The Expression of Amino Acid and Fatty Acid Receptors Show an Age-Dependent Pattern Involving Oral Cavity, Jejunum and Lower Gut Sensing in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Tissue Sampling
2.2. mRNA Isolation, cDNA Synthesis, and qPCR
2.3. Statistical Analysis
3. Results
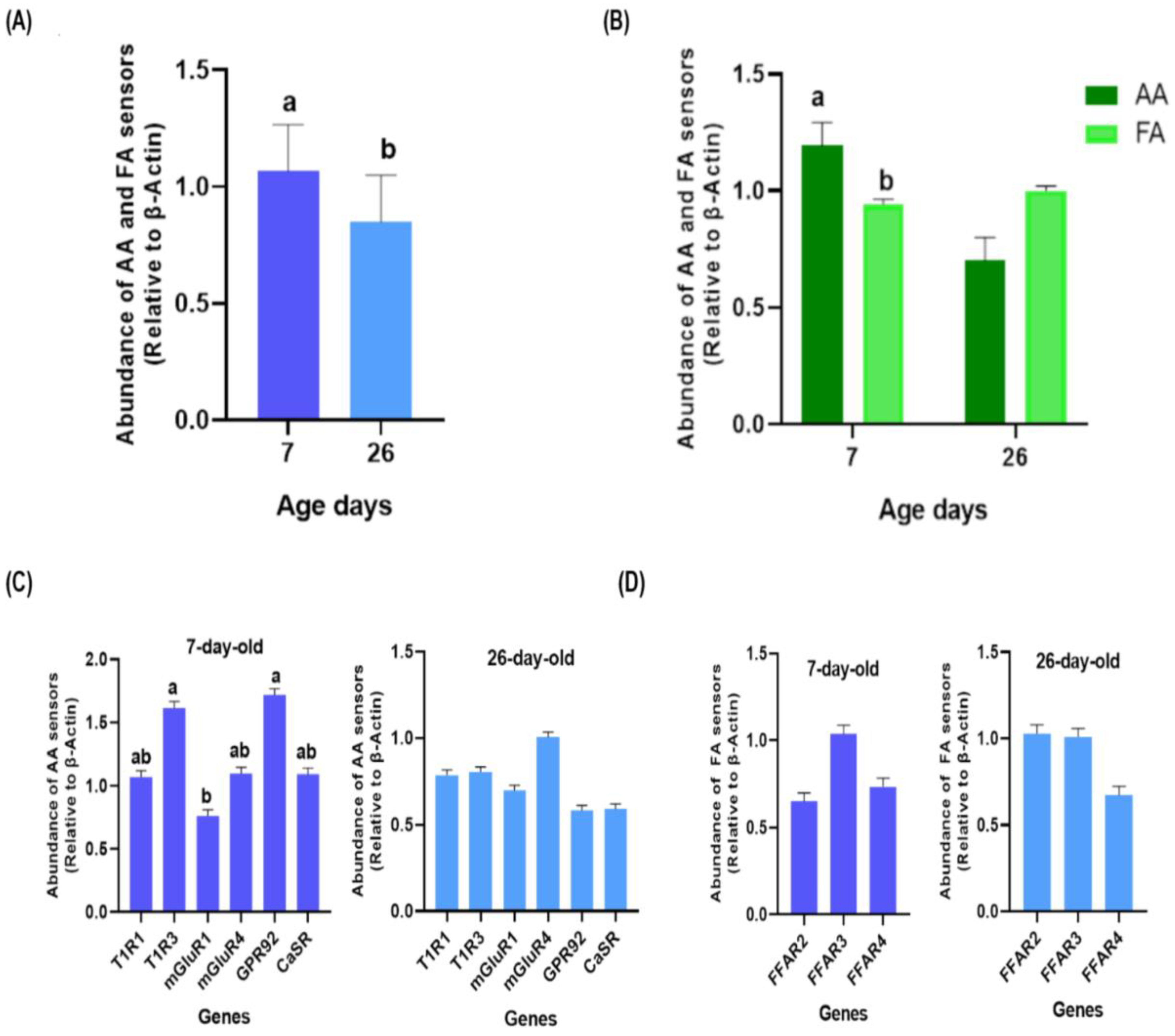
3.1. Differential Expression of AA and FA Sensors between 7 and 26 Days Post-Hatch
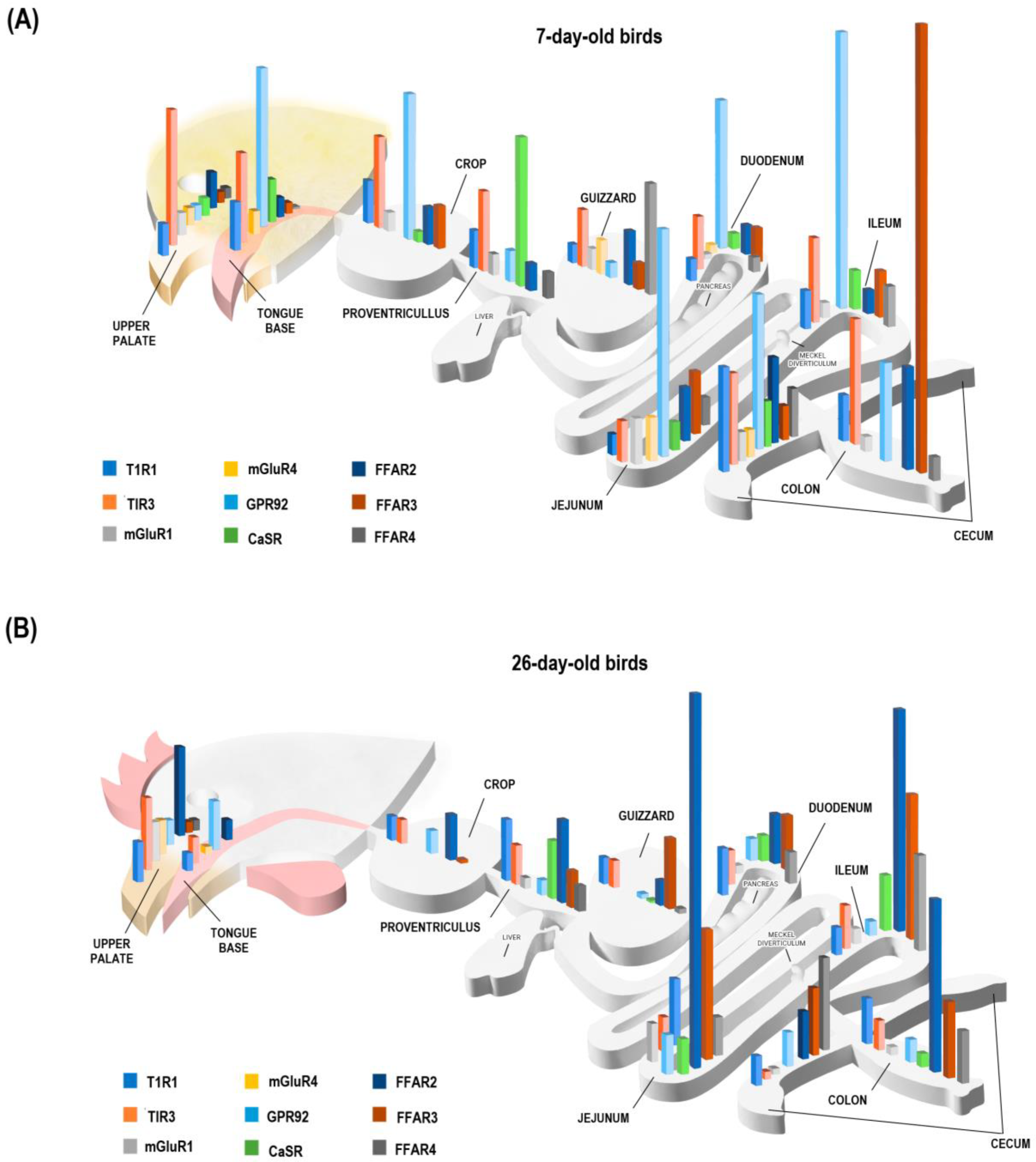
3.2. Differential Expression of AA and FA Sensors between Upper, Middle, and Lower GIT
3.3. Differential Expression of AA and FA Sensors between GIT Tissues
3.4. Correlations between Gene Expressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roura, E.; Navarro, M. Physiological and metabolic control of diet selecWtion. Festschrift for Prof John L Black: Celebrating 45 years of Excellence in Animal Production Science and Application. Anim. Prod. Sci. 2018, 58, 613–626. [Google Scholar] [CrossRef]
- Damak, S. Detection of intestinal nutrients. In Book Chemosensory Transduction; Zufall, F., Munger, S., Eds.; Elsiever: Berkeley, CA, USA, 2016; pp. 359–373. [Google Scholar] [CrossRef]
- Raka, F.; Farr, S.; Kelly, J.; Stoianov, A.; Adeli, K. Metabolic control via nutrient-sensing mechanisms: Role of taste receptors and the gut-brain neuroendocrine axis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Baldwin, M.W.; Klasing, K.C. The avian taste system: Potential implications in poultry nutrition. Anim. Feed. Sci. Technol. 2013, 180, 1–9. [Google Scholar] [CrossRef]
- Mellitzer, G.; Beucher, A.; Lobstein, V.; Michel, P.; Robine, S.; Kedinger, M.; Gradwohl, G. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J. Clin. Investig. 2010, 120, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wielen, N. Intestinal Nutrient Sensing: A Gut Feeling for Food. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2016. [Google Scholar]
- Niknafs, S.; Roura, E. Nutrient sensing, taste, and feed intake in avian species. Nutr. Res. Rev. 2016, 31, 256–266. [Google Scholar] [CrossRef]
- Cheled-Shoval, S.L.; Druyan, S.; Uni, Z. Bitter, sweet, and umami taste receptors and downstream signaling effectors: Expression in the embryonic and growing chicken gastrointestinal tract. Poult. Sci. J. 2015, 94, 1928–1941. [Google Scholar] [CrossRef]
- Baldwin, M.W.; Toda, Y.; Nakagita, T.; O’Connell, M.J.; Klasing, K.C.; Misaka, T.; Edwards, S.; Liberles, E. Evolution of sweet taste perception in hummingbirds by transformation of the ancestral umami receptor. Science 2014, 345, 929–933. [Google Scholar] [CrossRef]
- Meslin, C.; Desert, C.; Callebaut, I.; Djari, A.; Klopp, C.; Pitel, F.; Leroux, S.; Martin, p.; Froment, P.; Guilbert, E.; et al. Expanding duplication of free fatty acid receptor-2 (GPR43) genes in the chicken genome. Genome Biol. Evol. 2015, 7, 1332–1348. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kawabata, Y.; Kawabata, F.; Nishimura, S.; Tabata, S. Expression of multiple umami taste receptors in oral and gastrointestinal tissues, and umami taste synergism in chickens. Biochem. Biophys. Res. Commun. 2015, 466, 346–349. [Google Scholar] [CrossRef]
- Sukumarana, S.K.; Yee, K.K.; Iwata, S.; Kotha, R.; Quezada-Calvillo, R.; Nichols, B.L.; Mohan, S.; Pinto, B.M.; Shigemura, N.; Ninomiya, Y.; et al. Taste cell-expressed α-glucosidase enzymes contribute to gustatory responses to disaccharides. Proc. Natl. Acad. Sci. USA 2016, 113, 6035–6040. [Google Scholar] [CrossRef]
- Hui, Q.; Zhao, X.; Lu, P.; Liu, S.; Nyachoti, M.; Karmin, O.; Yang, C. Molecular distribution and localization of extracellular calcium-sensing Receptor (CASR) and Vitamin D Receptor (VDR) at three different laying stages in laying hens (Gallus Gallus Domesticus). Genet. Mol. Biol. 2021, 100, 101060. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.C.; De Jager, N.; Burgos, W.; Reverter, A.; Perez-Enciso, M.; Roura, E. Characterization of the Porcine GPCR Nutrient Sensor and Taste Receptor Gene Repertoire across international and local domestic breeds and wild populations. BMC Genom. 2014, 15, 1057. [Google Scholar] [CrossRef] [PubMed]
- Palomar, M.; Soler, M.; Roura, E.; Sala, R.; Piquer, O.; Garcés-Narro, C. Degree of saturation and free fatty acid content of fats determine dietary preference in hens. Animals 2020, 10, 2437. [Google Scholar] [CrossRef] [PubMed]
- Aviagen. Ross 308 Broiler: Nutrition Specifications. 2019. Available online: http://eu.aviagen.com/assets/Tech_Center/Ross_Broiler/RossBroilerNutritionSpecs2019-EN.pdf (accessed on 6 February 2022).
- OIE. World Organization for Animal Health. Animal Sacrifice. Health Code for Terrestrial Animals 2019, 7.5(7.5.1). Available online: https://doc.woah.org/dyn/portal/index.xhtml?page=alo&aloId=38680&espaceId=100 (accessed on 2 March 2022).
- Vandosempele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Steensels, S.; Depoortere, I. Chemoreceptores in the gut. Annu Rev. Physiol. 2018, 80, 117–141. [Google Scholar] [CrossRef]
- Roura, E.; Foster, S. Nutrient-sensing biology in mammals and birds. Annu. Rev. Anim. Biosci. 2018, 6, 197–225. [Google Scholar] [CrossRef]
- Gilbertson, T.; Yu, T.; Shah, B. Gustatory mechanisms for fat detection. In Book Fat Detection: Taste, Texture, and Post Ingestive Effects; Montmayeur, J., le Coutre, J., Eds.; CRP Press: Boca Raton, FL, USA, 2010; pp. 83–104. [Google Scholar]
- Venkatesan, N.; Rajapaksha, P.; Payne, J.; Goodfellow, F.; Wang, Z.; Kawabata, F.; Tabata, S.; Stice, S.; Beckstead, R.; Liu, H.X. Distribution of alpha-Gustducin and Vimentin in premature and mature taste buds in chickens. Biochem. Biophys. Res. Commun. 2016, 479, 305–311. [Google Scholar] [CrossRef]
- Dong, B. Molecular Characterization and Expression of Umami Receptors T1R1/T1R3 in Broiler Chickens. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2016; 99p. [Google Scholar]
- Rettenberger, A.; Schulze, W.; Breer, H.; Haid, D. Analysis of the protein related receptor GPR92 in G-cells. Front. Physiol. 2015, 6, 261. [Google Scholar] [CrossRef][Green Version]
- Rasoamanana, R.; Darcel, N.; Fromentin, G.; Tomé, D. Nutrient sensing and signaling by the gut. Proc. Nutr. Soc. 2012, 71, 446–455. [Google Scholar] [CrossRef]
- Apajalahti, J.; Kettunen, A.; Graham, H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. Worlds Poult. Sci. J. 2014, 60, 223–232. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Lam, T.K. Gut microbiota, nutrient sensing, and energy balance. Diabetes Obes. Metab. 2014, 16, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Vahjen, W.; Awad, W.A.; Zentek, J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 2007, 61, 319–335. [Google Scholar] [CrossRef]
- Fothergill, L.J.; Furness, J.B. Enteroendocrine cell diversity investigated at the cellular and subcellular level: The need for a new classification scheme. Histochem. Cell. Biol. 2018, 150, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Haid, D.; Widmayer, P.; Voigt, A.; Chaudhari, N.; Boehm, U.; Breer, H. Gustatory sensory cells express a receptor responsive to protein breakdown products (GPR92). Histoch. Cell Biol. 2013, 140, 137–145. [Google Scholar] [CrossRef]
- Lee, C.W.; Rivera, R.; Gardell, S.; Dubin, A.E.; Chun, J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 2006, 281, 23589–23597. [Google Scholar] [CrossRef]
- Deng, G.; Bi, J.; Qiao, J.; He, G.; Wu, D.; Zhang, M.; LV, N. Expression and tissue distribution of extracellular calcium-sensing receptor (CaSR) mRNA in chickens. Turk. J. Vet. Anim. Sci. 2010, 34, 249–254. [Google Scholar] [CrossRef]
- Bartoszek, A.; Von Moo, E.; Binienda, A.; Fabisiak, A.; Krajewska, J.B.; Mosińska, P.; Niewinna, K.; Tarasiuk, A.; Mar-temyanov, K.; Fichna, J. Free fatty acid receptors as new potential therapeutic target in inflammatory bowel diseases. Pharmacol. Res. 2020, 152, 104604. [Google Scholar] [CrossRef]
- Clench, M.H.; Mathias, J.R. The avian cecum: A review. Wilson. Bull. 1995, 107, 93–121. [Google Scholar]
- Rychlik, I. Composition and function of chicken gut microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, J.; Wigglesworth, M.; Kinghorn, I.; Fraser, N.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Hirano, K.; Tsujimoto, G. The SCFA Receptor GPR43 and energy metabolism. Front. Endocrinol. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Curto, E.; Milligan, G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem. Pharmacol. 2016, 114, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.; Al-rammahi, M.; Moran, A.; Marcello, M.; Ninomiya, Y.; Hhirazi-Beechey, S.P. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, 271–282. [Google Scholar] [CrossRef]



| Item | Starter | Grower |
|---|---|---|
| Ingredients (g/kg) | ||
| Corn | 554.2 | 630.9 |
| Soybean meal (47% protein) | 270.3 | 192.5 |
| Ground wheat | 50.0 | 50.0 |
| Rapeseed meal | 40.0 | 70.0 |
| Gluten meal (60% protein) | 30.0 | 0 |
| Olein oil | 16.5 | 20.3 |
| CaCO3 | 14.4 | 16.3 |
| CaHPO4 | 11.8 | 7.1 |
| NaCl | 4.4 | 4.1 |
| Lys | 2.2 | 1.6 |
| Met | 2.1 | 3.2 |
| Micofix plus 1 | 0.05 | 0.05 |
| Coccidiostat | 0.5 | 0.5 |
| Multivitamins–mineral–phytase ² | 2.0 | 2.0 |
| Formicit dry 3 | 1.0 | 1.0 |
| Analyzed nutrient composition (%) | ||
| Dry matter | 88.9 | 88.7 |
| Crude protein | 22.9 | 18.1 |
| Crude fiber | 3.4 | 4.3 |
| Ether extract | 3.8 | 4.9 |
| NNE | 53.7 | 55.0 |
| Ash | 5.1 | 6.4 |
| Gene | GenBank Accession No 2 | Forward Primer (5′ → 3′) | Reverse Primer (3′ → 5′) |
|---|---|---|---|
| T1R1 | XM_015297117.1 | CTA TGG TAG GGA TGG GCT CAA C | CTA AAG ACC AGT CCT CAG AGC C |
| T1R3 | KM091452 | GTC TTC GCC ACT CTG AGG AC | CTA AAG ACC AGT CCT CAG AGC C |
| mGluR1 | NC_052534.1 | CGC GCC AGG TTA AAA GTC AC | GGT TCC TCT TGG CTG CGT AT |
| mGluR4 | NC_052557.1 | GTG CAA GCC CTG ATT GAG AAG | GTG GAG GCA TAG CTG ATC TGG |
| CaSR | XM_416491.5 | TGG CTT CCA CCT TGT TGC TTA | GCA GCA GTG TTC CAG GTA AAC |
| GPR139 | NM_001321735.1 | TGC TGA CAT CCT CGT TCT CTT | GAG TGG ATG GCA CAC AGC TA |
| GPRC6A | XM_426177 | GGA GGT TTG TTT GCA GTT CAC A | TTT GGA CAG GAA CCT CAG AGC |
| GPR92 | XM_015293753.1 | GGA CAA ACC TGG CAC TCA GA | GCT AGG GGC TTT CTG TGG TT |
| FFAR2 | GCF_000002315.4 | GCC CCA TAG CAA ACT TCT | GGG CAG CCA TAA AGA GAG |
| FFAR3 | JQ927550.1 | GAA GGT GGT TTG GGA GTG AA | CAG AGG ATT TGA GGC TGG AG |
| FFAR4 | XM_040675455.1 | GCA CGG ACA GAA GGA AGA AG | CCA CCC CTG AAG TCT GAG AA |
| β-actin | NM_205518.1 | GAG AAA TTG TGC GTG ACA TCA | CCT GAA CCT CTC ATT GCC A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordero, P.; Díaz-Avilés, F.; Torres, P.; Guzmán, M.; Niknafs, S.; Roura, E.; Guzmán-Pino, S.A. The Expression of Amino Acid and Fatty Acid Receptors Show an Age-Dependent Pattern Involving Oral Cavity, Jejunum and Lower Gut Sensing in Broiler Chickens. Animals 2023, 13, 3120. https://doi.org/10.3390/ani13193120
Cordero P, Díaz-Avilés F, Torres P, Guzmán M, Niknafs S, Roura E, Guzmán-Pino SA. The Expression of Amino Acid and Fatty Acid Receptors Show an Age-Dependent Pattern Involving Oral Cavity, Jejunum and Lower Gut Sensing in Broiler Chickens. Animals. 2023; 13(19):3120. https://doi.org/10.3390/ani13193120
Chicago/Turabian StyleCordero, Paloma, Francisca Díaz-Avilés, Paulina Torres, Miguel Guzmán, Shahram Niknafs, Eugeni Roura, and Sergio A. Guzmán-Pino. 2023. "The Expression of Amino Acid and Fatty Acid Receptors Show an Age-Dependent Pattern Involving Oral Cavity, Jejunum and Lower Gut Sensing in Broiler Chickens" Animals 13, no. 19: 3120. https://doi.org/10.3390/ani13193120
APA StyleCordero, P., Díaz-Avilés, F., Torres, P., Guzmán, M., Niknafs, S., Roura, E., & Guzmán-Pino, S. A. (2023). The Expression of Amino Acid and Fatty Acid Receptors Show an Age-Dependent Pattern Involving Oral Cavity, Jejunum and Lower Gut Sensing in Broiler Chickens. Animals, 13(19), 3120. https://doi.org/10.3390/ani13193120









