Expressions of Insulin-like Growth Factor System among Different Breeds Impact Piglets’ Growth during Weaning
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Measurement of IGF System Components in Plasma
2.4. Expression Analysis of IGF System Component Genes
2.5. Statistical Analysis
3. Results
3.1. Weaning Effects on Organ Indices in Different Breeds of Piglets
3.2. Weaning Effects on Plasma IGF and IGFBP Concentration in Different Breeds of Piglets
3.3. Weaning Effects on IGF-Related Gene Expressions in Different Breeds of Piglets
3.4. Weaning Effects on IGF Receptor (IGF-R) Gene Expressions in Different Breeds of Piglets
3.5. Weaning Effects on IGF-Binding Protein (IGFBP) Expression in Different Breeds of Piglets
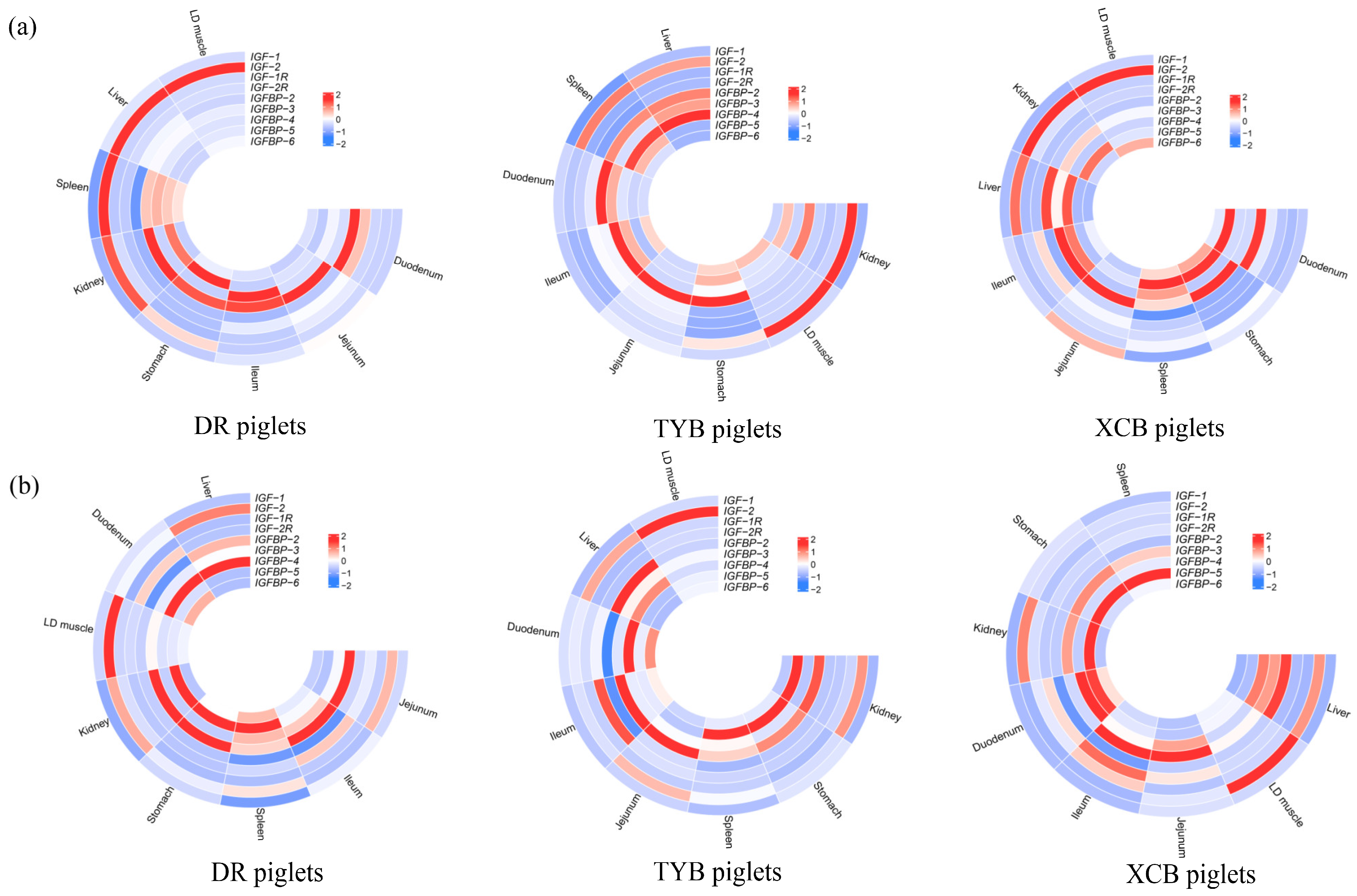
3.6. Weaning Effects on IGF Expressions in Different Tissues of the Same Breed of Piglets
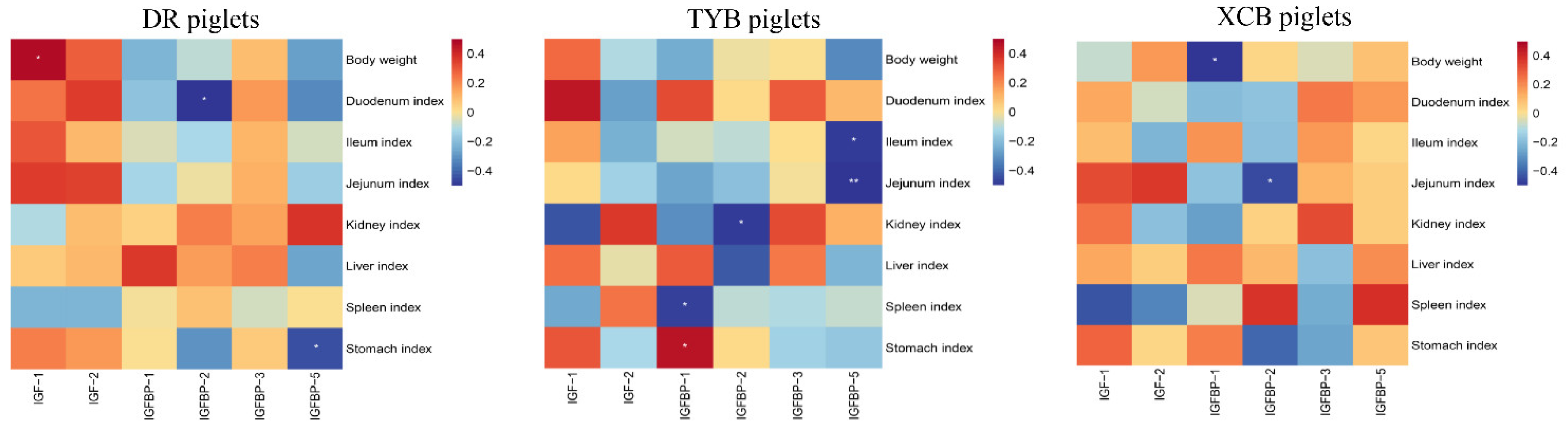
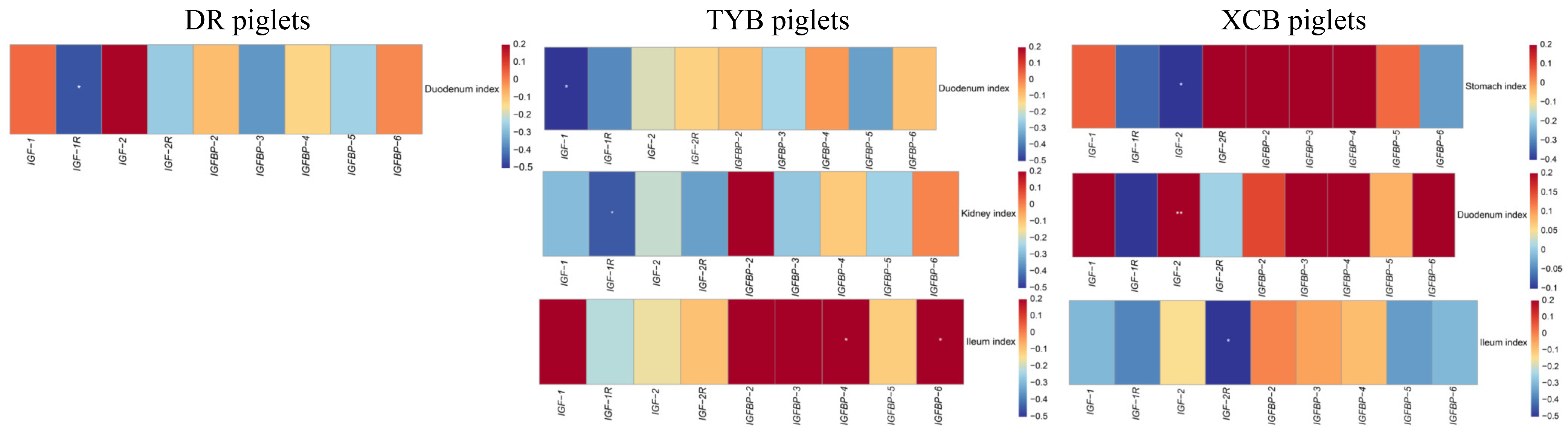
3.7. Correlations between Plasma Concentrations of IGF System Components, BW, and Organ Indices, as Well as IGF System Component Gene Expression and Organ Indices of Different Piglet Breeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pragna, P.; Sejian, V.; Bagath, M.; Krishnan, G.; Archana, P.R.; Soren, N.M.; Beena, V.; Bhatta, R. Comparative assessment of growth performance of three different indigenous goat breeds exposed to summer heat stress. J. Anim. Physiol. Anim. Nutr. 2018, 102, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, J.; Zhang, J.; Jin, Z. Dynamical analysis of the spread of African swine fever with the live pig price in China. Math. Biosci. Eng. 2021, 18, 8123–8148. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, X.; Zhao, D.; Dai, H. Pharmacological applications and action mechanisms of phytochemicals as alternatives to antibiotics in pig production. Front. Immunol. 2021, 12, 798553. [Google Scholar] [CrossRef]
- Van, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar]
- Hu, Y.; Cowling, B.J. Reducing antibiotic use in livestock, China. Bull. World Health Organ. 2020, 98, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Ables, E.T.; Armstrong, A.R. Nutritional, hormonal, and metabolic drivers of development. Dev. Biol. 2021, 476, 171–172. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: Roles and integration for cellular development and growth with exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef]
- Annunziata, M.; Granata, R.; Ghigo, E. The IGF system. Acta Diabetol. 2011, 48, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, M. The origination of growth hormone/insulin-like growth factor system: A story from ancient basal chordate amphioxus. Front. Endocrinol. 2022, 13, 825722. [Google Scholar] [CrossRef] [PubMed]
- Pierzchała, M.; Pareek, C.S.; Urbański, P.; Goluch, D.; Kamyczek, M.; Różycki, M.; Smoczynski, R.; Horbańczuk, J.O.; Kurył, J. Study of the differential transcription in liver of growth hormone receptor (GHR), insulin-like growth factors (IGF1, IGF2) and insulin-like growth factor receptor (IGF1R) genes at different postnatal developmental ages in pig breeds. Mol. Biol. Rep. 2012, 39, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A.; Adamek, A. The insulin-like growth factor (IGF) signaling axis and hepatitis C virus-associated carcinogenesis. Int. J. Oncol. 2012, 41, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Reindl, K.M.; Sheridan, M.A. Peripheral regulation of the growth hormone-insulin-like growth factor system in fish and other vertebrates. Comp. Biochem. Physiol. 2012, 163, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Furbeyre, H.; van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of oral supplementation with Spirulina and Chlorella on growth and digestive health in piglets around weaning. Animal 2018, 12, 2264–2273. [Google Scholar] [CrossRef]
- Matteri, R.L.; Dyer, C.J.; Touchette, K.J.; Carroll, J.A.; Allee, G.L. Effects of weaning on somatotrophic gene expression and circulating levels of insulin-like growth factor-1 (IGF-1) and IGF-2 in pigs. Domest. Anim. Endocrinol. 2000, 19, 247–259. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, N.; Ji, Y.; Tso, P.; Wu, Z. Weaning stress in piglets alters the expression of intestinal proteins involved in fat absorption. J. Nutr. 2022, 152, 2387–2395. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, P.; Zheng, X.; Zhou, L.; Wang, C.; Liu, J. Genome-wide detection of selection signatures in Duroc revealed candidate genes relating to growth and meat quality. G3-Genes Genom. Genet. 2020, 10, 3765–3773. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Cai, J.; Ni, Y.; Xiao, L.; Zhang, J. Transcriptome analysis in comparing carcass and meat quality traits of Jiaxing black pig and Duroc × Duroc × Berkshire × Jiaxing black pig crosses. Gene 2022, 808, 145978. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chu, H.; Jiang, Y.; Lin, C.; Li, S.; Li, K.; Weng, G.; Cheng, C.; Lu, D.; Ju, Y. Empirical selection of informative microsatellite markers within co-ancestry pig populations is required for improving the individual assignment efficiency. Asian-Australas. J. Anim. Sci. 2014, 27, 616–627. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Xie, H.; Liu, L.; Jiang, B.; Yu, Q.; He, J. Study on nutrient requirement of Xiangcun black pig at different growth stage. Acta Vet. Zootech. Sin. 2013, 44, 1400–1410. [Google Scholar]
- Cheng, Y.; Ding, S.; Azad, M.A.K.; Song, B.; Kong, X. Comparison of the pig breeds in the small intestinal morphology and digestive functions at different ages. Metabolites 2023, 13, 132. [Google Scholar] [CrossRef]
- Arnaud, E.A.; Gardiner, G.E.; Lawlor, P.G. Selected nutrition and management strategies in suckling pigs to improve post-weaning outcomes. Animals 2023, 13, 1998. [Google Scholar] [CrossRef] [PubMed]
- Wensley, M.R.; Tokach, M.D.; Woodworth, J.C.; Goodband, R.D.; Gebhardt, J.T.; DeRouchey, J.M.; McKilligan, D. Maintaining continuity of nutrient intake after weaning. II. Review of post-weaning strategies. Transl. Anim. Sci. 2021, 5, txab022. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.J.P.G.; Castro, T.L.P.; Aguiar, E.R.G.R.; Pacheco, L.G.C. Gene expression analysis in bacteria by RT-qPCR. Methods Mol. Biol. 2020, 2065, 119–137. [Google Scholar]
- Ding, S.; Cheng, Y.; Azad, M.A.K.; Zhu, Q.; Huang, P.; Kong, X. Developmental changes of immunity and different responses to weaning stress of Chinese indigenous piglets and Duroc piglets during suckling and weaning periods. Int. J. Mol. Sci. 2022, 23, 15781. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tan, X.; Wang, H.; Wang, Q.; Huang, P.; Li, Y.; Li, J.; Huang, J.; Yang, H.; Yin, Y. Effects of varying dietary folic acid during weaning stress of piglets. Anim. Nutr. 2021, 7, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Z.; Ren, G.; Zhao, Y.; Liu, D. Expression patterns of insulin-like growth factor system members and their correlations with growth and carcass traits in Landrace and Lantang pigs during postnatal development. Mol. Biol. Rep. 2013, 40, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Denley, A.; Cosgrove, L.J.; Booker, G.W.; Wallace, J.C.; Forbes, B.E. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005, 16, 421–439. [Google Scholar] [CrossRef]
- O’Kusky, J.; Ye, P. Neurodevelopmental effects of insulin-like growth factor signaling. Front. Neuroendocrinol. 2012, 33, 230–251. [Google Scholar] [CrossRef]
- LeRoith, D.; Holly, J.M.P.; Forbes, B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021, 52, 101245. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Yin, P.; Duan, C. IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J. Cell. Biol. 2008, 182, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Iresjö, B.M.; Diep, L.; Lundholm, K. Initiation of muscle protein synthesis was unrelated to simultaneously upregulated local production of IGF-1 by amino acids in non-proliferating L6 muscle cells. PLoS ONE 2022, 17, e0270927. [Google Scholar] [CrossRef]
- Liu, J.; Yakar, S.; LeRoith, D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology 2000, 141, 4436–4441. [Google Scholar] [CrossRef][Green Version]
- Upadhaya, S.D.; Kim, I.H. The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets-a review. Animals 2021, 11, 2418. [Google Scholar] [CrossRef]
- Zhong, H.; Lou, C.; Ren, B.; Zhou, Y. Insulin-like growth factor 1 injection changes gene expression related to amino acid transporting, complement and coagulation cascades in the stomach of tilapia revealed by RNA-seq. Front. Immunol. 2022, 13, 959717. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Long, W.; Ding, M.; Chen, X.; Chen, C. Detection of the RYR1 gene in Guizhou indigenous pigs and three-crossbred pigs. Biotechnol. Bull. 2015, 31, 66–70. [Google Scholar]
- Taguchi, A.; White, M.F. Insulin-like signaling, nutrient homeostasis, and life span. Annu. Rev. Physiol. 2008, 70, 191–212. [Google Scholar] [CrossRef]
- Hakuno, F.; Takahashi, S.I. IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef]
- Wang, Y.; MacDonald, R.G.; Thinakaran, G.; Kar, S. Insulin-like growth factor-II/cation-independent mannose 6-phosphate receptor in neurodegenerative diseases. Mol. Neurobiol. 2017, 54, 2636–2658. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, S.; Zhang, X.; Wu, Q.; Li, S.; Fu, H.; Dong, L.; Yu, H.; Hao, L. Expression profiles of IGF-1R gene and polymorphisms of its regulatory regions in different pig breeds. Protein J. 2016, 35, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Desbois-Mouthon, C.; Wendum, D.; Cadoret, A.; Rey, C.; Leneuve, P.; Blaise, A.; Housset, C.; Tronche, F.; Le Bouc, Y.; Holzenberger, M. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB J. 2006, 20, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Haywood, N.J.; Slater, T.A.; Matthews, C.J.; Wheatcroft, S.B. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 2019, 19, 86–96. [Google Scholar] [PubMed]
- Garcia de la Serrana, D.; Macqueen, D.J. Insulin-like growth factor-binding proteins of teleost fishes. Front. Endocrinol. 2018, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B. Insulin-like growth factor binding-protein-3 (IGFBP-3). Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 701–711. [Google Scholar] [CrossRef]
- Rajah, R.; Valentinis, B.; Cohen, P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-β1 on programmed cell death through a p53- and IGF-independent mechanism. J. Biol. Chem. 1997, 272, 12181–12188. [Google Scholar] [CrossRef] [PubMed]
- Frieten, D.; Gerbert, C.; Koch, C.; Dusel, G.; Eder, K.; Hoeflich, A.; Mielenz, B.; Hammon, H.M. Influence of ad libitum milk replacer feeding and butyrate supplementation on the systemic and hepatic insulin-like growth factor I and its binding proteins in Holstein calves. J. Dairy Sci. 2018, 101, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, K.; Suzuki, Y.; Kim, D.; Roh, S. Growth of rumen papillae in weaned calves is associated with lower expression of insulin-like growth factor-binding proteins 2, 3, and 6. Anim. Sci. J. 2019, 90, 1287–1292. [Google Scholar] [CrossRef]
- Nishihara, K.; Kato, D.; Suzuki, Y.; Kim, D.; Nakano, M.; Yajima, Y.; Haga, S.; Nakano, M.; Ishizaki, H.; Kawahara-Miki, R.; et al. Comparative transcriptome analysis of rumen papillae in suckling and weaned Japanese black calves using RNA sequencing. J. Anim. Sci. 2018, 96, 2226–2237. [Google Scholar] [CrossRef]
- Bach, L.A. Insulin-like growth factor binding proteins 4–6. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 713–722. [Google Scholar] [CrossRef]
| Gene Names | Accession No. | Primer Sequences (5′-3′) | Product Size (bp) |
|---|---|---|---|
| IGF-1 | XM_005664199.3 | F: CCAAGGCTCAGAAGGAAGTACA | 137 |
| R: ACTCGTGCAGAGCAAAGGAT | |||
| IGF-2 | NM_213883.2 | F: CGGCTTCTACTTCAGCAGGC | 219 |
| R: TGCTTCCAGGTGTCATAGCG | |||
| IGF-1R | XM_021082915.1 | F: ACGAGTGGAGAAATCTGCGG | 154 |
| R: TGAGCTTGGGAAAGCGGTAG | |||
| IGF-2R | NM_001244473.1 | F: ACAGAAGCTGGACGTCATCG | 150 |
| R: CTGTCAACGTCGAACCTGCT | |||
| IGFBP-1 | NM_001195105.1 | F: CTATCACAGCAAACAGTGCGAG | 181 |
| R: CACGTGAAGGAAGAGAGCCT | |||
| IGFBP-2 | NM_214003.1 | F: CGAGCAGGTTGCAGACAATG | 288 |
| R: GTGGAGATCCGTTCCAGGAC | |||
| IGFBP-3 | NM_001005156.1 | F: AAGAAAAAGCAGTGCCGCC | 208 |
| R: GATCGTGTCCTTGGCAGTCT | |||
| IGFBP-4 | NM_001123129.1 | F: CTGCTCCGAAGAGAAGCTGG | 279 |
| R: TCACCCTCGTCCTTGTCAGA | |||
| IGFBP-5 | NM_001315595.1 | F: GCAAGCCAAGATCGAGAGAG | 102 |
| R: GTGTGCTTGGGTCGGAAGAT | |||
| IGFBP-6 | NM_001100190.1 | F: CCCTCGGGGGAGAATCCTAA | 157 |
| R: GGCAAGGGCCCATCTCAG | |||
| β-Actin | XM_0210860471 | F: GATCTGGCACCACACCTTCTACAAC | 107 |
| R: TCATCTTCTCACGGTTGGCTTTGG |
| Organ Indices, g/kg | 21 Days of Age | 24 Days of Age | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DR | TYB | XCB | DR | TYB | XCB | Weaning | Breed | W × B | ||
| Stomach | 6.09 | 5.55 | 6.84 | 6.40 | 6.41 | 8.15 | 0.17 | 0.003 | <0.001 | 0.322 |
| Duodenum | 0.56 | 0.61 | 0.83 | 0.56 | 0.62 | 0.66 | 0.03 | 0.262 | 0.013 | 0.270 |
| Jejunum * | 14.66 | 17.67 | 18.96 | 15.51 | 15.03 | 17.91 | 0.64 | 0.454 | 0.098 | 0.529 |
| Ileum * | 16.75 | 20.07 | 20.09 | 17.80 | 15.25 | 19.55 | 0.56 | 0.185 | 0.120 | 0.076 |
| Kidney | 6.04 | 6.21 | 6.21 | 6.01 | 5.82 | 5.94 | 0.09 | 0.230 | 0.961 | 0.729 |
| Spleen * | 2.08 | 2.04 | 2.18 | 1.93 | 1.87 | 1.75 | 0.07 | 0.074 | 0.948 | 0.646 |
| Liver * | 23.96 | 25.98 | 27.37 | 26.85 | 25.64 | 26.59 | 0.38 | 0.427 | 0.207 | 0.097 |
| Index, µg/µL | 21 Days of Age | 24 Days of Age | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DR | TYB | XCB | DR | TYB | XCB | Weaning | Breed | W × B | ||
| IGF-1 | 15.95 | 15.17 | 17.88 | 15.50 | 15.06 | 17.86 | 0.39 | 0.797 | 0.012 | 0.971 |
| IGF-2 | 1.67 | 1.61 | 1.79 | 1.79 | 1.53 | 1.83 | 0.05 | 0.799 | 0.152 | 0.740 |
| IGFBP-1 | 38.90 | 42.82 | 43.29 | 44.42 | 45.41 | 42.31 | 1.42 | 0.425 | 0.794 | 0.668 |
| IGFBP-2 | 17.82 | 22.30 | 28.76 | 18.40 | 22.57 | 22.12 | 0.99 | 0.277 | 0.006 | 0.177 |
| IGFBP-3 | 36.53 b | 48.03 a | 35.66 b | 37.66 b | 34.05 b | 32.00 b | 1.35 | 0.029 | 0.065 | 0.045 |
| IGFBP-5 | 12.46 | 17.27 | 23.14 | 11.52 | 17.91 | 21.81 | 0.82 | 0.588 | <0.001 | 0.694 |
| Genes | Tissues | 21 Days of Age | 24 Days of Age | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DR | TYB | XCB | DR | TYB | XCB | Weaning | Breed | W × B | |||
| IGF-1 | Stomach | 1.03 | 2.10 | 2.13 | 2.29 | 1.66 | 1.88 | 0.19 | 0.610 | 0.735 | 0.134 |
| Duodenum | 1.07 | 1.34 | 1.29 | 1.00 | 1.26 | 0.70 | 0.08 | 0.122 | 0.227 | 0.314 | |
| Jejunum | 1.60 abc | 1.86 ab | 2.52 a | 1.49 bc | 1.60 abc | 0.78 c | 0.15 | 0.011 | 0.841 | 0.030 | |
| Ileum | 0.78 | 0.61 | 0.68 | 0.78 | 0.34 | 0.33 | 0.04 | 0.003 | 0.001 | 0.101 | |
| Kidney | 0.90 a | 0.58 b | 0.43 b | 0.60 b | 0.89 a | 0.62 b | 0.04 | 0.308 | 0.015 | 0.002 | |
| Spleen | 0.26 b | 1.25 a | 0.84 a | 0.17 b | 0.34 b | 0.21 b | 0.08 | <0.001 | 0.002 | 0.028 | |
| Liver | 5.80 ab | 7.66 a | 3.27 c | 3.77 bc | 1.75 c | 1.31 c | 0.45 | <0.001 | 0.004 | 0.033 | |
| LD muscle | 0.28 | 0.34 | 0.33 | 0.41 | 0.43 | 0.33 | 0.03 | 0.169 | 0.729 | 0.630 | |
| IGF-2 | Stomach | 3.62 | 4.00 | 2.74 | 3.76 | 1.42 | 1.66 | 0.27 | 0.018 | 0.042 | 0.080 |
| Duodenum | 1.07 | 0.97 | 1.12 | 1.13 | 1.35 | 0.67 | 0.09 | 0.985 | 0.440 | 0.158 | |
| Jejunum | 0.82 | 0.80 | 0.70 | 4.12 | 6.71 | 0.84 | 0.67 | 0.002 | 0.045 | 0.055 | |
| Ileum | 0.46 | 0.40 | 0.48 | 0.43 | 0.43 | 0.31 | 0.03 | 0.389 | 0.817 | 0.416 | |
| Kidney | 52.33 | 65.69 | 62.44 | 31.22 | 55.00 | 49.49 | 2.76 | 0.003 | 0.007 | 0.642 | |
| Spleen | 6.82 | 6.14 | 3.21 | 3.20 | 2.81 | 1.68 | 0.38 | <0.001 | 0.001 | 0.269 | |
| Liver | 74.11 | 104.84 | 125.37 | 79.73 | 61.70 | 64.75 | 10.06 | 0.117 | 0.761 | 0.382 | |
| LD muscle | 12.82 | 11.71 | 11.81 | 16.91 | 16.86 | 9.35 | 0.99 | 0.244 | 0.152 | 0.225 | |
| Genes | Tissues | 21 Days of Age | 24 Days of Age | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DR | TYB | XCB | DR | TYB | XCB | Weaning | Breed | W × B | |||
| IGF-1R | Stomach | 0.49 | 0.65 | 0.61 | 0.57 | 0.31 | 0.51 | 0.04 | 0.179 | 0.787 | 0.136 |
| Duodenum | 1.10 | 1.20 | 1.24 | 0.65 | 1.24 | 0.96 | 0.06 | 0.063 | 0.073 | 0.264 | |
| Jejunum | 1.18 | 0.99 | 0.92 | 1.16 | 1.22 | 0.51 | 0.08 | 0.671 | 0.052 | 0.271 | |
| Ileum | 0.45 | 0.40 | 0.62 | 0.50 | 0.37 | 0.63 | 0.04 | 0.927 | 0.023 | 0.866 | |
| Kidney | 11.63 | 8.27 | 10.64 | 11.74 | 12.06 | 13.71 | 0.45 | 0.006 | 0.116 | 0.154 | |
| Spleen | 1.35 | 1.77 | 2.41 | 1.05 | 1.08 | 1.27 | 0.13 | 0.003 | 0.070 | 0.312 | |
| Liver | 0.89 c | 2.28 a | 1.96 ab | 1.25 c | 1.80 b | 2.32 a | 0.10 | 0.521 | <0.001 | 0.013 | |
| LD muscle | 0.22 | 0.09 | 0.25 | 0.36 | 0.26 | 0.23 | 0.03 | 0.147 | 0.372 | 0.467 | |
| IGF-2R | Stomach | 0.63 | 0.75 | 0.68 | 1.18 | 0.96 | 1.41 | 0.07 | <0.001 | 0.362 | 0.167 |
| Duodenum | 1.06 | 1.81 | 1.65 | 1.41 | 1.42 | 1.23 | 0.09 | 0.387 | 0.245 | 0.159 | |
| Jejunum | 1.50 | 1.94 | 1.38 | 2.10 | 2.34 | 1.23 | 0.14 | 0.271 | 0.037 | 0.467 | |
| Ileum | 0.88 | 1.11 | 1.16 | 1.14 | 0.92 | 0.82 | 0.05 | 0.376 | 0.971 | 0.045 | |
| Kidney | 5.09 b | 4.82 b | 3.45 c | 6.75 a | 7.32 a | 8.05 a | 0.29 | <0.001 | 0.781 | 0.007 | |
| Spleen | 1.43 | 1.47 | 1.93 | 1.72 | 1.29 | 1.89 | 0.08 | 0.877 | 0.027 | 0.437 | |
| Liver | 3.28 | 5.29 | 5.50 | 4.28 | 4.86 | 5.03 | 0.19 | 0.914 | 0.001 | 0.111 | |
| LD muscle | 0.39 | 0.16 | 0.54 | 0.72 | 0.51 | 0.38 | 0.06 | 0.168 | 0.350 | 0.167 | |
| Genes | Tissues | 21 Days of Age | 24 Days of Age | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DR | TYB | XCB | DR | TYB | XCB | Weaning | Breed | W × B | |||
| IGFBP-1 | Liver | 1.39 | 18.66 | 14.45 | 11.27 | 15.90 | 16.88 | 1.58 | 0.294 | 0.014 | 0.253 |
| IGFBP-2 | Stomach | 0.66 | 0.93 | 0.54 | 1.03 | 0.65 | 0.92 | 0.06 | 0.207 | 0.753 | 0.056 |
| Duodenum | 2.63 bc | 7.48 a | 3.38 b | 0.44 c | 0.51 c | 0.48 c | 0.53 | <0.001 | 0.019 | 0.023 | |
| Jejunum | 0.48 | 2.23 | 0.70 | 1.39 | 2.06 | 0.58 | 0.20 | 0.568 | 0.004 | 0.406 | |
| Ileum | 0.16 b | 1.05 a | 0.33 b | 0.09 b | 0.15 b | 0.22 b | 0.08 | 0.016 | 0.031 | 0.039 | |
| Kidney | 0.34 c | 8.31 a | 5.44 b | 8.05 a | 6.60 ab | 8.24 a | 0.48 | <0.001 | <0.001 | <0.001 | |
| Spleen | 0.05 b | 5.91 a | 0.12 b | 0.08 b | 0.09 b | 0.09 b | 0.35 | <0.001 | <0.001 | <0.001 | |
| Liver | 10.36 c | 118.90 ab | 141.75 a | 60.67 bc | 111.30 ab | 85.11 ab | 9.88 | 0.775 | <0.001 | 0.034 | |
| LD muscle | 0.02 b | 0.02 b | 0.03 b | 0.07 a | 0.05 ab | 0.03 b | 0.00 | 0.010 | 0.270 | 0.022 | |
| IGFBP-3 | Stomach | 6.50 b | 9.45 ab | 6.24 b | 14.92 a | 5.47 b | 6.72 b | 1.01 | 0.385 | 0.168 | 0.032 |
| Duodenum | 1.14 b | 3.73 a | 1.47 b | 1.05 b | 1.30 b | 0.87 b | 0.22 | 0.004 | 0.002 | 0.018 | |
| Jejunum | 5.89 | 17.62 | 5.63 | 8.91 | 15.87 | 2.94 | 1.47 | 0.856 | 0.001 | 0.649 | |
| Ileum | 2.88 | 3.10 | 2.36 | 2.20 | 1.08 | 1.00 | 0.20 | <0.001 | 0.146 | 0.314 | |
| Kidney | 58.21 | 42.01 | 25.75 | 47.70 | 69.12 | 44.26 | 3.62 | 0.075 | 0.023 | 0.056 | |
| Spleen | 3.78 | 2.98 | 4.30 | 3.48 | 4.33 | 5.42 | 0.29 | 0.212 | 0.151 | 0.440 | |
| Liver | 11.34 c | 105.14 a | 58.72 b | 33.50 bc | 37.02 bc | 62.67 b | 7.10 | 0.248 | 0.005 | 0.009 | |
| LD muscle | 1.11 | 0.33 | 2.09 | 3.28 | 2.27 | 1.86 | 0.33 | 0.047 | 0.485 | 0.241 | |
| IGFBP-4 | Stomach | 1.15 | 3.30 | 1.33 | 1.66 | 1.59 | 1.46 | 0.26 | 0.476 | 0.145 | 0.161 |
| Duodenum | 1.28 | 1.66 | 1.48 | 2.47 | 3.01 | 1.87 | 0.19 | 0.008 | 0.306 | 0.505 | |
| Jejunum | 0.91 | 0.48 | 0.44 | 2.46 | 3.86 | 1.67 | 0.26 | <0.001 | 0.095 | 0.082 | |
| Ileum | 3.68 a | 1.68 b | 1.68 b | 1.13 bc | 0.47 c | 0.53 c | 0.19 | <0.001 | <0.001 | 0.008 | |
| Kidney | 9.40 | 8.85 | 5.44 | 8.47 | 6.30 | 8.36 | 0.49 | 0.843 | 0.209 | 0.061 | |
| Spleen | 4.10 | 6.91 | 5.60 | 3.92 | 3.25 | 2.35 | 0.46 | 0.009 | 0.493 | 0.203 | |
| Liver | 6.05 c | 153.59 a | 136.24 a | 122.78 a | 70.81 b | 71.83 b | 9.25 | 0.405 | 0.005 | <0.001 | |
| LD muscle | 0.66 | 0.77 | 0.53 | 1.10 | 0.62 | 0.34 | 0.08 | 0.833 | 0.074 | 0.179 | |
| IGFBP-5 | Stomach | 8.38 | 5.11 | 6.18 | 12.87 | 9.57 | 12.09 | 0.71 | <0.001 | 0.097 | 0.856 |
| Duodenum | 1.03 | 1.33 | 3.89 | 0.96 | 1.37 | 1.78 | 0.28 | 0.159 | 0.010 | 0.153 | |
| Jejunum | 1.01 | 0.85 | 0.95 | 0.86 | 0.96 | 0.53 | 0.06 | 0.156 | 0.314 | 0.161 | |
| Ileum | 0.48 b | 0.52 b | 0.49 b | 0.83 a | 0.46 b | 0.45 b | 0.04 | 0.224 | 0.064 | 0.030 | |
| Kidney | 47.43 b | 30.93 b | 42.19 b | 46.07 b | 81.66 a | 74.67 a | 3.66 | <0.001 | 0.177 | 0.001 | |
| Spleen | 3.70 | 5.15 | 8.81 | 6.74 | 11.69 | 15.23 | 0.87 | <0.001 | 0.001 | 0.508 | |
| Liver | 1.67 c | 1.28 c | 1.42 c | 2.01 c | 3.51 b | 5.75 a | 0.29 | <0.001 | 0.001 | <0.001 | |
| LD muscle | 0.90 | 0.88 | 1.22 | 1.59 | 1.83 | 1.35 | 0.13 | 0.020 | 0.937 | 0.377 | |
| IGFBP-6 | Stomach | 1.99 bc | 4.39 ab | 4.37 ab | 4.78 a | 1.84 c | 1.55 c | 0.38 | 0.220 | 0.880 | 0.002 |
| Duodenum | 1.16 | 1.48 | 1.64 | 1.57 | 2.14 | 1.21 | 0.15 | 0.495 | 0.444 | 0.350 | |
| Jejunum | 0.64 b | 0.58 b | 0.55 b | 1.22 a | 1.70 a | 0.50 b | 0.10 | 0.001 | 0.007 | 0.014 | |
| Ileum | 0.74 b | 1.44 a | 0.85 b | 0.73 b | 0.57 bc | 0.41 c | 0.06 | <0.001 | 0.001 | <0.001 | |
| Kidney | 8.10 | 8.81 | 7.69 | 6.37 | 7.09 | 6.29 | 0.31 | 0.010 | 0.410 | 0.968 | |
| Spleen | 3.26 | 3.04 | 4.38 | 3.93 | 2.17 | 2.93 | 0.23 | 0.207 | 0.098 | 0.128 | |
| Liver | 1.52 | 1.13 | 1.08 | 1.14 | 1.59 | 1.62 | 0.08 | 0.177 | 0.990 | 0.033 | |
| LD muscle | 1.67 b | 3.99 a | 4.93 a | 2.21 b | 2.07 b | 1.27 b | 0.29 | 0.001 | 0.082 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, M.; Azad, M.A.K.; Cheng, Y.; Ding, S.; Liu, Y.; Song, B.; Kong, X. Expressions of Insulin-like Growth Factor System among Different Breeds Impact Piglets’ Growth during Weaning. Animals 2023, 13, 3011. https://doi.org/10.3390/ani13193011
Dou M, Azad MAK, Cheng Y, Ding S, Liu Y, Song B, Kong X. Expressions of Insulin-like Growth Factor System among Different Breeds Impact Piglets’ Growth during Weaning. Animals. 2023; 13(19):3011. https://doi.org/10.3390/ani13193011
Chicago/Turabian StyleDou, Mengying, Md. Abul Kalam Azad, Yating Cheng, Sujuan Ding, Yang Liu, Bo Song, and Xiangfeng Kong. 2023. "Expressions of Insulin-like Growth Factor System among Different Breeds Impact Piglets’ Growth during Weaning" Animals 13, no. 19: 3011. https://doi.org/10.3390/ani13193011
APA StyleDou, M., Azad, M. A. K., Cheng, Y., Ding, S., Liu, Y., Song, B., & Kong, X. (2023). Expressions of Insulin-like Growth Factor System among Different Breeds Impact Piglets’ Growth during Weaning. Animals, 13(19), 3011. https://doi.org/10.3390/ani13193011






