Seroconversion in Galapagos Sea Lions (Zalophus wollebaeki) Confirms the Presence of Canine Distemper Virus in Rookeries of San Cristóbal Island
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. Laboratory and Data Analysis
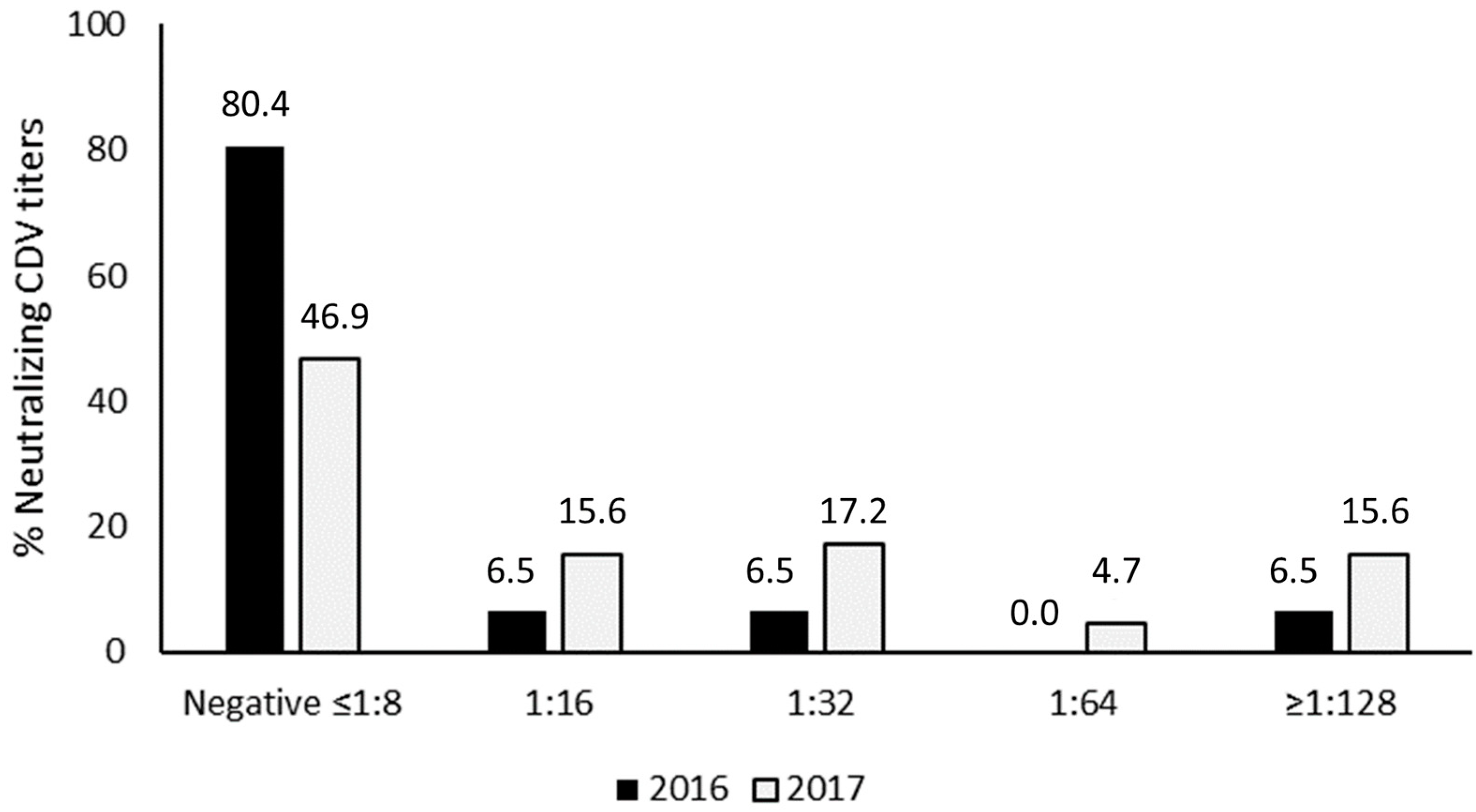
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trillmich, F. Zalophus wollebaeki. The IUCN Red List of Threatened Species. 2015, p. e.T41668A45230540. Available online: https://www.iucnredlist.org/species/41668/45230540 (accessed on 1 November 2023).
- Kalberer, S.; Meise, K.; Trillmich, F.; Krüger, O. Reproductive performance of a tropical apex predator in an unpredictable habitat. Behav. Ecol. Sociobiol. 2018, 72, 108. [Google Scholar] [CrossRef]
- Páez-Rosas, D.; Torres, J.; Espinoza, E.; Marchetti, A.; Seim, H.; Riofrío-Lazo, M. Declines and recovery in endangered Galapagos pinnipeds during the El Niño event. Sci. Rep. 2021, 11, 8785. [Google Scholar] [CrossRef] [PubMed]
- Páez-Rosas, D.; Guevara, N. Management strategies and conservation status of Galapagos sea lion populations at San Cristóbal Island, Galapagos, Ecuador. In Tropical Pinnipeds: Bio-Ecology, Threats and Conservation; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 159–175. [Google Scholar]
- Howitt, B. Save Galapagos species by managing cats and dogs. Vet. Rec. 2019, 185, 733–734. [Google Scholar] [CrossRef]
- Walsh, S.J.; Mena, C.F. Perspectives for the study of the Galapagos Islands: Complex systems and human–environment interactions. In Science and Conservation in the Galapagos Islands: Frameworks & Perspectives; Springer: Berlin/Heidelberg, Germany, 2012; pp. 49–67. [Google Scholar]
- INEC. Censo de Población y Vivienda-Galápagos. 2015. Available online: https://www.ecuadorencifras.gob.ec/censo-de-poblacion-y-vivienda-galapagos/ (accessed on 1 November 2023).
- Hernandez, J.A.; Yoak, A.J.; Walden, H.S.; Thompson, N.; Zuniga, D.; Criollo, R.; Duque, V.; Cruz, M. Dog overpopulation on Santa Cruz Island, Galapagos 2018. Conserv. Sci. Pract. 2020, 2, e201. [Google Scholar] [CrossRef]
- Culda, C.A.; Dionnet, R.; Barbu, A.C.; Cârstolovean, A.S.; Dan, T.; Grijalva, J.; Espin, P.; Vinueza, R.L.; Cruz, M.; Páez-Rosas, D.; et al. The Presence of Dirofilaria immitis in Domestic Dogs on San Cristobal Island, Galapagos. Pathogens 2022, 11, 1287. [Google Scholar] [CrossRef]
- Diaz, N.M.; Mendez, G.S.; Grijalva, C.J.; Walden, H.S.; Cruz, M.; Aragon, E.; Hernandez, J.A. Dog overpopulation and burden of exposure to canine distemper virus and other pathogens on Santa Cruz Island, Galapagos. Prev. Vet. Med. 2016, 123, 128–137. [Google Scholar] [CrossRef]
- Denkinger, J.; Guevara, N.; Ayala, S.; Murillo, J.C.; Hirschfeld, M.; Montero-Serra, I.; Fietz, K.; Goldstein, T.; Ackermann, M.; Barragán, V.; et al. Pup mortality and evidence for pathogen exposure in Galapagos sea lions (Zalophus wollebaeki) on San Cristobal island, Galapagos, Ecuador. J. Wildl. Dis. 2017, 53, 491–498. [Google Scholar] [CrossRef]
- Vega-Mariño, P.; Olson, J.; Howitt, B.; Criollo, R.; Figueroa, L.; Orlando, S.A.; Cruz, M.; Garcia-Bereguiain, M.A. A recent distemper virus outbreak in the growing canine populations of Galapagos Islands: A persistent threat for the endangered Galapagos Sea Lion. Front. Vet. Sci. 2023, 10, 1154625. [Google Scholar] [CrossRef] [PubMed]
- Gregory, T.M.; Parker, M.; Deresienski, D.; Alarcón-Ruales, D.; Muñoz-Pérez, J.P.; Torres, J.; Gavilanes, G.I.; Lewbart, G.A.; Páez-Rosas, D. Evaluating the Possibility of Transfusion Medicine, Through Crossmatching in Juvenile Galapagos Sea Lions (Zalophus wollebaeki). Front. Vet. Sci. 2022, 9, 830272. [Google Scholar] [CrossRef] [PubMed]
- Sarzosa, M.S.; Duignan, P.; DeRango, E.J.; Field, C.; Ríos, C.; Sanchez, S.; Espinoza, E.; Loyola, A.; Rueda, D.; Páez-Rosas, D. Occurrence of Mycoplasmas in Galapagos Sea Lions (Zalophus wollebaeki) and their Association with Other Respiratory Pathogens. J. Wildl. Dis. 2021, 57, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Duque-Valencia, J.; Sarute, N.; Olarte-Castillo, X.A.; Ruíz-Sáenz, J. Evolution and Interspecies Transmission of Canine Distemper Virus—An Outlook of the Diverse Evolutionary Landscapes of a Multi-Host Virus. Viruses 2019, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Echeverry-Bonilla, D.F.; Buriticá-Gaviria, E.F.; Orjuela-Acosta, D.; Chinchilla-Cardenas, D.J.; Ruiz-Saenz, J. The First Report and Phylogenetic Analysis of Canine Distemper Virus in Cerdocyon thous from Colombia. Viruses 2022, 14, 1947. [Google Scholar] [CrossRef] [PubMed]
- Duque-Valencia, J.; Forero-Muñoz, N.R.; Díaz, F.J.; Martins, E.; Barato, P.; Ruiz-Saenz, J. Phylogenetic evidence of the intercontinental circulation of a Canine distemper virus lineage in the Americas. Sci. Rep. 2019, 9, 15747. [Google Scholar] [CrossRef] [PubMed]
- Fuques, E.; Tomás, G.; Grecco, S.; Condon, E.; Techera, C.; Marandino, A.; Sarute, N.; Aldaz, J.; Enciso, J.; Benech, A.; et al. Origin and spreading of canine morbillivirus in South America. Virus Res. 2022, 319, 198858. [Google Scholar] [CrossRef] [PubMed]
- Sarute, N.; Pérez, R.; Aldaz, J.; Alfieri, A.A.; Alfieri, A.F.; Name, D.; Llanes, J.; Hernandez, M.; Francia, L.; Panzera, Y. Molecular typing of canine distemper virus strains reveals the presence of a new genetic variant in South America. Virus Genes 2014, 48, 474–478. [Google Scholar] [CrossRef]
- Deem, S.L.; Spelman, L.H.; Yates, R.A.; Montali, R.J. Canine Distemper in Terrestrial Carnivores: A Review. J. Zoo Wildl. Med. 2000, 31, 441–451. [Google Scholar]
- Jo, W.K.; Osterhaus, A.D.; Ludlow, M. Transmission of morbilliviruses within and among marine mammal species. Curr. Opin. Virol. 2018, 28, 133–141. [Google Scholar] [CrossRef]
- Levy, J.K.; Crawford, P.C.; Lappin, M.R.; Dubovi, E.J.; Levy, M.G.; Alleman, R.; Tucker, S.J.; Clifford, E.L. Infectious Diseases of Dogs and Cats on Isabela Island, Galapagos. J. Vet. Intern. Med. 2008, 22, 60–65. [Google Scholar] [CrossRef]
- Wilkes, R.P. Canine Distemper Virus in Endangered Species: Species Jump, Clinical Variations, and Vaccination. Pathogens 2023, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Riofrío-Lazo, M.; Arreguín-Sánchez, F.; Páez-Rosas, D. Population abundance of the endangered Galapagos sea lion Zalophus wollebaeki in the southeastern Galapagos archipelago. PLoS ONE 2017, 12, e0168829. [Google Scholar] [CrossRef]
- Burleson, F.G.; Chambers, T.M.; Wiedbrauk, D.L. Assays to measure virus neutralization test In Virology: A Laboratory Manual; Academic Press: Cambridge, MA, USA, 1992; pp. 123–168. [Google Scholar]
- Riofrío-Lazo, M.; Páez-Rosas, D. Galapagos Sea Lions and Fur Seals, Adapted to a Variable World. In Ethology and Behavioral Ecology of Otariids and the Odobenid; Campagna, C., Harcourt, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 643–661. [Google Scholar] [CrossRef]
- Riofrío-Lazo, M.; Reck, G.; Páez-Rosas, D.; Zetina-Rejón, M.J.; Del Monte-Luna, P.; Reyes, H.; Murillo-Posada, J.C.; Hernández-Padilla, J.C.; Arreguín-Sánchez, F. Food web modeling of the southeastern Galapagos shelf ecosystem. Ecol. Indic. 2021, 132, 108270. [Google Scholar] [CrossRef]
- Mos, L.; Ross, P.S.; McIntosh, D.; Raverty, S. Canine distemper virus in river otters in British Columbia as an emergent risk for coastal pinnipeds. Vet. Rec. 2003, 152, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Grachev, M.; Kumarev, V.; Mamaev, L.; Zorin, V.; Baranova, L.; Denikina, N.; Belikov, S.; Petrov, E.; Kolesnik, V.; Kolesnik, R. Distemper virus in Baikal seals. Nature 1989, 338, 209. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Kuiken, T.; Jepson, P.D.; Deaville, R.; Forsyth, M.; Barrett, T.; Van de Bildt, M.; Osterhaus, A.; Eybatov, T.; Duck, C. Mass die-off of Caspian seals caused by canine distemper virus. Emerg. Infect. Dis. 2000, 6, 637. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, J.L.; Boveng, P.; Franzen, U.; Have, P.; Heide-Jørgensen, M.; Härkönen, T. Antibodies to canine distemper virus in Antarctic seals. Mar. Mammal Sci. 1991, 7, 85–87. [Google Scholar] [CrossRef]
- Barrett, T.; Wohlsein, P.; Bidewell, C.; Rowell, S. Canine distemper virus in a Californian sea lion (Zalophus californianus). Vet. Rec. 2004, 154, 334. [Google Scholar] [CrossRef]
- Pope, J.P.; Miller, D.L.; Riley, M.C.; Anis, E.; Wilkes, R.P. Characterization of a novel canine distemper virus causing disease in wildlife. J. Vet. Diagn. Investig. 2016, 28, 506–513. [Google Scholar] [CrossRef]
- Loots, A.K.; Mitchell, E.; Dalton, D.L.; Kotzé, A.; Venter, E.H. Advances in canine distemper virus pathogenesis research: A wildlife perspective. J. Gen. Virol. 2017, 98, 311–321. [Google Scholar] [CrossRef]
- Sykes, J.E. Greene’s Infectious Diseases of the Dog and Cat, 5th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Ceballos, G.; Pompa, S.; Espinoza, E.; García, A. Extralimital distribution of Galapagos (Zalophus wollebaeki) and northern (Eumetopias jubatus) sea lions in Mexico. Aquat. Mamm. 2010, 36, 188–194. [Google Scholar] [CrossRef]
- Elorriaga-Verplancken, F.R.; Morales-Vázquez, J.E.; Ortega-Ortiz, C.D.; Llamas-González, M.; Meza-Yáñez, R.; Páez-Rosas, D. Northernmost Record of the Galapagos Sea Lion (Zalophus wollebaeki): Sightings along the Mexican Central Pacific and the Gulf of California During La Niña Conditions. Aquat. Mamm. 2022, 48, 478–484. [Google Scholar] [CrossRef]
- Visser, I.; vd Bildt, M.; Brugge, H.; Reijnders, P.; Vedder, E.; Kuiper, J.; de Vries, P.; Groen, J.; Walvoort, H.; UytdeHaag, F. Vaccination of harbour seals (Phoca vitulina) against phocid distemper with two different inactivated canine distemper virus (CDV) vaccines. Vaccine 1989, 7, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Visser, I.; Vedder, E.; Van de Bildt, M.; Örvell, C.; Barrett, T.; Osterhaus, A. Canine distemper virus ISCOMs induce protection in harbour seals (Phoca vitulina) against phocid distemper but still allow subsequent infection with phocid distemper virus-1. Vaccine 1992, 10, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Quinley, N.; Mazet, J.A.; Rivera, R.; Schmitt, T.L.; Dold, C.; McBain, J.; Fritsch, V.; Yochem, P.K. Serologic response of harbor seals (Phoca vitulina) to vaccination with a recombinant canine distemper vaccine. J. Wildl. Dis. 2013, 49, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Carter, S.; Robinson, I.; Clarke, D.; Clarke, C. Anti-canine distemper virus antibodies in common and grey seals. Vet. Rec. 1992, 130, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Strobel, M.M.; Rosen, D.A.; Nielsen, O. Serologic Response to Vaccination with Recombinant Canine Distemper Vaccine in Steller Sea Lions (Eumetopias jubatus) over an 8-Year Period. In Proceedings of the 50th Annual IAAAM Conference, Durban, South Africa, 18–22 May 2019. [Google Scholar]
- Jessup, D.A.; Murray, M.J.; Casper, D.R.; Brownstein, D.; Kreuder-Johnson, C. Canine distemper vaccination is a safe and useful preventive procedure for southern sea otters (Enhydra lutra nereis). J. Zoo Wildl. Med. 2009, 40, 705–710. [Google Scholar] [CrossRef]
- Pujol, J.; Rousseau, C.; Vergneau-Grosset, C. Serologic response and adverse effects of recombinant canine distemper vaccination in three aquarium-housed walruses (Odobenus rosmarus). J. Zoo Wildl. Med. 2023, 54, 131–136. [Google Scholar] [CrossRef]
- Duignan, P.J.; Van Bressem, M.-F.; Baker, J.D.; Barbieri, M.; Colegrove, K.M.; De Guise, S.; De Swart, R.L.; Di Guardo, G.; Dobson, A.; Duprex, W.P.; et al. Phocine Distemper Virus: Current Knowledge and Future Directions. Viruses 2014, 6, 5093–5134. [Google Scholar] [CrossRef]
- Baker, J.D.; Harting, A.L.; Barbieri, M.M.; Robinson, S.J.; Gulland, F.M.D.; Littnan, C.L. Modeling a morbillivirus outbreak in Hawaiian monk seals (Neomonachus schauinslandi) to aid in the design of mitigation programs. J. Wildl. Dis. 2017, 53, 736–748. [Google Scholar] [CrossRef]
- Robinson, S.J.; Barbieri, M.M.; Murphy, S.; Baker, J.D.; Harting, A.L.; Craft, M.E.; Littnan, C.L. Model recommendations meet management reality: Implementation and evaluation of a network-informed vaccination effort for endangered Hawaiian monk seals. Proc. R. Soc. B Biol. Sci. 2018, 285, 20171899. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Saenz, J.; Barragan, V.; Grijalva-Rosero, C.J.; Diaz, E.A.; Páez-Rosas, D. Seroconversion in Galapagos Sea Lions (Zalophus wollebaeki) Confirms the Presence of Canine Distemper Virus in Rookeries of San Cristóbal Island. Animals 2023, 13, 3657. https://doi.org/10.3390/ani13233657
Ruiz-Saenz J, Barragan V, Grijalva-Rosero CJ, Diaz EA, Páez-Rosas D. Seroconversion in Galapagos Sea Lions (Zalophus wollebaeki) Confirms the Presence of Canine Distemper Virus in Rookeries of San Cristóbal Island. Animals. 2023; 13(23):3657. https://doi.org/10.3390/ani13233657
Chicago/Turabian StyleRuiz-Saenz, Julian, Veronica Barragan, Colón Jaime Grijalva-Rosero, Eduardo A. Diaz, and Diego Páez-Rosas. 2023. "Seroconversion in Galapagos Sea Lions (Zalophus wollebaeki) Confirms the Presence of Canine Distemper Virus in Rookeries of San Cristóbal Island" Animals 13, no. 23: 3657. https://doi.org/10.3390/ani13233657
APA StyleRuiz-Saenz, J., Barragan, V., Grijalva-Rosero, C. J., Diaz, E. A., & Páez-Rosas, D. (2023). Seroconversion in Galapagos Sea Lions (Zalophus wollebaeki) Confirms the Presence of Canine Distemper Virus in Rookeries of San Cristóbal Island. Animals, 13(23), 3657. https://doi.org/10.3390/ani13233657







