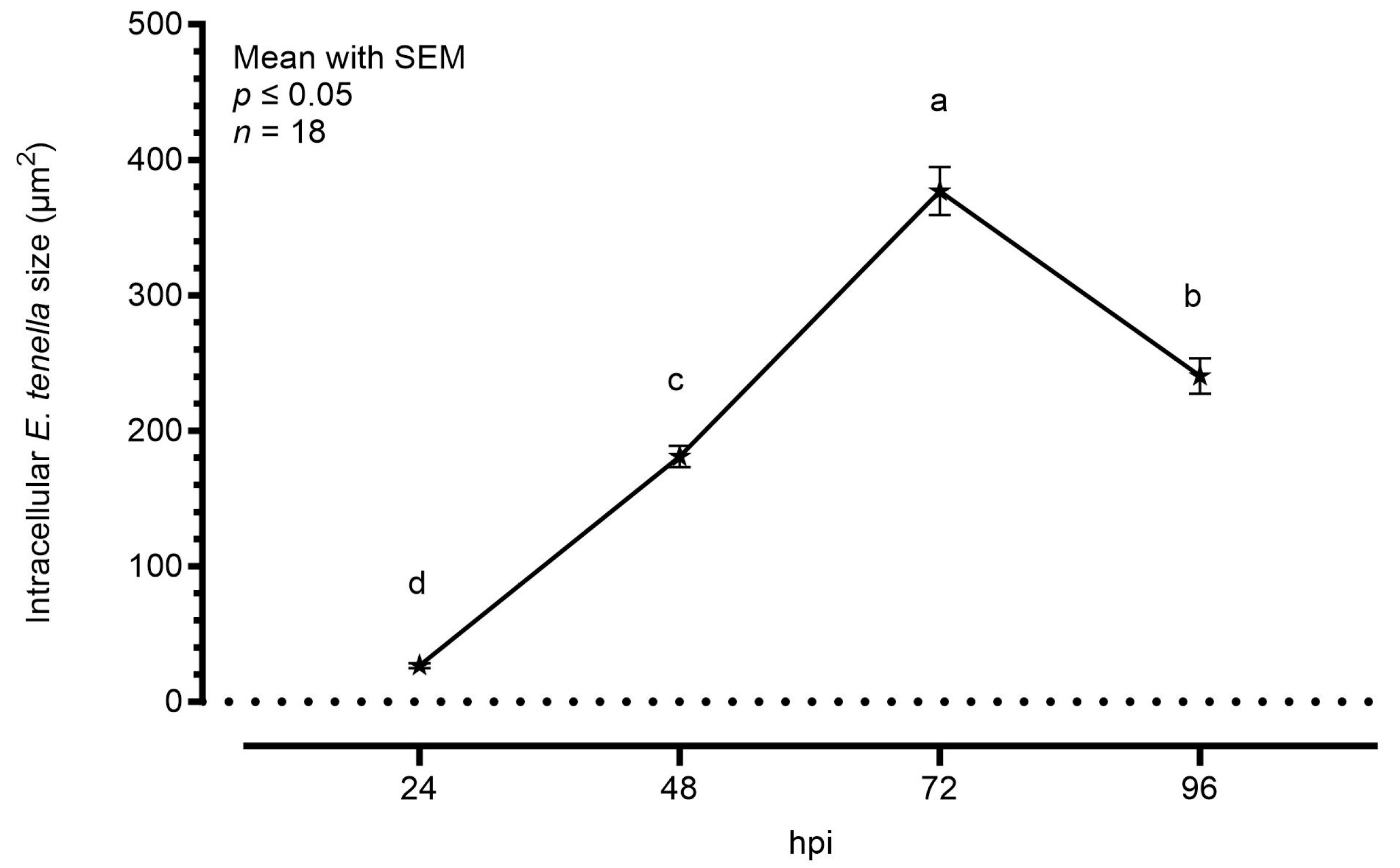
Figure 2 shows the average area increase of intracellular parasites during the time course, considering sporozoites at 24 hpi and only schizonts at later timepoints. Starting from an area of around 30.0 μm2, the intracellular sporozoites started their maturation in schizonts. At 48 hpi, the average area was significantly bigger (181.0 μm2), but the highest increase was visible at 72 hpi, as the schizonts’ area reached an average of 377.0 μm2. At 96 hpi, the area was slightly smaller than the previous timepoint (240.5 μm2).
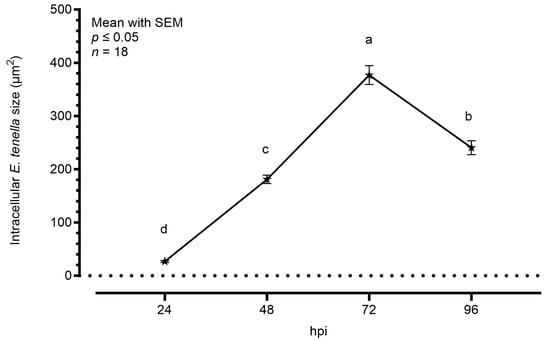
Figure 2.
Average size of E. tenella intracellular stages measured by semi-automated imaging software. Data are represented as mean ± SEM (n = 18). The plotted values are 26.70 ± 1.72 at 24 hpi, 181.00 ± 7.94 at 48 hpi, 377.00 ± 17.91 at 72 hpi, and 240.50 ± 13.01 at 96 hpi. Different letters indicate significant differences among timepoints (p ≤ 0.05).
3.2. Effects of Natural Compounds on Intracellular Growth
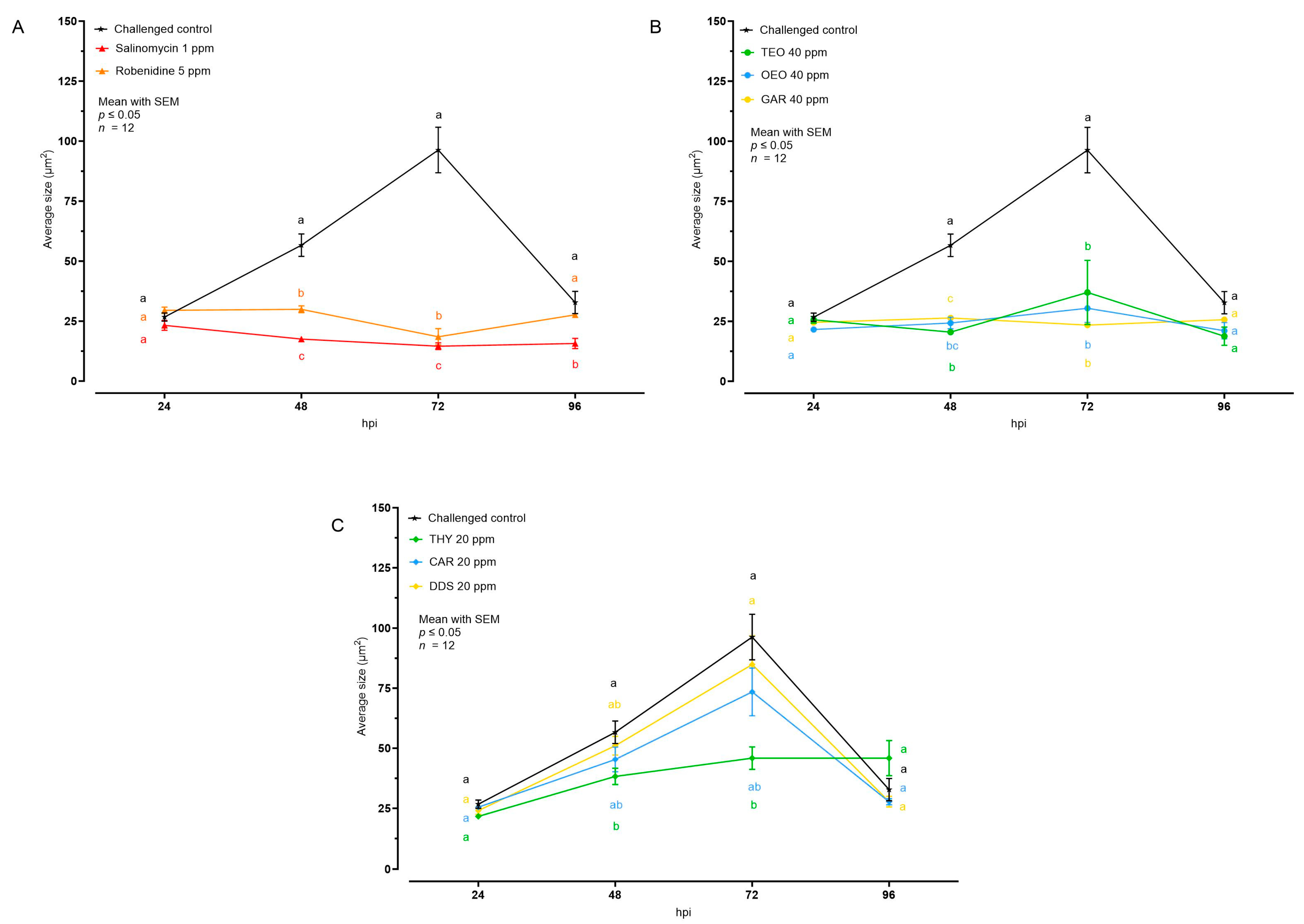
The effect of the different tested compounds on the average size of intracellular E. tenella stages is shown in Figure 3 and Table 1. Concerning AC and all the EO, no intracellular development was visible; in the cell monolayer only internalized sporozoites, characterized by an average size of 30 μm2, were observed. In the case of NIC, THY 20 ppm allowed a low degree of intracellular development at 48 and 72 hpi, while CAR and DDS did not cause any significant reduction of the average E. tenella size compared to the challenged control.
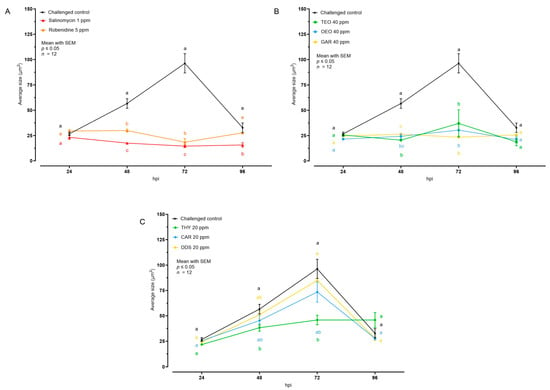
Figure 3.
Average size of intracellular E. tenella after treatment with anticoccidial drugs (A), essential oils (B), or nature-identical compounds (C). Data are represented as mean with SEM (n = 12), and the values are listed in Table 1. Different letters indicate significant differences among groups within the same time point and the same graph (p ≤ 0.05). Thyme essential oil (TEO); oregano essential oil (OEO); garlic oil (GAR); thymol (THY); carvacrol CAR); diallyl-disulfide (DDS).

Table 1.
Average size of intracellular E. tenella after treatment. Data are represented as mean with SEM (n = 12). Values are represented as graph in Figure 3.
To better understand if the growth velocity was affected by the treatments, the rate of growth was calculated, considering average area values between 24 and 72 hpi (Table 2). In the challenged control group, the intracellular parasites grew by 1.21 μm2/h. All AC and EO managed to stop growth, keeping the growth rate close to 0. Among NIC, only THY was significantly lower than the challenged control.

Table 2.
Rate of growth of intracellular E. tenella after treatment (between 24 and 72 hpi). Data are reported as mean with SEM (n = 12).
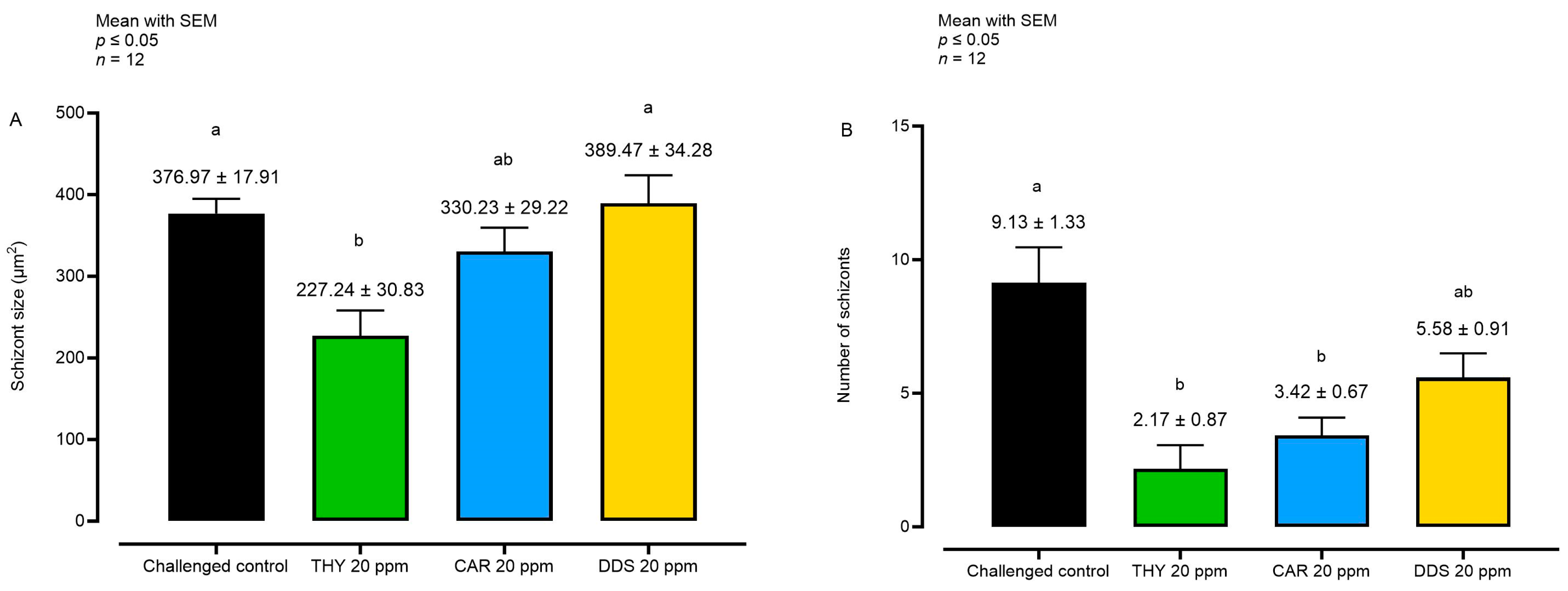
The sizes and numbers of E. tenella schizonts were also assessed after treatment in cases where a certain degree of intracellular growth was observed. This was done to examine the impact of these substances specifically on the schizonts at the peak of development, observed at 72 hpi (Figure 4).
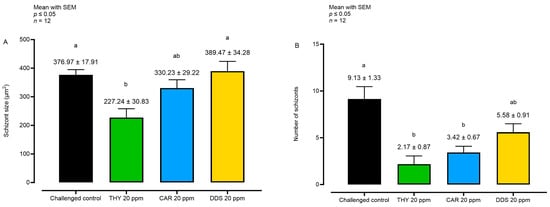
Figure 4.
Analysis of schizont size (A) and number (B) at 72 hpi. Data are represented as mean with SEM (n = 12). Different letters indicate significant differences among groups (p ≤ 0.05). Thymol (THY); carvacrol CAR); diallyl-disulfide (DDS).
Considering the schizont size at 72 hpi, only THY reduced the parameter compared to the challenged control. However, a reduction in the number of sporozoites per field was observable with both THY and CAR, while with DDS, only a non-significant numerical reduction was visible.
3.3. Effects of Natural Compounds on Parasite Invasion and Replication
Almost all the compounds significantly reduced the internalization of sporozoites at 24 hpi (Table 3). AC and EO lead to the most significant decrease of invasion, with values above 50% for both groups. Among NIC, only CAR significantly inhibited invasion regarding the control, with a level of 43%.

Table 3.
Level of inhibition of invasion 24 hpi. Data are reported as mean with SEM (n = 8).
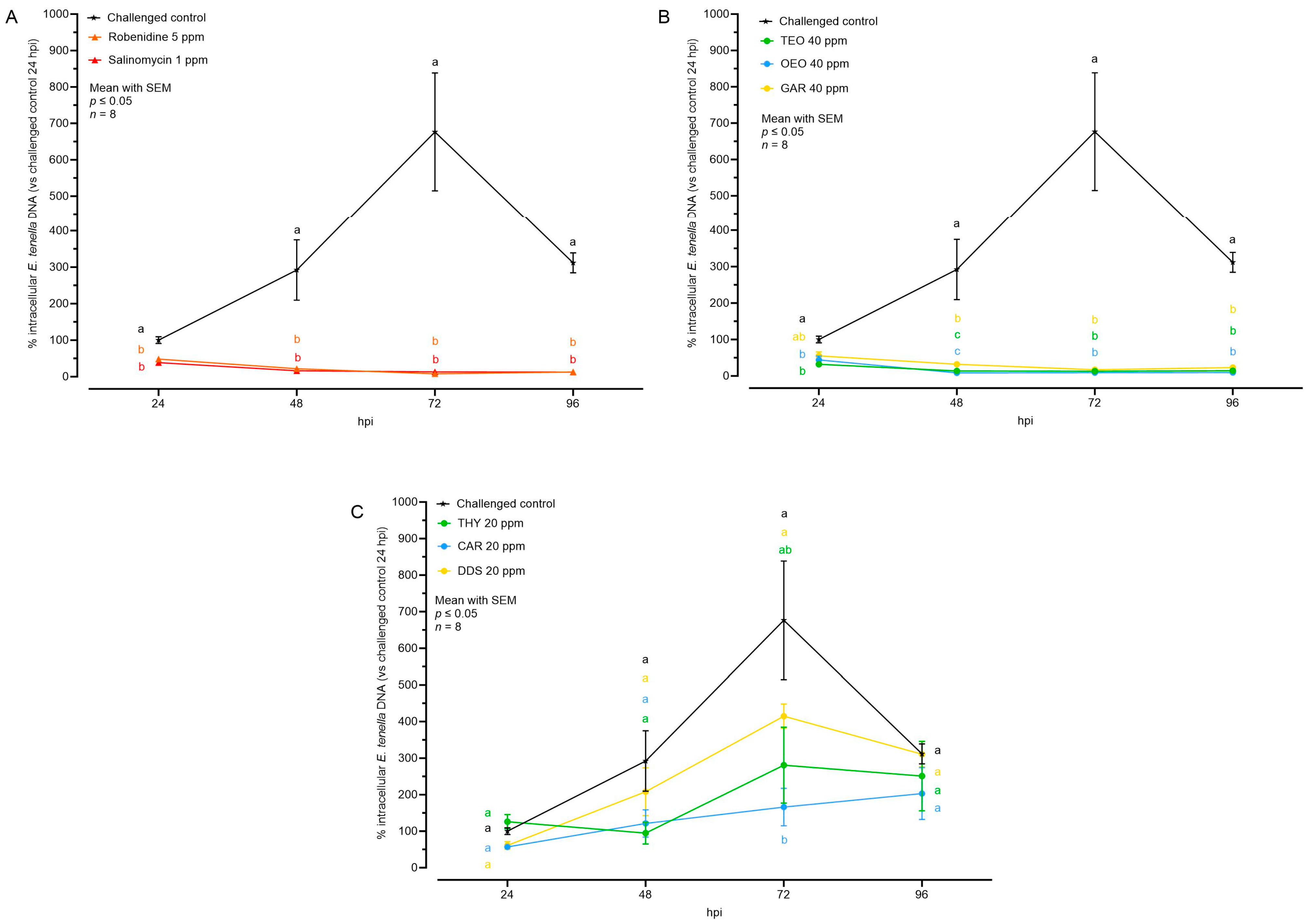
Figure 5 shows the quantification of E. tenella intracellular genomes as a percentage in relation to the challenged control at 24 hpi. Parasite replication was evident in the challenged control, as the number of genomes nearly tripled every 24 h, peaking at 72 hpi. However, at 96 hpi, the number of intracellular genomes dropped significantly (probably caused by schizont breakage, with release of merozoites into the washed supernatants).
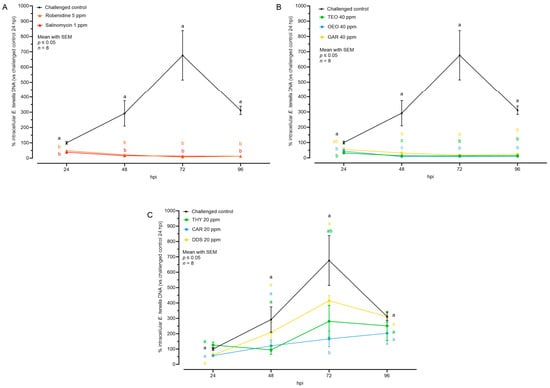
Figure 5.
Quantification of intracellular E. tenella DNA by qPCR after treatment with anticoccidial drugs (A), essential oils (B), or nature-identical compounds (C). Values are expressed as percentage over the control at 24 hpi. Data are represented as mean with SEM (n = 8) and the values are listed in Table 4. Significant differences among groups within the same time point and the same graph are indicated with different letters (p ≤ 0.05). Thyme essential oil (TEO); oregano essential oil (OEO); garlic oil (GAR); thymol (THY); carvacrol CAR); diallyl-disulfide (DDS).
The ACs and the EOs successfully reduced the number of intracellular parasite genomes starting from the moment of infection (24 hpi). Subsequently, there was no further detected nuclear replication in any of these groups during the subsequent time points (Figure 5). Concerning NIC, a partial inhibition of replication was noticed in the groups THY and CAR. These groups did not exhibit a distinct peak of growth at 72 hpi, suggesting a certain level of efficacy in inhibiting parasite replication. The DDS group did not show any significant difference, but their numbers at the peak (72 hpi) were lower than in the challenged control, potentially influenced by the lower (but not significant) number of invading sporozoites (Figure 5 and Table 4).

Table 4.
Quantification of intracellular E. tenella DNA by qPCR after treatment. Data are represented as mean with SEM (n = 8). Values are represented as a graph in Figure 5.
The rate of replication was calculated to investigate the intracellular development velocity of between 24 and 72 hpi (Table 5). In the challenged control group, the replication rate was 6.44 genomes/h. Most of the compounds demonstrated a capacity to reduce this rate. Specifically, AC and EO significantly reduced it, reaching values below 0 (usually caused by non-developing sporozoites observed to leave host cells over time). Meanwhile, THY and CAR managed to decrease the rate to almost half of the challenged control, but not significantly.

Table 5.
Rate of E. tenella DNA replication (between 24 and 72 hpi). Data are reported as mean with SEM (n = 8).
4. Discussion
Anticoccidials of any nature should be safe for the host, easy to produce and apply, and, preferentially, they should interfere with more than one stage of the parasite’s lifecycle, with a deep and comprehensive characterization of their mode of action [27]. For this purpose, in vitro systems that employ invasion and development assays on cell cultures have been increasingly used, as they allow a closer look at the undergoing processes and higher throughput testing compared to in vivo systems [27]. Eimeria spp., in particular E. tenella, are able to invade different cell lines and to complete the first round of schizogony in MDBK cells. Moreover, transgenic parasites expressing the YFP fluorescent marker have become a powerful tool for studies on Eimeria, especially to study intracellular development in cell cultures, as they allow the direct visualization of intracellular stages (i.e., schizonts) of development [18,27].
Previous research has explored the anticoccidial effects of thyme, oregano, and garlic essential oils, as well as their main bioactive molecules, both in vivo and in vitro, focusing primarily on the inhibition of sporozoite invasion [23,28]. Considering that parasite pathogenesis is related to intracellular stages, more extensive evaluation is required to comprehensively assess the impact of these novel compounds. [27].
4.1. Anticoccidial Drugs
In the present study, the efficacy of salinomycin and robenidine (commercial anticoccidials) has been confirmed, as both were able to decrease the number of sporozoites invading the cell monolayer and also to prevent the formation of schizonts. As previously reported, a pre-treatment with these molecules, before infection, is already enough to cause sporozoites death, thus stopping any further maturation [18]. In a similar study, the impact of 1 h of pre-incubation with salinomycin and robenidine on intracellular development was investigated by qPCR [25]. Pre-treatment with salinomycin 1 ppm lowered the level of invasion by 31%. Additionally, both robenidine and salinomycin pre-treatment managed to decrease the rate of replication between 24 and 44 hpi [25]. In the present study, the treatment with anticoccidials occurred along invasion and continued for the whole time course. This resulted in higher inhibition of invasion at 24 hpi and in no observed replication, suggesting that the anticoccidial power could be time-dependent.
4.2. Essential Oils
Similar to anticoccidials, thyme, oregano, and garlic essential oils were very effective in preventing the formation of schizonts. Inhibition of invasion was observed at 24 hpi with essential oils, suggesting that the action on sporozoites is so detrimental that internalized sporozoites struggle to undergo further development after meeting these compounds. The effect of similar essential oils on invasion has been explored previously by Sidiropoulou and colleagues [9,28]. E. tenella sporozoites were pre-treated for 1 h with different concentrations of garlic, oregano, and thyme essential oils from Greece. They recorded effective inhibition of invasion for oregano and garlic essential oils, in particular, at higher concentrations (100 ppm), whereas thyme had more consistent effects at lower concentrations [9,28]. Also, a previous study conducted on primary chicken enterocytes showed that 40 ppm of garlic, oregano, and thyme essential oils effectively reduced invasion at 24 hpi by around 45% [23]. In the first case, differences can be attributed to a different composition of the essential oils. In the second, where the source of the essential oils was the same as that in the current study, the levels of inhibition were slightly lower in the chicken enterocytes compared to MDBK cells. This could be due to differences in the protocols of invasion, but also, since a better predisposition of E. tenella to chicken enterocytes has been observed, differences caused by treatments could be dampened [23]. In addition to the cell model used, the effect of these essential oils on invasion and development was clear in the present study. To our knowledge, this is the first study to explore the impact of essential oils and bioactive molecules on Eimeria endogenous development in vitro, extending beyond invasion and reaffirming their potential as anticoccidial agents.
4.3. Nature-Identical Compounds
The power of essential oils lies in their diverse composition, which encompasses a wide range of bioactive molecules like terpenes, phenols, aldehydes, thiol compounds, and others. Often, these derivatives alone can drive the main effects of the original oil, as they maintain a more uniform composition than essential oils, which depends on specific extraction protocols; therefore, they have become a target of study for their therapeutic effects. In this study, thymol, carvacrol, and diallyl-disulfide, major components of thyme, oregano, and garlic essential oils, respectively, have been investigated to understand if these compounds solely drive the observed effects.
Diallyl-disulfide had no significant impact on development, in contrast to the relative essential oil (garlic), suggesting that the inhibitory effect is likely to be mediated by other molecules within the mixture. Garlic oil is rich in sulfur species that are able to oxidize thiols in protein residues, leading to loss of function. Other studies on sporozoite invasion in vitro evidenced that allicin, found in garlic species too, is a very strong anticoccidial. It shares the main chemical structure with diallyl-disulfide; however, allicin has a thiosulfinate group that makes the molecule very unstable and reactive [29]. A poorer anticoccidial performance of diallyl-disulfide compared to garlic oil was also observed in previous research on E. tenella invasion [23], concluding that thiosulfinates could be key effectors to drive the anticoccidial effects.
Thymol and carvacrol slightly reduced the intracellular number of E. tenella genomes; in particular, carvacrol reduced by 43% the level of invasion at 24 hpi. A similar effect on inhibition of invasion was observed in a previous study, where a mild level of inhibition was observed on chicken enterocytes, especially for carvacrol [23]. However, in the present study, thymol and carvacrol did not completely stop replication. Nevertheless, they proved to be effective in reducing both the number and size of developed schizonts at 72 hpi, factors associated with decreased pathogenicity, as seen in attenuated Eimeria strains [30]. Hence, these treatments may not completely halt development, but they can still result in a decreased severity of infection. When compared to thyme and oregano essential oils, thymol and carvacrol exhibit a milder impact on development. This suggests that a portion of sporozoites treated with these compounds managed to survive and develop within cells, whereas sporozoites treated with the essential oils ultimately succumbed.
Natural alternatives operate through a combination of synergistic effects among compounds found in complex mixtures, such as essential oils, or rely on the concentrated nature of single bioactive compounds. In this study, the pure bioactives proved to be less effective than the complex mixture, underscoring the importance of synergistic interaction between natural compounds in enhancing anticoccidial effects. Therefore, formulations combining bioactive compounds might offer a better alternative; however, this will need to be tested in vitro, and if high efficacy is shown, in vivo models will be necessary for efficacy validation.
5. Conclusions
In conclusion, this study has shed light on the promising potential of essential oils and their bioactive compounds as anticoccidial candidates. These findings highlight that these natural substances not only inhibit sporozoite invasion but also interfere with the formation of schizonts, a critical phase in the parasite’s lifecycle. Further research on the mechanisms and applications of these natural compounds is necessary, holding promise for safer and more comprehensive anticoccidial solutions in the future.
Author Contributions
Conceptualization, M.F. and V.M.-H.; investigation, M.F. and C.D.H.-T.; writing—original draft preparation, M.F.; writing—review and editing, V.M.-H., B.T. and E.G.; supervision, V.M.-H., A.P. and E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Procedures used in this study for the generation of E. tenella oocysts were carried out in accordance with the Animals (Scientific Procedures) Act 1986 (United Kingdom Parliament Act). All animal studies and protocols have been approved by the Royal Veterinary College Animal Welfare and Ethical Review Board (AWERB) and performed under the United Kingdom Government Home Office project license PDAAD5C9D.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Andrea Piva serves as a professor at the University of Bologna and is a member of the board of directors of Vetagro S.p.A. (Reggio Emilia, Italy). Ester Grilli serves as an assistant professor at the University of Bologna and is a member of the board of directors of Vetagro, Inc. The remaining authors declare that research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.T.; Hofacre, C.L.; Payne, A.M.; Anderson, D.B.; Kaiser, P.; Mackie, R.I.; Gaskins, H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.E.; Van Diemen, P.M.; Martineau, H.; Stevens, M.P.; Tomley, F.M.; Stabler, R.A.; Blake, P. Impact of Eimeria tenella Coinfection on Campylobacter jejuni Colonization of the Chicken. J. Hepatol. 2019, 87, e00772-18. [Google Scholar] [CrossRef] [PubMed]
- Rimet, C.S.; Maurer, J.J.; Berghaus, R.D.; Jordan, B.J.; Antoniassi Da Silva, L.H.; Stabler, L.J.; Johnson, K.K.; Tensa, L.R.; Segovia, K.M.; França, M.S. The Contribution of Eimeria Coinfection and Intestinal Inflammation to Cecal Colonization and Systemic Spread of Salmonella Typhimurium Deficient in Tetrathionate Reductase or Type III Secretion Systems Salmonella Pathogenicity Island 1 or 2. Avian Dis. 2019, 63, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Muthamilselvan, T.; Kuo, T.F.; Wu, Y.C.; Yang, W.C. Herbal remedies for coccidiosis control: A review of plants, compounds, and anticoccidial actions. Evid.-Based Complement. Altern. Med. 2016, 2016, 2657981. [Google Scholar] [CrossRef] [PubMed]
- Gordillo Jaramillo, F.X.; Kim, D.H.; Lee, S.H.; Kwon, S.K.; Jha, R.; Lee, K.W. Role of oregano and Citrus species-based essential oil preparation for the control of coccidiosis in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 47. [Google Scholar] [CrossRef]
- Chang, L.Y.; Di, K.Q.; Xu, J.; Chen, Y.F.; Xi, J.Z.; Wang, D.H.; Hao, E.Y.; Xu, L.J.; Chen, H.; Zhou, R.Y. Effect of natural garlic essential oil on chickens with artificially infected Eimeria tenella. Vet. Parasitol. 2021, 300, 109614. [Google Scholar] [CrossRef]
- Sidiropoulou, E.; Skoufos, I.; Marugan-Hernandez, V.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Blake, D.P.; Tzora, A. In vitro Anticoccidial Study of Oregano and Garlic Essential Oils and Effects on Growth Performance, Fecal Oocyst Output, and Intestinal Microbiota in vivo. Front. Vet. Sci. 2020, 7, 420. [Google Scholar] [CrossRef]
- Gholami-Ahangaran, M.; Ahmadi-Dastgerdi, A.; Azizi, S.; Basiratpour, A.; Zokaei, M.; Derakhshan, M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022, 8, 267–288. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Sarti, M.D.; Campieri, M.; Valerii, M.C. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: Implications in colonic pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef] [PubMed]
- Reza Youssefi, M.; Moghaddas, E.; Abouhosseini Tabari, M.; Moghadamnia, A.A.; Hosseini, S.M.; Hosseini Farash, B.R.; Amin Ebrahimi, M.; Nabavi Mousavi, N.; Fata, A.; Maggi, F.; et al. In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum Mohammad. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Uscanga, A.; Aycart, D.F.; Li, K.; Witola, W.H.; Andrade Laborde, J.E. Anti-protozoal activity of Thymol and a Thymol ester against Cryptosporidium parvum in cell culture. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, S.M.A.; Ojha, S.; Kalasz, H.; Adeghate, E. Chemical constituents and medicinal properties of Allium species. Mol. Cell. Biochem. 2021, 476, 4301–4321. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Chand, N.; Khan, R.U.; Naz, S.; Gul, S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019, 47, 79–84. [Google Scholar] [CrossRef]
- Felici, M.; Tugnoli, B.; Piva, A.; Grilli, E. In vitro assessment of anticoccidials: Methods and molecules. Animals 2021, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Patton, W.H. Eimeria tenella: Cultivation of the Asexual Stages in Cultured Animal Cells. Science 1965, 150, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Marugan-Hernandez, V.; Jeremiah, G.; Aguiar-Martins, K.; Burrell, A.; Vaughan, S.; Xia, D.; Randle, N.; Tomley, F. The Growth of Eimeria tenella: Characterization and Application of Quantitative Methods to Assess Sporozoite Invasion and Endogenous Development in Cell Culture. Front. Cell. Infect. Microbiol. 2020, 10, 579833. [Google Scholar] [CrossRef]
- McDougald, L.R.; Galloway, R.B. Anticoccidial drugs: Effects on infectivity and survival intracellularly of Eimeria tenella sporozoites. Exp. Parasitol. 1976, 40, 314–319. [Google Scholar] [CrossRef]
- Clark, J.D.; Billington, K.; Bumstead, J.M.; Oakes, R.D.; Soon, P.E.; Sopp, P.; Tomley, F.M.; Blake, D.P. A toolbox facilitating stable transfection of Eimeria species. Mol. Biochem. Parasitol. 2008, 162, 77–86. [Google Scholar] [CrossRef]
- Shirley, M.W. Eimeria species and strains of chickens. In Guidelines on Techniques in Coccidiosis Research; Office for Official Publications of the European Communities: Luxembourg, 1995; pp. 1–25. ISBN 9282749703. [Google Scholar]
- Pastor-Fernández, I.; Pegg, E.; Macdonald, S.E.; Tomley, F.M.; Blake, D.P.; Marugán-Hernández, V. Laboratory Growth and Genetic Manipulation of Eimeria tenella. Curr. Protoc. Microbiol. 2019, 53, e81. [Google Scholar] [CrossRef] [PubMed]
- Felici, M.; Tugnoli, B.; Ghiselli, F.; Baldo, D.; Ratti, C.; Piva, A.; Grilli, E. Investigating the effects of essential oils and pure botanical compounds against Eimeria tenella in vitro. Poult. Sci. 2023, 102, 102898. [Google Scholar] [CrossRef] [PubMed]
- Marugan-Hernandez, V.; Cockle, C.; Macdonald, S.; Pegg, E.; Crouch, C.; Blake, D.P.; Tomley, F.M. Viral proteins expressed in the protozoan parasite Eimeria tenella are detected by the chicken immune system. Parasites Vectors 2016, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Arias-Maroto, S.; Aguiar-Martins, K.; Regidor-Cerrillo, J.; Ortega-Mora, L.; Marugan-Hernandez, V. Reduction of chickens use to perform in vitro pre-screening of novel anticoccidials by miniaturisation and increased throughput of the current Eimeria tenella compound-screening model. F1000Research 2022, 11, 1135. [Google Scholar] [CrossRef]
- Thabet, A.; Alnassan, A.A.; Daugschies, A.; Bangoura, B. Combination of cell culture and qPCR to assess the efficacy of different anticoccidials on Eimeria tenella sporozoites. Parasitol. Res. 2015, 114, 2155–2163. [Google Scholar] [CrossRef]
- Müller, J.; Hemphill, A. In vitro culture systems for the study of apicomplexan parasites in farm animals. Int. J. Parasitol. 2013, 43, 115–124. [Google Scholar] [CrossRef]
- Sidiropoulou, E.; Marugán-Hernández, V.; Skoufos, I.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Papagrigoriou, T.; Fotou, K.; Grigoriadou, K.; et al. In Vitro Antioxidant, Antimicrobial, Anticoccidial, and Anti-Inflammatory Study of Essential Oils of Oregano, Thyme, and Sage from Epirus, Greece. Life 2022, 12, 1783. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, D.K. Pharmacological effects of garlic (Allium sativum L.). Annu. Rev. Biomed. Sci. 2008, 10, 6–26. [Google Scholar] [CrossRef]
- Long, P.L. Endogenous stages of a ‘chick embryo-adapted’ strain of Eimeria tenella. Parasitology 1973, 66, 55–62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).