Simple Summary
Toxoplasma gondii and Neospora caninum are intracellular parasites with a great impact on human and animal health, respectively. This work aims to investigate the presence of antibodies against T. gondii and N. caninum in client-owned cats from Portugal and to identify risk factors. A total of 183 domestic cats were sampled and their owners answered an online questionnaire designed to obtain background information. The overall anti-T. gondii and anti-N. caninum seroprevalences were 13.1% and 3.8%, respectively. An indoor lifestyle was identified as a significant protection factor against T. gondii infection, while the presence of a chronic disease and the presence of antibodies against N. caninum were identified as significant risk factors to T. gondii seroprevalence. To the best of our knowledge, this is the first serosurvey on N. caninum seroprevalence in cats from Portugal.
Abstract
Toxoplasma gondii and Neospora caninum are obligate intracellular protozoan parasites infecting a wide range of hosts worldwide. However, information on the epidemiology of toxoplasmosis and neosporosis in cats from Portugal is limited. Thus, this study aims to evaluate anti-T. gondii and anti-N. caninum seroprevalence in client-owned cats from Portugal and to identify risk factors using a panel of well-characterized sera. A total of 183 domestic cats were sampled and screened for antibodies against T. gondii and N. caninum using commercial ELISA assays, and their owners answered an online questionnaire designed to obtain background information. The overall anti-T. gondii and anti-N. caninum seroprevalences were 13.1% (CI: 8.97–18.77) and 3.8% (CI: 1.87–7.68), respectively. Univariate analysis revealed that living strictly indoors was a significant protection factor (cOR: 0.053; CI: 0.005–0.627), and the presence of a chronic disease a significant risk factor (cOR: 3.106; CI: 1.062–9.082) to T. gondii seroprevalence. When performing multivariate analysis, only chronic disease (aOR: 57.527; CI: 1.7–1976.7) and seropositivity to N. caninum (aOR: 7.929; CI:0.8–82.9) were found to be a significant risk factor to anti-T. gondii antibodies. To the best of our knowledge, this is the first report of N. caninum seropositivity in cats from Portugal.
1. Introduction
Toxoplasma gondii and Neospora caninum are closely related obligate intracellular protozoan parasites of the phylum Apicomplexa [1]. Due to morphological similarities, N. caninum was initially misidentified as T. gondii. Subsequently, the two parasites were distinguished based on host preferences, clinical picture, ultrastructural morphology, and serological, immunohistochemical [2,3], genomic, and proteomic methods [4].
Both parasites have heteroxenous life cycles, with sexual development in the intestine of definitive hosts and asexual development in extraintestinal tissues of the intermediate hosts. Felids (domestic and wild) are the only known definitive hosts of T. gondii, but the parasite infects almost all warm-blooded vertebrates, including human populations that act as the intermediate host. To date, only domestic dogs (Canis lupus familiaris), coyotes (Canis latrans), wolves (Canis lupus), and dingoes (Canis lupus dingo) have been identified as definitive hosts of N. caninum [5,6,7,8]. Furthermore, N. caninum infects a restricted range of intermediate hosts, including cattle (Bos taurus), sheep (Ovis aries), and probably other warm-blooded animals [6,9,10,11,12,13]. At present there is no evidence that N. caninum can successfully infect humans [14].
The infectious stages of Toxoplasma and Neospora are tachyzoites, bradyzoites, and sporozoites. Tachyzoites are rapidly dividing stages of the parasite, capable of infecting a wide range of host cell types. They are located within a parasitophorous vacuole in the host cell cytoplasm and are the hallmark of active infection. Bradyzoites are the slowly multiplying encysted stage of both parasites and maintain chronic infection. Tachyzoite- and bradyzoite-containing cysts may be found in intermediate and definitive hosts. Sporozoites are enclosed within resistant oocysts and are disseminated in the environment by the definitive host through their feces [15,16].
Felids become infected with T. gondii by predating small mammals and birds, or by ingestion of raw or undercooked meat with bradyzoite-containing cysts. Queens infected for the first-time during gestation can infect offspring through the placenta or through breastfeeding [17,18]. After primoinfection, T. gondii sexual stages develop in the intestine of felids, which can excrete oocysts in feces for a short period of time. After elimination, oocysts become infectious after sporulation in the environment. Oocysts are very resistant and can survive, remaining infectious for months or years in soil and water [19,20,21,22]. Once seropositive, cats generally do not excrete oocysts again [16].
Intermediate hosts, as well as felids, harbor extraintestinal T. gondii stages. During the acute phase of infection, T. gondii-tachyzoites undergo rapid asexual multiplication into nucleated cells until the host’s humoral immune response contains parasite replication and promotes the formation of bradyzoite-containing cysts [15,23,24].
Clinical toxoplasmosis develops during replication and dissemination of tachyzoites. Primoinfection in pregnant women may cause health-threatening sequelae for the fetus, or even intrauterine death, and the reactivation of a latent infection in immunocompromised individuals can cause fatal encephalitis [25]. In cats, clinical toxoplasmosis is more severe after intrauterine infection, and kittens frequently develop hepatitis or cholangiohepatitis, pneumonia, and encephalitis. In adult cats, unspecific clinical signs can be observed [26].
In cats, T. gondii seroprevalence varies geographically, among countries, within different areas of a country, and within the same city [27] due to a variety of factors, such as climate, geographical region, animal age, and lifestyle [28], among others.
Dogs play an important role in the epidemiology of N. caninum, representing an important risk factor for the occurrence of abortus and neonatal mortality in cattle and other intermediate hosts, through environmental contamination [29,30], although the parasite is efficiently transmitted vertically [10,31]. N. caninum infection was related to abortion outbreaks in dairy herds in the North of Portugal [32,33]. Seroprevalence registered in the Northern and Central regions of the country was 28.0%, being particularly high in cows with a history of abortion (46.0%) [34].
Regarding dogs, in the first seroprevalence study performed in Portugal, 7.9% of the domestic canids presented antibodies against N. caninum, but significant differences were observed among regions, with a higher prevalence in Lisboa and North regions [35]. Canine infection can result in clinical disease, characterized by paralysis that progresses to rigid contracture of the muscles, particularly in young dogs [29,36,37]. Higher N. caninum seroprevalences were observed in dogs with musculoskeletal and neurological signs (21.4%) [35].
Experimental studies demonstrated that cats are susceptible to N. caninum infection, developing more severe lesions if treated with corticosteroids, or infected in neonatal and prenatal periods, resembling those observed in dogs [15,38]. However, there are only a few reports of naturally acquired seropositivity to N. caninum among cats worldwide [27,39,40,41,42,43]. In Portugal, N. caninum seroprevalence in cats is unknown.
Thus, the aim of this study was to evaluate the presence of specific anti-T. gondii and anti-N. caninum antibodies in client-owned cats from Portugal and identify risk factors for seropositivity using a panel of well-characterized sera from different geographical locations.
2. Materials and Methods
2.1. Animal Recruiting and Sampling
Most of the samples analyzed in this work were obtained for conducting a previous study [44]. Briefly, convenience sampling was used to select veterinary centers (clinics and hospitals). Eighteen veterinary centers from mainland Portugal were invited by email to participate in this study. Veterinary centers that agreed to collaborate (8/18) received detailed instructions for sample collection and storage, informed consent, and a link to access an online questionnaire for owners. Most serum samples (65.0%) were obtained between June and August 2021, although the collection period was extended until the end of January 2022, when seven additional serum samples were obtained.
During health care visits, veterinary practitioners from collaborating veterinary centers invited cat owners to participate in the study. Cat owners who agreed to collaborate answered an online questionnaire designed to collect background information. Blood samples were collected according to veterinary norms into dry tubes and then centrifugated at 500 rcf for 10 min. Supernatants were transferred to 2 mL microtubes and stored at −20 °C before being sent to Escola Superior Agrária de Viseu (ESAV) laboratory. At ESAV, serum samples were stored frozen at −20 °C in a temperature-controlled freezer and thawed no more than 2 times prior to serological testing.
2.2. Background Data Collection
A questionnaire was developed using an online platform (Google Forms®, Google LLC, San José, CA, USA) to collect background data. From the original questionnaire that was prepared in the Portuguese language, 17 questions (dichotomic, multiple choice and open-ended) were analyzed. The questions covered two main topics, specifically: characterization of cat owners (3 questions) and characterization of sampled cats, including cat origin, full signalment, lifestyle, prophylactic, and medical history (14 questions) (Appendix A, Table A1: questionnaire with English translation). For internal validation, the questionnaire was evaluated by the authors.
2.3. Detection of Anti-T. gondii and N. caninum IgG Antibodies
Serum samples were screened for antibodies against the P30 antigen of T. gondii using a commercial and already validated indirect multi-species ELISA (ID Screen®, Toxoplasmosis Indirect Multi-species, ID.Vet, Grabels, France). Testing was performed following the manufacturer’s instructions. Optical density (OD) was measured at a wavelength of 450 nm on a microplate reader MB 580 (Heales, Shenzhen Huisong Technology Development Co., Ltd., Shenzhen, China). For each sample, S/P% was calculated as follows: (OD sample-OD negative control)/(OD positive control-OD negative control) ×100, with serum samples presenting S/P% ≥ 50% being considered as positive, between 40% and 50% doubtful and ≤40% negative.
Serum samples were screened for antibodies against N. caninum using a commercial competition multi-species ELISA (ID Screen®, Neospora caninum Competition, ID.Vet, Grabels, France), according to manufacturer’s instructions. Overnight incubation protocol was used, and optical density (OD) was measured on a microplate reader MB 580 (Heales, Shenzhen Huisong Technology Development Co., Ltd., Shenzhen, China) at a wavelength of 450 nm. For each sample, the competition percentage (S/N%) was calculated: (OD sample)/(OD negative control) ×100, with serum samples presenting S/N% ≤ 50% being considered as positive, 50% < S/N% ≤ 60% doubtful and >60% negative.
2.4. Data Processing and Statistical Analysis
The background data collected from Google Forms® and the results of serologic analyses were downloaded in a database (Microsoft Excel 2016®; Microsoft Corp., Redmond, WA, USA). Statistical analysis was performed with IBM SPSS v.28.0.0.0 (IBM Corp., Armonk, NY, USA, 2020) using descriptive statistics and univariate and multivariate logistic regression. The association between the detection of anti-T. gondii and anti-N. caninum antibodies and the variables origin, breed, gender, reproductive state, age, lifestyle, cohabitants, vaccination, deworming, chronic disease and undergoing treatment, and seropositivity to N. caninum and T. gondii was evaluated using binomial logistic regression (univariate) and multinomial logistic regression (multivariate) analysis. Associations were considered significant with p < 0.05.
2.5. Ethics
The questionnaire was approved by the ethics committee of the Instituto Politécnico de Viseu (IPV), Viseu, Portugal. The blood collection was made according to the veterinary norms. Animal sampling was approved by the committee for Animal Welfare (ORBEA) of IPV. Cat owners were informed about the study and signed a written consent form.
3. Results
3.1. Sample Characterization
A total of 183 cats were sampled. Background information on the cat owners was obtained using an online questionnaire. Most cat owners were females (87.1%) and aged between 30 and 40 years (45.5%). According to the educational qualification, 28.7% of the owners stated that they had a degree, 24.8% had secondary education, and 20.8% had a master’s degree.
Serum samples were obtained from cats living in 11 of the 18 districts of mainland Portugal. Almost half of the samples (46.5%) were obtained in the Porto district, 14.9% in Évora, and 11.9% in the Braga district. According to their owners, cats were rescued from the street (62.3%), adopted from an association or official shelter (21.3%), or offered by a family member or friend (9.8%).
Most of the sampled cats were female (52.5%), non-fertile (93.4%), and European shorthair (83.6%), with a predominance of young adults, aging between 1–5 years (45.4%). Regarding animal environment, owners stated that 79.2% of the cats had an indoor lifestyle and 43.7% cohabited with other animals, namely dogs and cats.
Concerning medical prophylactic measures and health status, most cat owners stated that their cats were annually vaccinated (78.1%) and dewormed four times a year (67.8%), and 80.3% did not have chronic diseases (Table 1).

Table 1.
Characterization of sampled cats, including origin, residence district, signalment, environment, and clinical status.
3.2. Epidemiological Analysis of T. gondii and N. caninum-Seropositive Cats
The overall anti-T. gondii and anti-N. caninum seroprevalences were 13.1% (CI: 8.97–18.77) and 3.8% (CI: 1.87–7.68), respectively.
The 24 T. gondii-seropositive cats were from the districts of Braga (41.7%), Porto (29.2%), Évora (12.5%), Aveiro (8.3%), Setúbal (4.2%), and Viana do Castelo (4.2%). According to the Nomenclature of Territorial Units for Statistics (NUTS) II regions and considering the number of sampled cats per region, seroprevalence in the North (Viana do Castelo, Braga, Porto, Aveiro, and Viseu districts) was 13.3%, in Lisbon Metropolitan Area (Lisboa and Setúbal districts) was 10.0% and in Alentejo (Portalegre, Évora, and Beja districts) was 14.3%.
The seven N. caninum-seropositive cats were from Braga (12.5%), Porto (8.3%), and Viana do Castelo (8.3%). Considering the number of sampled cats, the seroprevalence in NUTS II North region was 4.7% (Figure 1).
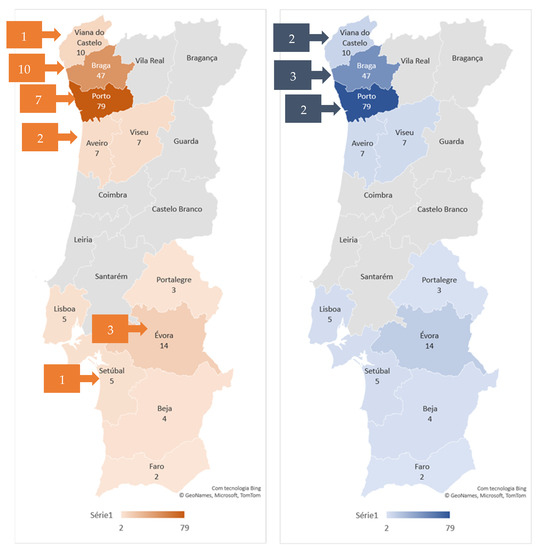
Figure 1.
Geographical distribution of T. gondii (on the left) and N. caninum-seropositive (on the right) cats. The number of sampled cats by district is shown by the color gradient, as indicated in the legend. The number of seropositive cats by district is presented in the squares.
Most of the T. gondii-seropositive cats were rescued by their current owners from the street (70.8%). Regarding signalment, most T. gondii-seropositive cats were European shorthair (87.5%), females (3%), and non-fertile (91.7%). The age of seropositive cats was variable, but T. gondii antibodies were more frequent in adult cats aged between 6 and 10 years (50.0%). Concerning the environment, most seropositive cats had an indoor lifestyle (58.3%) and contact with other animals (91.7%), namely cats and dogs (37.5%). According to their owners, most seropositive cats were vaccinated (75.0%) and dewormed every 3 months (58.3%). Furthermore, most T. gondii-seropositive cats had no chronic diseases (62.5%) and were N. caninum-seronegative (91.7%).
Most N. caninum-seropositive cats were rescued (71.4%). Regarding signalment, all seropositive cats were European shorthair and females, and most of them were non-fertile (71.4%) and aged between 1 and 5 years (71.4%). Concerning the environment, all cats had an indoor lifestyle and contact with other animals, namely cats and dogs (85.7%). According to their owners, all N. caninum-seropositive cats were vaccinated and frequently dewormed (every 3 months). Most of them had no chronic diseases (85.7%) and were T. gondii-seronegative (71.4%) (Table 2).

Table 2.
Epidemiological characterization of T. gondii and N. caninum-seropositive cats.
3.3. Binary and Multinominal Logistic Regression
Univariate analysis revealed that living strictly indoors was a significant protection factor for T. gondii seropositivity with a p value of 0.02 and a crude odds ratio (cOR) of 0.053 (CI: 0.005–0.627). However, living outdoors was not found to significantly increase the risk of T. gondii seropositivity. The presence of a chronic disease was found to be a significant risk factor with a p value of 0.038 and a cOR of 3.106 (CI: 1.062–9.082) (increasing by 3.106 times the probability for being seropositive to T. gondii). In the univariate analysis, being positive for N. caninum was not found to increase the risk to T. gondii seropositivity. When performing multivariate analysis, only chronic disease (p = 0.025, aOR = 57.527 (CI: 1.7–1976.7) and seropositivity for N. caninum (p = 0.084, aOR = 7.929 (CI:0.8–82.9) were found to be significant risk factors to anti-T. gondii antibodies.
Regarding N. caninum, significant variables were found in neither univariate nor multivariate analysis (protective or risk factors) (Table 3).

Table 3.
Binary and multinominal logistic regression analysis of T. gondii and N. caninum seropositivity in client-owned cats from Portugal.
4. Discussion
A considerable number of studies have examined the seroprevalence and risk factor of T. gondii infection worldwide and some of them have compared the seroprevalence of T. gondii and N. caninum [40,41,42,43,45,46,47,48]. However, in Portugal, the number of serosurveys on T. gondii is limited and there are no studies on the seroprevalence of N. caninum in cats.
The overall T. gondii and N. caninum seroprevalences obtained in this study were 13.1% and 3.8%, respectively. However, it must be considered that these values did not reflect mainland Portugal seroprevalences, since most samples were collected in Porto, Évora, and Braga districts. The seroprevalence of T. gondii was much higher than that of N. caninum, indicating that the cat population had more exposure in the natural environment to T. gondii than to N. caninum, as observed in previous studies worldwide [41,42,43,44,45,46,47], with some exceptions [48]
Although tempting, the direct comparison of results obtained by different studies is a challenge, due to different sample sizes, animal sampling (age, outside access, exposure to other risk factors) and environmental factors. Still, considering the same sample, the seroprevalence obtained can be very different, depending on the characteristics of the serologic testing used. For example, T. gondii seroprevalences of 18.0% and 26.0% were obtained by two commercial agglutination test kits: an Indirect Hemagglutination Test (IHAT), and a Modified Agglutination Test (MAT), respectively [49]. Even when using the same serologic testing method, the lack of standardization and cut-off titers considered compromise comparison between studies [41].
The pooled global T. gondii seroprevalence estimated by meta-analysis in domestic cats was 35.0%, and in Europe was 43.0% [50]. However, T. gondii seroprevalences in domestic cats varied between 18.2% in the Netherlands and 81.3% in Poland (19.2% in Scotland, 20.8% in Greece, 32.3% in Cyprus, 34.8% in Spain, 41.0% in Norway, 47.6% in Hungary, 62.3% in Albania, and 63.1% in Estonia) [41,46,51,52,53].
In Portugal, T. gondii seroprevalences ranged between 20.5% and 44.2% [54,55,56,57]. However, considering that our sample is exclusively composed of owned cats, living mostly indoor, the low seroprevalence obtained (13.1%) is not surprising. Higher seroprevalences were obtained from stray cats from Lisbon city (44.2%) [56] and from Lisbon Metropolitan Area (24.2%) [55] using MAT but with different cut-offs (1:20 and ≥1:80, respectively), which may explain the discrepancies observed. A lower seroprevalence (20.5%) was found in domestic cats living in apartments in Lisbon city, also using MAT and considering a cut-off of 1:40 [57]. However, in the Trás-os-Montes e Alto Douro region, an overall seroprevalence of 35.8% was found in domestic cats (MAT, cut-off 1:20), but infection levels were significantly different according to cat lifestyle. Cats that had outdoors access (45.4%) presented higher seroprevalence than those living totally indoors (13.8%) [54].
Contrary to all T. gondii seroprevalence studies previously performed in Portugal, which used MAT with different cut-offs, our study was accomplished using a commercially validated indirect multispecies ELISA. The ID Screen Toxoplasmosis Indirect Multi-species uses the P30 antigen, considered the major surface antigen on the external surface of the plasma membrane of T. gondii. The agreement between this ELISA kit and IFAT on cat sera was high, with only 2/110 discordant results [58].
Infection of cats that usually do not have access to outdoors, as seems to be the case for most of the cats in this study, can occur through the diet, including raw meat, as well as other types of food (e.g., vegetables) contaminated with oocysts, or oocysts accidentally carried inside by their owners [57,59]. Background information collected in this study does not allow evaluating cat diet. However, most of the seropositive animals were rescued from the street, where they were exposed to a high infectious pressure [60], at least in the early stages of their lives. Vertical transmission of T. gondii via the placenta or their ingestion through milk are also possibilities [17,18,23,61,62].
The univariate analysis carried out in this study revealed that living strictly indoors was a significant protection factor against T. gondii seropositivity, but living outdoors was not found to significantly increase the risk of T. gondii seropositivity, probably due to the characteristics of our sampling and the reduced number of cats with an outdoor lifestyle. However, several studies point to an increased T. gondii seroprevalence in cats with outdoor access, which can be explained by the exposure to environmental contamination with oocysts and mainly the opportunity of outdoor cats to exert their predatory behavior and ingest potentially infected rodents and birds that are intermediate hosts of T. gondii [27,54,59,60].
The presence of a chronic disease was found to be a significant risk factor, both in univariate and multivariate analysis, increasing by three times the probability for being seropositive to T. gondii. Although concomitant infectious diseases, such as Bartonella spp., Leishmania spp., Feline Leukemia Virus (FeLV), and Dirofilaria immitis can modify the clinical outcome of T. gondii infection [27,63,64], the evidence that these diseases affect seropositivity is scarce, except for Feline Immunodeficiency Virus (FIV) [54,65]. To our knowledge, this study is the first one to identify the presence of infectious and non-infectious chronic diseases as a risk factor to T. gondii seropositivity. Indeed, chronic diseases, especially if induce immunosuppression, can render cats more susceptible to T. gondii infection. Furthermore, immunosuppression conditions can lead to reactivation of tissue cysts in chronically infected cats with subsequent release of bradyzoites, resulting in increased antigenemia and stimulation of specific humoral immune response [66]. However, the association between the presence of chronic disease and seropositivity to T. gondii may be a consequence of greater veterinary surveillance. In fact, all cats in this study were sampled during a veterinary visit, which reflects their owners’ concern with veterinary care.
Regarding N. caninum, the low seroprevalence (3.8%) found in this study may be explained by the resistance of cats to oral infection [9]. Furthermore, the low level of natural seroconversion observed in cats is in line with the seroprevalence registered in dogs (7.9%) in Portugal [35]. Although convenience sampling was carried out, with a greater representation of the districts located in the North of the country, the geographical distribution of N. caninum-seropositive cats shown here is in accordance with previous findings on canine [35] and bovine seroprevalence [28,34], underlining the role of dogs and cows in the life cycle of the parasite.
In Hungary, a N. caninum seroprevalence of 0.6% (titer 1:40) was found in a sample of urban, suburban, and rural cats, employing Indirect Fluorescent Test (IFAT) [41]. The low levels of seroconversion observed in Hungary contrast with the results obtained in Albania, where 10.3% of free-rooming cats from suburban areas were found positive with IFAT with a titer ≥ 1:100 [46]. The characteristics of sampled cats may justify the discrepancy observed in these seroprevalence. Higher seroprevalences (24.8%) were observed in stray cats from North Italy with Neospora Agglutination Test (NAT) considering titers ≥ 1:80 [39]. In this case, the differences may be partly explained by the higher sensitivity but lower specificity of the NAT when compared to the IFAT in the serodiagnosis of N. caninum infection [67]. Seroprevalence to N. caninum in feral cats from Majorca (Spain) assayed using a commercial competitive inhibition enzyme-linked immunosorbent assay (cELISA) and confirmed with IFAT (cut-off ≥ 1:50), considered the reference test for neosporosis, was 6.8% [42]. Surprisingly, Sedlák et al. (2014) [68] obtained seroprevalences of 33.0% and 3.9% with cELISA and IFAT, respectively, in domestic cats from different parts of the Czech Republic. In our study, samples were assayed using a commercial cELISA validated for the detection of antibodies against N. caninum in serum and plasma from ruminants, dogs, and other susceptible species, and had already been used in other serological studies, revealing a good inter-rater agreement (Kappa value) with N. caninum immunoblot [69,70].
How cats become naturally infected with N. caninum is not fully understood [29]. Experimental studies have proved N. caninum transplacental transmission during the acute and chronic stages of infection [38]. Although the ingestion of infected tissues is the most likely source of infection for carnivores, viable N. caninum parasites has not been isolated from potential cat prey, such as birds and small rodents [29]. Despite that, N. caninum DNA has been frequently demonstrated in neural and extra neural tissues of mice and rats [39,71,72] and bird tissues [73,74,75,76].
Considering the background information collected from the sampled cats, the source of infection is hard to establish. However, we can consider the possibility of vertical transmission, since most N. caninum-seropositive cats were rescued and probably come from litters born on the street, where exposure of mothers to sources of infection is probably higher. However, cats may become infected postpartum, while still on the street, or after being rescued by their current owners. In the latter case and considering that most seropositive cats had an indoor lifestyle, we can raise the possibility of infection through the ingestion of contaminated meat, occasionally offered by the owners [57]. As most seropositive cats co-habited with dogs, the final host of N. caninum [9], the possibility of environmental contamination and ingestion of oocysts cannot be discarded, as suggested by Hornok et al. (2008) [41].
The characterization of N. caninum-seropositive cats was very similar to that of T. gondii-seropositive animals, except for age. Cats with detectable antibodies against N. caninum were younger than T. gondii-seropositive cats. This finding may be explained by the ability of N. caninum to be vertically transmitted [4], from the queen to their offspring, while T. gondii seropositivity increases with age, in line with the assumption that infection is lifelong [60] and can be transmitted post-natally [27].
The association between seroprevalence of both N. caninum and T. gondii has previously been documented [40]. In our study, multivariate analysis identified N. caninum seroprevalence as a significant risk factor to T. gondii seroprevalence. The similarities between these protozoa and the epidemiological role of rodents and birds in the biological cycles of both protozoa may explain this finding [41].
5. Conclusions
In conclusion, our data report the exposure of client-owned cats from Portugal to T. gondii and N. caninum and identified the presence of a chronic disease and the seropositivity to N. caninum as significant risk factors to T. gondii seroprevalence. A strict indoor lifestyle appeared to be a significant protection factor to T. gondii seroprevalence. While the role of cats as definitive hosts of T. gondii is well defined, the significance of cats in the epidemiology of neosporosis still needs to be outlined. To the best of our knowledge, this is the first serosurvey on N. caninum seroprevalence in cats from Portugal.
Author Contributions
Conceptualization, M.A.P. and T.L.M.; methodology, M.A.P.; formal analysis, M.A.P. and D.A.; investigation, M.A.P., A.O. and C.N.; writing—original draft preparation, M.A.P., D.A. and C.C.; writing—review and editing, M.A.P., C.N., T.L.M., D.A., A.O., C.C., R.C., P.O., A.F.-R., M.J.P., J.R.M. and H.V.; funding acquisition, C.N., P.O., A.F.-R. and M.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
The participation of M.A.P. was supported by Global Health and Tropical Medicine (GHTM) under the project UID/04413/2020, by the Centre for Natural Resources, Environment and Society (CERNAS) through the project UIDB/00681/2020, and by the project PTDC/CVT-CVT/0228/2020, all founded by Portuguese Foundation for Science and Technology (FCT). The participation of T.L.M. was supported by the projects UIDB/CVT/00772/2020 and LA/P/0059/2020, funded by FCT. The participation of H.V. was supported by the Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB) through the project UIDB/04033/2020 and by CERNAS through the project UIDB/00681/2020, both funded by FCT. The participation of C.N., P.O., A.F.-R. and M.J.P., were supported by CITAB through the project UIDB/04033/2020.
Institutional Review Board Statement
The animal study protocol was approved by the Committee for Animal Welfare of the Instituto Politécnico de Viseu (IPV) (01/ORBEA/2021, 30 April 2021). The questionnaire was approved by the ethics committee of IPV. Written consent from each owner was collected after they were informed about the study.
Informed Consent Statement
Informed consent was obtained from all cat owners involved in the study.
Data Availability Statement
Data available on request due to privacy and ethical restrictions.
Acknowledgments
The authors acknowledge veterinary centers (Hospital Veterinário de Gaia, Hospital Veterinário Bom Jesus, Vetviana, Clilegre, Hospital Veterinário da Arrábida, Hospital Veterinário Muralha de Évora, TermasVet, Clínica Veterinária de Loulé), particularly the veterinary practitioners and veterinary nurses who participated in this study, for recruiting the cats and their owners, namely Ana Prata, Lina Costa, Ricardo Oliveira, Marta Braguez, Susana Costa, Clara Barbosa, Tiago Abreu, and Sandrina Vieira.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Questionnaire with English translation.
Table A1.
Questionnaire with English translation.
| Question | Options | Type of Question |
|---|---|---|
| Owner’s gender | Male/Female/I don’t want to answer | Multiple choice |
| Owner’s age | From 18 to 30 years old/From 30 to 50 years old/From 50 to 70 years old/Over 70 years old | Multiple choice |
| Owner’s Academic qualifications: | Elementary School (1st Cycle) Elementary School (2nd Cycle) Elementary School (3rd Cycle) Secondary Education/Graduation Bachelor’s degree/master’s degree PhD (Doctorate)/Other | Multiple choice |
| Origin | Bought Adopted (association, official pet collection center) Gifted by a private person (relative, friend, etc.) Rescued (from the street, from another home, …) Other | |
| District of residence | Viana do Castelo/Braga/Vila Real/Bragança/Porto/Aveiro/Viseu Guarda/Coimbra/Castelo Branco Leiria/Lisboa/Santarém/Portalegre Setúbal/Évora/Beja/Faro | Multiple choice |
| Gender | Female/Male | Dichotomic |
| Reproductive Status | Non-neutered/Non-spayed/Neutered/Spayed | Multiple choice |
| Age | Under 1 year/From 1 to 5 years/From 5 to 10 years/More than 10 years | Multiple choice |
| Breed | Persian/Maine Coon/Siamese/Scottish Fold/Norwegian Forest/British Shorthair/Bengal/European Shorthaired/Other | Multiple choice |
| Is the cat mainly indoor (stays inside) or outdoor (have access to the road)? | Indoor/Outdoor/Indoor and Outdoor | Multiple choice |
| Do you have other pets? | Yes/No | Dichotomic |
| If the answer is yes, please select the species | Dog Cat Ferret Birds Other | Multiple choice |
| Do you usually vaccinate your cat once a year? | Yes/No | Dichotomic |
| What about deworming? How often do you do it? | Every 3 months/Every 6 months/Every year/When possible/I do not usually do it | Multiple choice |
| Does your animal present any chronic disease? | Yes/No/I don’t know | Multiple choice |
| If the answer is yes, which one/what one? | Diabetes/Kidney/hepatic disease/Joint disease/FIV/FeLV/Heart disease/Hypothyroidism /Gingivostomatitis/Other | Multiple choice |
| If the answer is yes, what is the name of the medicine? | Open-ended |
References
- Dubey, J.P.; Hattel, A.L.; Lindsay, D.S.; Topper, M.J. Neonatal Neospora caninum infection in dogs: Isolation of the causative agent and experimental transmission. J. Am. Vet. Med. Assoc. 1988, 193, 1259–1263. [Google Scholar]
- Lindsay, D.S.; Dubey, J.P. Immunohistochemical diagnosis of Neospora caninum in tissue sections. Am. J. Vet. Res. 1989, 50, 1981–1983. [Google Scholar] [PubMed]
- Dubey, J.P.; Barr, B.C.; Barta, J.R.; Bjerkås, I.; Björkman, C.; Blagburn, B.L.; Bowman, D.D.; Buxton, D.; Ellis, J.T.; Gottstein, B.; et al. Redescription of Neospora caninum and its differentiation from related coccidia. Int. J. Parasitol. 2002, 32, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Vermont, S.J.; Cotton, J.A.; Harris, D.; Hill-Cawthorne, G.A.; Könen-Waisman, S.; Latham, S.M.; Mourier, T.; Norton, R.; Quail, M.A.; et al. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog. 2012, 8, e1002567. [Google Scholar] [CrossRef]
- Gondim, L.F.; McAllister, M.M.; Pitt, W.C.; Zemlicka, D.E. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int. J. Parasitol. 2004, 34, 159–161. [Google Scholar] [CrossRef] [PubMed]
- King, J.S.; Slapeta, J.; Jenkins, D.J.; Al-Qassab, S.E.; Ellis, J.T.; Windsor, P.A. Australian dingoes are definitive hosts of Neospora caninum. Int. J. Parasitol. 2010, 40, 945–950. [Google Scholar] [CrossRef]
- Dubey, J.P.; Jenkins, M.C.; Rajendran, C.; Miska, K.; Ferreira, L.R.; Martins, J.; Kwok, O.C.; Choudhary, S. Gray wolf (Canis lupus) is a natural definitive host for Neospora caninum. Vet. Parasitol. 2011, 181, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Donahoe, S.L.; Lindsay, S.A.; Krockenberger, M.; Phalen, D.; Šlapeta, J. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int. J. Parasitol. Parasites Wildl. 2015, 4, 216–238. [Google Scholar] [CrossRef]
- McAllister, M.M.; Jolley, W.R.; Wills, R.A.; Lindsay, D.S.; McGuire, A.M.; Tranas, J.D. Oral inoculation of cats with tissue cysts of Neospora caninum. Am. J. Vet. Res. 1998, 59, 441–444. [Google Scholar]
- Davison, H.C.; Guy, F.; Trees, A.J.; Ryce, C.; Ellis, J.T.; Otter, A.; Jeffrey, M.; Simpson, V.R.; Holt, J.J. In vitro isolation of Neospora caninum from a stillborn calf in the UK. Res. Vet. Sci. 1999, 67, 103–105. [Google Scholar] [CrossRef]
- Dubey, J.P.; Liddell, S.; Mattson, D.; Speert, C.A.; Howe, D.K.; Jenkins, M.C. Characterization of the Oregon isolate of Neospora hughesi from a horse. J. Parasitol. 2001, 87, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Rosypal, A.C.; Lindsay, D.S. The sylvatic cycle of Neospora caninum: Where do we go from here? Trends Parasitol. 2005, 21, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Schares, G.; Ortega-Mora, L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007, 20, 323–367. [Google Scholar] [CrossRef] [PubMed]
- McCann, C.M.; Vyse, A.J.; Salmon, R.L.; Thomas, D.; Williams, D.J.; McGarry, J.W.; Pebody, R.; Trees, A.J. Lack of serologic evidence of Neospora caninum in humans, England. Emerg. Infect. Dis. 2008, 14, 978–980. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S.; Lipscomb, T.P. Neosporosis in cats. Vet. Pathol. 1990, 27, 335–339. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis of Animals and Humans; CRC Press: Boca Raton, NW, USA, 2010. [Google Scholar]
- Powell, C.C.; Lappin, M.R. Clinical ocular toxoplasmosis in neonatal kittens. Vet. Ophthalmol. 2001, 4, 87–92. [Google Scholar] [CrossRef]
- Powell, C.C.; Brewer, M.; Lappin, M.R. Detection of Toxoplasma gondii in the milk of experimentally infected lactating cats. Vet. Parasitol. 2001, 102, 29–33. [Google Scholar] [CrossRef]
- Yilmaz, S.M.; Hopkins, S.H. Effects of different conditions on duration of infectivity of Toxoplasma gondii oocysts. J. Parasitol. 1972, 58, 938–939. [Google Scholar] [CrossRef]
- Frenkel, J.K.; Ruiz, A.; Chinchilla, M. Soil survival of toxoplasma oocysts in Kansas and Costa Rica. Am. J. Trop. Med. Hyg. 1975, 24, 439–443. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. J. Parasitol. 2009, 95, 1019–1020. [Google Scholar] [CrossRef]
- Vanwormer, E.; Conrad, P.A.; Miller, M.A.; Melli, A.C.; Carpenter, T.E.; Mazet, J.A. Toxoplasma gondii, source to sea: Higher contribution of domestic felids to terrestrial parasite loading despite lower infection prevalence. EcoHealth 2013, 10, 277–289. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M.; Dubey, J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009, 39, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Saadatnia, G.; Golkar, M. A review on human toxoplasmosis. Scand. J. Infect. Dis. 2012, 44, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Calero-Bernal, R.; Gennari, S.M. Clinical Toxoplasmosis in Dogs and Cats: An Update. Front. Vet. Sci. 2019, 6, 54. [Google Scholar] [CrossRef]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Kwok, O.C.H.; Yang, Y.R.; Su, C. All about toxoplasmosis in cats: The last decade. Vet. Parasitol. 2020, 283, 109145. [Google Scholar] [CrossRef]
- Waap, H.; Bärwald, A.; Nunes, T.; Schares, G. Seroprevalence and Risk Factors for Toxoplasma gondii and Neospora caninum in Cattle in Portugal. Animals 2022, 12, 2080. [Google Scholar] [CrossRef]
- Dubey, J.P.; Schares, G. Neosporosis in animals--the last five years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef]
- Almería, S.; López-Gatius, F. Bovine neosporosis: Clinical and practical aspects. Res. Vet. Sci. 2013, 95, 303–309. [Google Scholar] [CrossRef]
- Williams, D.J.; Hartley, C.S.; Björkman, C.; Trees, A.J. Endogenous and exogenous transplacental transmission of Neospora caninum—How the route of transmission impacts on epidemiology and control of disease. Parasitology 2009, 136, 1895–1900. [Google Scholar] [CrossRef]
- Thompson, G.; Canada, N.; Topa, M.; Silva, E.; Vaz, F.; Rocha, A. First confirmed case of Neospora caninum associated abortion outbreak in Portugal. Reprod. Domest. Anim. 2001, 36, 309–312. [Google Scholar] [CrossRef]
- Canada, N.; Meireles, C.S.; Rocha, A.; Sousa, S.; Thompson, G.; Dubey, J.P.; Romand, S.; Thulliez, P.; Costa, J.M. First Portuguese isolate of Neospora caninum from an aborted fetus from a dairy herd with endemic neosporosis. Vet. Parasitol. 2002, 110, 11–15. [Google Scholar] [CrossRef]
- Canada, N.; Carvalheira, J.; Meireles, C.S.; Correia da Costa, J.M.; Rocha, A. Prevalence of Neospora caninum infection in dairy cows and its consequences for reproductive management. Theriogenology 2004, 62, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Cortes, H.; Brancal, H.; Lopes, A.P.; Pimenta, P.; Campino, L.; Cardoso, L. Prevalence and correlates of antibodies to Neospora caninum in dogs in Portugal. Parasite 2014, 21, 29. [Google Scholar] [CrossRef]
- Anvari, D.; Saberi, R.; Sharif, M.; Sarvi, S.; Hosseini, S.A.; Moosazadeh, M.; Hosseininejad, Z.; Chegeni, T.N.; Daryani, A. Seroprevalence of Neospora caninum Infection in Dog Population Worldwide: A Systematic Review and Meta-analysis. Acta Parasitol. 2020, 65, 273–290. [Google Scholar] [CrossRef]
- Silva, R.C.; Machado, G.P. Canine neosporosis: Perspectives on pathogenesis and management. Vet. Med. 2016, 7, 59–70. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S. Transplacental Neospora caninum infection in cats. J. Parasitol. 1989, 75, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Ferroglio, E.; Guiso, P.; Pasino, M.; Accossato, A.; Trisciuoglio, A. Antibodies to Neospora caninum in stray cats from north Italy. Vet. Parasitol. 2005, 131, 31–34. [Google Scholar] [CrossRef]
- Bresciani, K.D.; Gennari, S.M.; Serrano, A.C.; Rodrigues, A.A.; Ueno, T.; Franco, L.G.; Perri, S.H.; Amarante, A.F. Antibodies to Neospora caninum and Toxoplasma gondii in domestic cats from Brazil. Parasitol. Res. 2007, 100, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Edelhofer, R.; Joachim, A.; Farkas, R.; Berta, K.; Repasi, A.; Lakatos, B. Seroprevalence of Toxoplasma gondii and Neospora caninum infection of cats in Hungary. Acta Vet. Hung. 2008, 56, 81–88. [Google Scholar] [CrossRef]
- Millán, J.; Cabezón, O.; Pabón, M.; Dubey, J.P.; Almería, S. Seroprevalence of Toxoplasma gondii and Neospora caninum in feral cats (Felis silvestris catus) in Majorca, Balearic Islands, Spain. Vet. Parasitol. 2009, 165, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Coelho, W.M.; do Amarante, A.F.; Apolinário Jde, C.; Coelho, N.M.; de Lima, V.M.; Perri, S.H.; Bresciani, K.D. Seroepidemiology of Toxoplasma gondii, Neospora caninum, and Leishmania spp. infections and risk factors for cats from Brazil. Parasitol. Res. 2011, 109, 1009–1013. [Google Scholar] [CrossRef]
- Oliveira, A.; Pereira, M.A.; Mateus, T.L.; Mesquita, J.R.; Vala, H. Seroprevalence of SARS-CoV-2 in Client-Owned Cats from Portugal. Vet. Sci. 2022, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Braga Mdo, S.; André, M.R.; Jusi, M.M.; Freschi, C.R.; Teixeira, M.C.; Machado, R.Z. Occurrence of anti-Toxoplasma gondii and anti-Neospora caninum antibodies in cats with outdoor access in São Luís, Maranhão, Brazil. Rev. Bras. De Parasitol. Veterinária 2012, 21, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Knaus, M.; Rapti, D.; Kusi, I.; Shukullari, E.; Hamel, D.; Pfister, K.; Rehbein, S. Survey of Toxoplasma gondii and Neospora caninum, haemotropic mycoplasmas and other arthropod-borne pathogens in cats from Albania. Parasites Vectors 2014, 7, 62. [Google Scholar] [CrossRef]
- Sousa, K.C.; Herrera, H.M.; Domingos, I.H.; Campos, J.B.; Santos, I.M.; Neves, H.H.; Machado, R.Z.; André, M.R. Serological detection of Toxoplasma gondii, Leishmania infantum and Neospora caninum in cats from an area endemic for leishmaniasis in Brazil. Rev. Bras. De Parasitol. Veterinária 2014, 23, 449–455. [Google Scholar] [CrossRef]
- Yang, J.; Ai, J.; Qi, T.; Ni, X.; Xu, Z.; Guo, L.; Sun, Y.; Li, Y.; Kang, M.; Li, J. Toxoplasma gondii and Neospora caninum Infections in Stray Cats and Dogs in the Qinghai-Tibetan Plateau Area, China. Animals 2022, 12, 1390. [Google Scholar] [CrossRef]
- Fernandes, S.; Brilhante-Simões, P.; Coutinho, T.; Cardoso, L.; Dubey, J.P.; Lopes, A.P. Comparison of indirect and modified agglutination tests for detection of antibodies to Toxoplasma gondii in domestic cats. J. Vet. Diagn. Invest. 2019, 31, 774–777. [Google Scholar] [CrossRef]
- Montazeri, M.; Mikaeili Galeh, T.; Moosazadeh, M.; Sarvi, S.; Dodangeh, S.; Javidnia, J.; Sharif, M.; Daryani, A. The global serological prevalence of Toxoplasma gondii in felids during the last five decades (1967-2017): A systematic review and meta-analysis. Parasites Vectors 2020, 13, 82. [Google Scholar] [CrossRef]
- Attipa, C.; Yiapanis, C.; Tasker, S.; Diakou, A. Seroprevalence of Toxoplasma gondii in Cats from Cyprus. Pathogens 2021, 10, 882. [Google Scholar] [CrossRef]
- Tharaka, K.L.D.; Liyanage, D.; Wiethoelter, A.; Hufschmid, J.; Jabbar, A. Descriptive Comparison of ELISAs for the Detection of Toxoplasma gondii Antibodies in Animals: A Systematic Review. Pathogens. 2021, 10, 605. [Google Scholar] [CrossRef]
- Gardner, I.A.; Greiner, M.; Dubey, J.P. Statistical evaluation of test accuracy studies for Toxoplasma gondii in food animal intermediate hosts. Zoonoses Public Health 2010, 57, 82–94. [Google Scholar] [CrossRef]
- Lopes, A.P.; Cardoso, L.; Rodrigues, M. Serological survey of Toxoplasma gondii infection in domestic cats from northeastern Portugal. Vet. Parasitol. 2008, 155, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Castro, I.; Pereira da Fonseca, I.M.; Almeida, V.; Madeira de Carvalho, L.M.; Meireles, J.; Fazendeiro, M.I.; Tavares, L.; Vaz, Y. Survey of infectious and parasitic diseases in stray cats at the Lisbon Metropolitan Area, Portugal. J. Feline Med. Surg. 2010, 12, 441–446. [Google Scholar] [CrossRef]
- Waap, H.; Cardoso, R.; Leitão, A.; Nunes, T.; Vilares, A.; Gargaté, M.J.; Meireles, J.; Cortes, H.; Ângelo, H. In vitro isolation and seroprevalence of Toxoplasma gondii in stray cats and pigeons in Lisbon, Portugal. Vet. Parasitol. 2012, 187, 542–547. [Google Scholar] [CrossRef]
- Esteves, F.; Aguiar, D.; Rosado, J.; Costa, M.L.; de Sousa, B.; Antunes, F.; Matos, O. Toxoplasma gondii prevalence in cats from Lisbon and in pigs from centre and south of Portugal. Vet. Parasitol. 2014, 200, 8–12. [Google Scholar] [CrossRef]
- Pourquier, P.; Macrì, G.; Scarpulla, M.; Salvato, L. Comparison of indirect immunofluorescence and ID Screen® Toxoplasmosis indirect ELISA for the detection of antibodies against Toxoplasma gondii in cat and dog sera. Poster Presented at the WAVLD Meeting, Madrid, 2009. Wild & Domestic Animals.
- Schreiber, N.; Basso, W.; Riond, B.; Willi, B.; Torgerson, P.R.; Deplazes, P. Antibody kinetics and exposure to Toxoplasma gondii in cats: A seroepidemiological study. Int. J. Parasitol. 2021, 51, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Dámek, F.; Swart, A.; Waap, H.; Jokelainen, P.; Le Roux, D.; Deksne, G.; Deng, H.; Schares, G.; Lundén, A.; Álvarez-García, G.; et al. Systematic Review and Modelling of Age-Dependent Prevalence of Toxoplasma gondii in Livestock, Wildlife and Felids in Europe. Pathogens 2023, 12, 97. [Google Scholar] [CrossRef]
- Dubey, J.P.; Beattie, C.P. Toxoplasmosis of Animals and Man; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Dubey, J.P.; Mattix, M.E.; Lipscomb, T.P. Lesions of neonatally induced toxoplasmosis in cats. Vet. Pathol. 1996, 33, 290–295. [Google Scholar] [CrossRef]
- Heidel, J.R.; Dubey, J.P.; Blythe, L.L.; Walker, L.L.; Duimstra, J.R.; Jordan, J.S. Myelitis in a cat infected with Toxoplasma gondii and feline immunodeficiency virus. J. Am. Vet. Med. Assoc. 1990, 196, 316–318. [Google Scholar]
- Pena, H.F.J.; Evangelista, C.M.; Casagrande, R.A.; Biezus, G.; Wisser, C.S.; Ferian, P.E.; Moura, A.B.; Rolim, V.M.; Driemeier, D.; Oliveira, S.; et al. Fatal toxoplasmosis in an immunosuppressed domestic cat from Brazil caused by Toxoplasma gondii clonal type I. Rev. Bras. Parasitol. Vet. 2017, 26, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Dorny, P.; Speybroeck, N.; Verstraete, S.; Baeke, M.; De Becker, A.; Berkvens, D.; Vercruysse, J. Serological survey of Toxoplasma gondii, feline immunodeficiency virus and feline leukaemia virus in urban stray cats in Belgium. Vet. Rec. 2002, 151, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R.; Gasper, P.W.; Rose, B.J.; Powell, C.C. Effect of primary phase feline immunodeficiency virus infection on cats with chronic toxoplasmosis. Vet. Immunol. Immunopathol. 1992, 35, 121–131. [Google Scholar] [CrossRef]
- Packham, A.E.; Sverlow, K.W.; Conrad, P.A.; Loomis, E.F.; Rowe, J.D.; Anderson, M.L.; Marsh, A.E.; Cray, C.; Barr, B.C. A modified agglutination test for Neospora caninum: Development, optimization, and comparison to the indirect fluorescent-antibody test and enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 1998, 5, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, K.; Bartova, E.; Machacova, T. Seroprevalence of Neospora caninum in cats from the Czech Republic. Acta Parasitol. 2014, 59, 359–361. [Google Scholar] [CrossRef]
- Basso, W.; Sollberger, E.; Schares, G.; Küker, S.; Ardüser, F.; Moore-Jones, G.; Zanolari, P. Toxoplasma gondii and Neospora caninum infections in South American camelids in Switzerland and assessment of serological tests for diagnosis. Parasites Vectors 2020, 13, 256. [Google Scholar] [CrossRef]
- Manca, R.; Ciccarese, G.; Scaltrito, D.; Chirizzi, D. Detection of Anti-Neospora caninum Antibodies on Dairy Cattle Farms in Southern Italy. Vet. Sci. 2022, 9, 87. [Google Scholar] [CrossRef]
- Jenkins, M.C.; Parker, C.; Hill, D.; Pinckney, R.D.; Dyer, R.; Dubey, J.P. Neospora caninum detected in feral rodents. Vet. Parasitol. 2007, 143, 161–165. [Google Scholar] [CrossRef]
- Barratt, J.; Al Qassab, S.; Reichel, M.P.; Ellis, J.T. The development and evaluation of a nested PCR assay for detection of Neospora caninum and Hammondia heydorni in feral mouse tissues. Mol. Cell Probes. 2008, 22, 228–233. [Google Scholar] [CrossRef]
- Du, L.; Yang, D.; Zhai, T.; Gong, P.; Zhang, X.; Li, J. Detection of Neospora caninum-DNA in brain tissues from pigeons in Changchun, Jilin (China). Vet. Parasitol. 2015, 214, 171–173. [Google Scholar] [CrossRef]
- de Barros, L.D.; Miura, A.C.; Minutti, A.F.; Vidotto, O.; Garcia, J.L. Neospora caninum in birds: A review. Parasitol. Int. 2018, 67, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Nardoni, S.; Poli, A.; Varvaro, I.; Rocchigiani, G.; Ceccherelli, R.; Mancianti, F. Detection of Neospora Caninum DNA in Wild Birds from Italy. Pathogens 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Karakavuk, M.; Can, H.; Aldemir, D.; Döndüren, Ö.; Karakavuk, T.; Karakavuk, E.; Özdemir, H.G.; Muz, M.N.; Gürüz, A.Y.; Döşkaya, M. Presence of Neospora caninum DNA of Wild Birds from Turkey. Turk. Parazitolojii Derg. 2021, 45, 231–236. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).