Simple Summary
Canine vector-borne diseases (CVBDs) can affect the health of domestic and wild animals, and their prevalence is increasing worldwide. As potential reservoirs of zoonotic pathogens, dogs might transfer these pathogens to humans. There is limited knowledge about the vector-borne pathogens circulating in dogs in China. To investigate the current epidemiological status and genetic characteristics of Ehrlichia spp., Hepatozoon spp., and Mycoplasma spp., blood samples were collected from healthy pet dogs in four regions of China. There was no evidence of Ehrlichia spp. or Mycoplasma spp., and only Hepatozoon canis was detected in these dogs. High haplotype diversity and the occurrence of genetic variation were observed among these H. canis isolates. These results will be useful for developing effective control approaches against CVBDs in companion animals.
Abstract
Canine vector-borne diseases are widely distributed around the world. They are transmitted by arthropods, and many seriously threaten the health of animals and humans. In China, our knowledge of Ehrlichia, Hepatozoon, and Mycoplasma species circulating in dogs is still poorly understood. Therefore, the aim of this study was to understand the prevalence and genetic characteristics of canine Ehrlichia spp., Hepatozoon spp., and Mycoplasma spp. in Chongqing (southwest), Fujian (southeast), Shandong (southeast), and Hubei (central) Provinces of China. Blood samples from healthy pet dogs were processed to detect Ehrlichia, Hepatozoon, and Mycoplasma DNA with PCR. Haplotype and phylogenetic analyses were performed on 18S rRNA sequences. Among 306 dogs, no Ehrlichia spp. or Mycoplasma spp. were detected, whereas one Hepatozoon sp. was detected in 10 (3.27%) of the animals. Only Hepatozoon canis was identified and was endemic to Chongqing (2.46%) and Hubei (8.77%). A haplotype analysis identified eight haplotypes among the H. canis isolates. A phylogenetic analysis showed that the H. canis isolates in this study clustered into four clades, together with isolates from different countries and hosts, forming a large group that was clearly separate from other Hepatozoon species. These findings provided new information on the epidemiological characteristics of canine vector-borne diseases in China and will be helpful in the development of efficient measures to safeguard the health and well-being of companion animals and their owners.
1. Introduction
Canine vector-borne diseases (CVBDs) pose a risk to the health of both domestic and wild animals, and many CVBDs are zoonotic [1]. The pathogens involved in CVBDs include viruses, bacteria, protozoans, and helminths, which are transmitted by hematophagous arthropods such as ticks, mosquitoes, fleas, and lice [2]. CVBDs are widely distributed in tropical, subtropical, and temperate areas, which are suitable for the survival of these vectors [3,4,5]. In recent years, increasing numbers of families have kept pets, and contact with animals is common, increasing the opportunities for the transmission of zoonotic infectious diseases to humans [6]. Therefore, monitoring CVBD infections is important for understanding the health status of pets and their owners and for improving pet welfare.
Hepatozoonosis is a tick-borne disease caused by Hepatozoon americanum and Hepatozoon canis, which are mainly transmitted by Amblyomma maculatum and Rhipicephalus sanguineus, respectively [7,8]. Other tick species have been identified as vectors or suspected vectors of H. canis, including A. ovale, Dermacentor marginatus, Haemaphysalis (Hae) flava, Hae. longicornis, and R. turanicus [9,10,11,12]. The life cycle of these two Hepatozoon species is heteroxenous in that they undergo a sporogonic stage in the definitive invertebrate host (e.g., mite, mosquito, or tick), and merogonic and gamontogonic stages in the intermediate vertebrate host [13]. In contrast to other tick-borne pathogens that are transmitted through the tick salivary glands, the transmission of Hepatozoon occurs through the ingestion of ticks containing mature oocysts [14]. Other routes by which Hepatozoon is transmitted to dogs have been confirmed, including transplacental infections and predation on infected animals [15,16]. Hepatozoon americanum is mainly endemic in the United States, and its infection causes fever, leukocytosis, musculoskeletal pain, and often fatal disease [13]. Hepatozoon canis is widely distributed and has been described in dogs in Asia, Africa, America, and Europe [17,18,19,20]. Its infections cause lethargy and anemia, but most cases have subclinical symptoms [21]. Wild canids are possible reservoirs of H. canis and usually display no clinical signs [22]. H. canis is a common parasite in red foxes in Poland (45.7%), Portugal (75.6%), and Spain (91%) [22,23,24]. In Italy, the prevalence of H. canis in red foxes (13.4%) is higher than in dogs (3.6%) [25,26], and similar rates have been observed in foxes (27.9%) and dogs (1.8%) in Germany [27]. H. canis has also been detected in golden jackals in Israel and Hungary [28,29]. In Israel, the prevalence of H. canis in golden jackals (46%) and red foxes (43%) is similar to that in dogs (33.1%) [28,30]. In China, there is little information on H. canis infections in wild canids, but they have been sporadically reported in dogs, such as in Beijing, Henan, Jiangsu, Shaanxi, and Xinjiang [31,32].
Canine monocytic ehrlichiosis (CME) is another tick-borne disease caused by Ehrlichia canis, a gram-negative bacterium of the family Anaplasmataceae. E. canis is transmitted by R. sanguineus and is mainly distributed in tropical, subtropical, and Mediterranean climates [33,34,35]. E. canis is inoculated into the host by ticks through their salivary glands during blood meals. This pathogen can be transmitted by a tick within 3 h of its attachment to a host. E. canis primarily infects monocytes in dogs, and its infection may cause thrombocytopenia. After an incubation period of 1–3 weeks, CME has three phases: acute, subclinical, and chronic [36]. In the acute phase, dogs are characterized by fever, anorexia, lethargy, lymphadenomegaly, and splenomegaly. Dogs are likely to be infested with ticks during this phase. In the subclinical or chronic phase, the dog seems to be healthy but becomes a pathogen carrier. Ophthalmic lesions are common and often include anterior uveitis, papilledema, chorioretinitis, and retinal hemorrhage [37]. E. canis occasionally infects humans and has been confirmed as causing human disease in Venezuela [38]. All dog breeds are susceptible to CME. However, German Shepherds are more likely to have severe clinical symptoms and a poor prognosis [39]. In clinical cases, detecting E. canis morula is rarely used in a blood smear because it occurs at a low incidence (4–6%). Usually, E. canis can be diagnosed with serological (e.g., IFAT or ELISA) or molecular (e.g., PCR) techniques. In Europe, E. canis has been detected in dogs in countries such as Italy (46%), Portugal (0.7%), Romania (2.1%), and Serbia (18.2%) [35,40,41,42]. In Asia, E. canis has been reported in dogs in India (16.1%), Korea (4.7%), and Pakistan (24.5%) [43,44,45]. In China, the prevalence of E. canis is 1.3% in dogs and 10.2% in ticks in southeastern regions [46]. In Xinjiang, E. canis is common, with a prevalence of 12.12% in dogs and 15.23% in ticks [47]. In Hong Kong, E. canis has been detected in stray dogs (8%) and pet dogs (6%) [48]. Similarly, in another study of 1508 dogs examined with real-time PCR, 7.4% were positive for E. canis [49].
Haemotropic mycoplasmas are small, unculturable, and cell wall-deficient bacteria that cause erythrocytic infections in domestic and wild mammals. Mycoplasma haemocanis and “Candidatus Mycoplasma haematoparvum” are two major species of hemoplasmas infecting dogs. Infections with canine hemoplasma are often asymptomatic, and the outcomes, including acute hemolytic anemia and fatal disease, may be associated with immunosuppression or coinfection with other pathogens. Canine hemoplasmas may be transmitted by bloodsucking arthropods. Particularly, R. sanguineus may play an important role as a vector in the transmission of these pathogens. In cats, blood transfusions are a common source of feline hemoplasma infections [50]. Because blood smears stained with Giemsa have low sensitivity and specificity, specific conventional or quantitative real-time PCR is used to examine the canine hemoplasma species [51]. In Europe, canine hemoplasma infections have shown a higher prevalence in countries with Mediterranean climate than in Switzerland [52,53]. M. haemocanis (8.45%) and C. M. haematoparvum (2.11%) have been detected in dogs in Portugal [35], and similar rates of M. haemocanis (9.9%) and C. M. haematoparvum (2.9%) have been detected in Cambodia [54]. In another study, the prevalence of M. haemocanis (26.2%) was higher than that of C. M. haematoparvum (6.7%) in Turkey [55]. M. haemocanis (38.2%) and C. M. haematoparvum (43.2%) are commonly found in Korea [44]. In Italy, the prevalence of M. haemocanis (13.1%) is similar to that of C. M. haematoparvum (11.4%) in hunting dogs [56]. The prevalence of canine hemoplasmas is low in Australia (1.6%) [57]. Coinfection with both hemoplasma species is found in dogs in Turkey (5.3%) and Italy (4.6%) [55,56]. In China, the prevalence of M. haemocanis is 1.85% in Jiangxi [58]. “Candidatus Mycoplasma haemominutum”, a major feline hemoplasma species, has been detected in China and Japan [59,60]. “Candidatus Mycoplasma haemobos” has also been reported in dogs in China [61].
Our understanding of the canine vector-borne pathogens in China remains scant. Therefore, in this study, we investigated the distribution and genetic characteristics of Ehrlichia spp., Hepatozoon spp., and Mycoplasma spp. in domestic dogs from Chongqing municipality and Fujian, Hubei, and Shandong provinces to update the current epidemic status of canine vector-borne diseases in China and to provide valuable reference information for the prevention and control of these diseases.
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
Blood samples from 306 owned dogs were taken from pet clinics in the southwestern (Chongqing municipality), central (Hubei Province), and southeastern (Fujian and Shandong Provinces) regions of China. These regions have different geographical and environmental characteristics, such as temperature, humidity, and annual rainfall. The sample size was 203 dogs in Chongqing, 23 dogs in Fujian, 57 dogs in Hubei, and 23 dogs in Shandong. All samples were randomly collected from apparently healthy dogs, and no ectoparasites were found. Approximately 300 µL of whole blood were obtained in sterile EDTA vacutainer tubes and transported in iceboxes to the laboratory. Genomic DNA was extracted from 250 µL of blood using the Blood DNA Mini Kit (Omega, Norcross, GA, USA) following the manufacturer′s instructions and then stored at −20 °C until use.
2.2. PCR Amplification and Sequencing
The presence of Ehrlichia, Hepatozoon, and Mycoplasma DNA was screened by conventional PCR. The primers EHR16SD: 5′-GGTACCYACAGAAGAAGTCC-3′ and EHR16SR: 5′-TAGCACTCATCGTTTACAGC-3′ were used to amplify the 345 bp fragment of the 16S rRNA gene for detection of Ehrlichia spp. [62]. A fragment of 666 bp of the 18S rRNA gene of Hepatozoon spp. was amplified using the primers: Hep F: 5′-ATACATGAGCAAAATCTCAAC-3′ and Hep R: 5′-CTTATTATTCCATGCTGCAG-3′ [63]. A PCR targeting approximately 560 bp of the 16S rRNA gene was performed using primers: Myco322s: 5′-GCCCATATTCCTACGGGAAGCAGCAGT-3′ and Myco938as: 5′-CTCCACCACTTG TTCAGGTCCCCGTC-3′ [64]. PCR reaction mixtures of 25 µl were prepared containing 2.5 µL of 10 × PCR buffer, 2.0 µL of 2.5 mM dNTP, 0.3 µL of 5 U/µL Taq DNA polymerase (Takara, Dalian, China), 0.1 µM of each primer, and 2 µL of DNA template under the reaction conditions as described previously with an annealing temperature of 53 °C for Ehrlichia, 58 °C for Hepatozoon, and 68 °C for Mycoplasma species [62,63,64]. The positive products were purified using a Hipure Gel Pure DNA Mini Kit (Magen, Guangzhou, China) and cloned into the pMD19-T vector (TaKaRa, Dalian, China). Sequencing reactions were performed with M13-F/M13-R primers, and the reaction products were separated and detected using an automated sequencer, the ABI 3730XL. DNA extracted from a dog infected with H. canis and distilled water were used as positive and negative controls, respectively.
2.3. Sequence Analysis
The 18S rRNA sequences obtained in this study were assembled and edited with the Lasergene program (DNASTAR Inc., Madison, WI, USA). To identify highly similar sequences, all these sequences were analyzed with the NCBI BLASTn program (https://blast.ncbi.nlm.nih.gov, accessed on 25 January 2022 and 20 June 2022). The accession numbers for the Hepatozoon isolates detected are OM392073–OM392078 and ON810476–ON810479.
ClustalW in the MEGA 11 software was used to align the sequences obtained [65]. A nucleotide sequence analysis was performed with the GeneDoc program [66]. To estimate the genetic relationships between different regions among H. canis isolates, a haplotype TCS network was constructed with the PopArt software [67,68]. The number of haplotypes, haplotype diversity, and nucleotide diversity were calculated with DnaSP 5.1 [69]. To infer the evolutionary relationships of the H. canis isolates, the sequences obtained in this study were compared with those from different countries and hosts downloaded from the GenBank database. A phylogenetic analysis with the neighbor-joining method was implemented in the MEGA 11 software [65]. The best Tamura 3-parameter model was selected. Branch support was assessed with bootstrap values calculated with 1000 replicates. A homologous sequence of Adelina bambarooniae (accession number AF494058) was used as an outgroup.
3. Results
3.1. Detection and Identification of Vector-Borne Pathogens
In the 306 samples analyzed, the prevalence of Hepatozoon spp. was 3.27%, but no Ehrlichia spp. or Mycoplasma spp. were detected, as shown in Table 1. Hepatozoon spp. were found in Chongqing and Hubei, with prevalence rates of 2.46% and 8.77%, respectively. BLAST analysis indicated that the 10 positive samples were closely related to H. canis from dogs in China (MT107091), Malaysia (KT267958), Zambia (LC331053), and Venezuela (DQ439540) with 96.3% to 100% sequence identity. The sequences obtained in this study shared 96.1% to 100% nucleotide identity with each other.

Table 1.
Geographical distribution and prevalence of Hepatozoon canis in dogs in four regions of China.
3.2. Sequence Analysis
Nucleotide sequence variations were observed within the 18S rRNA sequences determined. Compared with the reference sequence (MT107091), nucleotide substitutions occurred at 34 positions, as shown in Figure S1. A haplotype analysis identified eight haplotypes in 10 individuals. The haplotype diversity and nucleotide diversity were 0.956 and 0.013, respectively.
3.3. Phylogenetic Analysis and Haplotype Network
A phylogenetic tree was constructed from the 18S rRNA sequences of the H. canis isolates together with related homologous sequences retrieved from GenBank, as shown in Figure 1. The neighbor-joining tree showed that the 10 H. canis isolates clustered into four clades. Seven H. canis isolates (CQ39, CQ72, HB2, HB13, HB14, HB15, and HB16) belonged to clade 1, and CQ33, CQ31, and CQ11 to clades 2, 3, and 4, respectively. The phylogenetic tree also indicated that H. canis isolates from various geographic regions or different hosts clustered in a large group with a bootstrap value of 99% but were clearly separated from those of other Hepatozoon species.
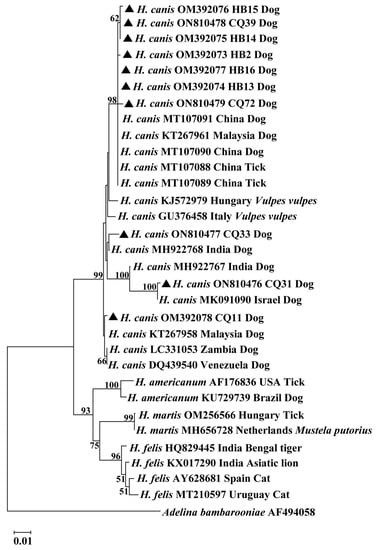
Figure 1.
Phylogenetic tree constructed using 18S rRNA sequences from Hepatozoon isolates based on the neighbor-joining method. The sequences obtained from this study were compared with related sequences deposited in GenBank. The bootstrap values of >50% were exhibited at each branch point. GenBank accession numbers, the isolate, countries, and host were shown alongside species names. The sequence of Adelina bambarooniae (AF494058) was used as an outgroup. Representative isolates in this study were indicated by bold triangles. Abbreviations: CQ (Chongqing) and HB (Hubei).
The haplotype network showed that haplotypes Hap1, Hap2, and Hap4 originated in Hubei, haplotypes Hap5–Hap8 in Chongqing, and haplotype Hap3 in Chongqing and Hubei, as shown in Figure 2. Other haplotypes from India, Korea, Malaysia, and Thailand are shown in Figure 2. Haplotype Hap2 was shared by China, Korea, and Malaysia. The random distribution of eight haplotypes from China across the haplotype network did not display any particular genetic structure among H. canis isolates. The haplotype network also showed that there was no distinct grouping of H. canis isolates according to the geographical region in Asia.

Figure 2.
Haplotype network of H. canis isolates. The size of the circle represents the frequency of each haplotype. The different colored dots represent haplotypes from different locations.
4. Discussion
In China, pet ownership is rapidly growing in some cities. Keeping pets can not only enrich people’s spiritual lives, especially for empty-nesters, but also promote communication among pet owners and increase their happiness. An important problem should be noted: these pets may carry some pathogens, especially zoonotic pathogens. Thus, health issues for pet animals, their owners, or the public should not be neglected. In recent decades, the distribution and prevalence of CVBDs have been continuously expanding. This may be related to the interactions between pathogens, hosts, and vectors, which in turn, are influenced by environmental climate change and anthropogenic factors. In particular, arthropod vectors are more easily affected by climate change because high temperatures and low humidity contribute to their growth. Canine vector-borne pathogens are transmitted by arthropods, and many are zoonotic and threaten the health of animals and humans. Importantly, domestic dogs can act as reservoir hosts for zoonotic agents, and may transfer these pathogens to humans.
In this study, the prevalence of common vector-borne pathogens was investigated in dogs in four regions of China. H. canis was identified in the collected samples by sequencing the 18S rRNA genes and was detected in 2.46% and 8.77% of the samples collected from Chongqing and Hubei, respectively. In previous studies, the prevalence of H. canis in dogs was 4.35% in Hanzhong [32], 4.5% in Beijing, 2.3% in Nanjing, 1.2% in Urumchi, 8.9% in Yangling, and 4.9% in Zhengzhou [31]. On a global scale, the prevalence of H. canis in China is lower than that in other countries such as Brazil (66.45%), Iran (23.07%), Pakistan (45.5%), and Portugal (20.42%) [35,70,71,72]. On the contrary, the prevalence of H. canis in some other countries, such as India (0.26%), Qatar (1.6%), and Thailand (1.81%), is lower than that in China [73,74,75]. The prevalence of H. canis in this study was also similar to that in guard dogs in Nigeria (6%) [76]. These differences in the distribution of H. canis could be related to differences in the abundance and geographical distributions of tick species, such as Hae. longicornis and R. sanguineus, differences in the characteristics of specific dog populations (e.g., age, sex, breed, and health), or the methods of sampling [77]. In China, more than 20 tick species have been recorded in Hubei, but relatively few in Chongqing [78]. In the present study, the prevalence of H. canis was higher in Hubei, where R. sanguineus is not endemic but Hae. longicornis is both endemic and common. In another study, a low prevalence of Hepatozoon sp. was found in Hae. longicornis in northeastern China [79]. Therefore, the role of Hae. longicornis as a vector transmitting hepatozoonosis should be investigated in future studies. No H. canis was detected in Fujian or Shandong, where R. sanguineus is considered endemic [78]. A possible explanation was that the sampled dogs had little opportunity to contact ticks or that the sample size was too small to detect their prevalence. H. canis is also the most prevalent pathogen in wild canids and has been described in red foxes in Poland, Portugal, Spain, Italy, and Germany [22,23,24,25,27], in golden jackals in Israel and Hungary [28,29], and in maned wolves in Brazil [80]. H. canis infections in foxes and dogs have both been detected in Italy and Germany [25,26,27], suggesting that it is transferred from wild canids to domestic animals living in the same areas. Currently, there is little information on H. canis in wild canids in China, and the relationship between H. canis infections in wild and domestic animals should be investigated in follow-up studies.
The four regions surveyed were negative for Ehrlichia spp. and Mycoplasma spp. These bacteria can be zoonotic and potentially fatal, and they are known to be transmitted by ticks. Among the three Ehrlichial species that infect dogs, E. canis, E. chaffeensis, and E. ewingii, E. canis is most common. The clinical manifestations of E. canis infection are variable, depending on the virulence of the strain, the immune status of hosts, and coinfection with other pathogens. E. canis has been reported in dogs in India, Italy, Korea, Pakistan, Portugal, Romania, and Serbia [35,40,41,42,43,44,45]. The prevalence of E. canis is high in India (16.1%) and Italy (46%) [40,43], indicating that dogs living outdoors are more susceptible to contact ticks compared with pet dogs living indoors. In addition, E. canis has also been examined in wild canids, including red foxes (52%), and gray wolves (50%), in Italy [81]. In general, R. sanguineus is considered the main vector for CME transmission, but Hae. longicornis is a common tick species responsible for transmitting CME in East Asian countries [82]. In China, E. canis has been detected in dogs in areas such as Beijing, Jiangsu, Xinjiang, and Hong Kong [31,47,48] and has been detected in other hosts, including ticks, goats, and deer [46,83,84]. E. canis has been identified both in R. sanguineus and Hae. longicornis in China [46]. M. haemocanis and C. M. haematoparvum are also common in dogs and have been detected in Australia, Cambodia, Italy, Korea, Portugal, and Turkey [35,44,54,55,56,57]. For M. haemocanis and C. M. haematoparvum, the prevalence of dogs living outdoors is higher than that living indoors [44,56], which are similar to E. canis infections in dogs. In Europe, R. sanguineus is mainly found in areas with Mediterranean climate, and a higher prevalence of canine hemoplasma infection was observed in these regions [52,53]. M. haemocanis, C. M. haemominutum, and C. M. haemobos have been identified in dogs in China [58,59,61]. However, our knowledge of Ehrlichia and Mycoplasma infections in dogs is still relatively limited, and large-scale epidemiological research is required in endemic or non-endemic areas for ticks in further studies. In this study, the failure to detect these pathogens could be related to the fact that the dogs sampled were domestic dogs maintained in good health and living conditions. Although these dogs spent most of their time indoors and had limited opportunities to contact ticks, H. canis was still detected. As a tick-borne disease, the prevalence of hepatozoonosis could be associated with the ability of ticks to transmit the pathogen. However, no ticks were detected on the bodies of these pet dogs or in the environment in which they lived, so it was difficult to identify the origins of the initial infections. Therefore, how these dogs were exposed to Hepatozoon infection and the routes of transmission of H. canis should be investigated in further studies.
A haplotype analysis indicated high haplotype diversity (0.956), consistent with the findings for H. canis isolates from different continents in a previous study [85]. A haplotype network showed that haplotype Hap2 was shared by China, Korea, and Malaysia, which could be attributable to its evolution from other haplotypes in China or its introduction from neighboring countries. A phylogenetic tree showed that the H. canis 18S rRNA sequences clustered into four clades. This finding was similar to reports from Pakistan and Germany, where H. canis isolates were also classified into different clusters [71,86]. These sequence data suggested that genetic variation existed within H. canis isolates. In a phylogenetic analysis, H. canis did not clearly cluster according to geographic region. This result was supported by a recent study that showed no phylogeographic grouping when H. canis populations were analyzed by continent [85]. Collectively, these results imply the presence of minor strain variations in H. canis, but there may be some gene flow between different geographic regions. Possible reasons for this are that the transmission intensity of H. canis and the dispersal of ticks are affected by human activities and that the spread of ticks and tick-borne pathogens is influenced by migratory birds [85,87]. Further studies that evaluate the genetic characteristics of H. canis in large-scale samples from China are essential.
5. Conclusions
In this study, H. canis was detected in Chongqing and Hubei. High haplotype diversity and the occurrence of genetic variation were observed among these H. canis isolates. These findings provided a foundation for studying epidemiology and genetic characteristics of H. canis in China and will also be useful in understanding the health status of companion animals and minimizing the risk of infection in animals and humans.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13111867/s1. Figure S1. Nucleotide sequence analysis of H. canis isolates in dogs. The sequences obtained from this study were compared with the related sequences deposited in GenBank in China (MT107091), Malaysia (KT267958), Zambia (LC331053), and Venezuela (DQ439540). The nucleotide substitutions occurred at 34 positions. Abbreviations: CQ (Chongqing) and HB (Hubei).
Author Contributions
F.Y. and F.L. conceived the project. D.L. collected blood samples. F.Y., C.G. and Z.T. carried out laboratory work. F.Y. performed the data analysis. F.Y. prepared the manuscript with the support of F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 32102695) and the Fundamental Research Funds for the Central Universities (Grant No. SWU-KT22014).
Institutional Review Board Statement
This study was approved by the Institutional Animal Care and Use Committee of Southwest University (20211125-01). All pet dogs were handled in accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China. All samples were collected with the permission of the owners of pet dogs.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
Thanks to the contributors who submitted sequences to GenBank and all the veterinary practitioners for assistance with sample collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Day, M.J. One health: The importance of companion animal vector-borne diseases. Parasit. Vectors 2011, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-Torres, F.; Breitschwerdt, E.B. Managing canine vector-borne diseases of zoonotic concern: Part one. Trends Parasitol. 2009, 25, 157–163. [Google Scholar] [CrossRef]
- Colella, V.; Nguyen, V.L.; Tan, D.Y.; Lu, N.; Fang, F.; Zhijuan, Y.; Wang, J.; Liu, X.; Chen, X.; Dong, J.; et al. Zoonotic vectorborne pathogens and ectoparasites of dogs and cats in Eastern and Southeast Asia. Emerg. Infect. Dis. 2020, 26, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Angelou, A.; Gelasakis, A.I.; Verde, N.; Pantchev, N.; Schaper, R.; Chandrashekar, R.; Papadopoulos, E. Prevalence and risk factors for selected canine vector-borne diseases in Greece. Parasit. Vectors 2019, 12, 283. [Google Scholar] [CrossRef]
- Miro, G.; Wright, I.; Michael, H.; Burton, W.; Hegarty, E.; Rodon, J.; Buch, J.; Pantchev, N.; von Samson-Himmelstjerna, G. Seropositivity of main vector-borne pathogens in dogs across Europe. Parasit. Vectors 2022, 15, 189. [Google Scholar] [CrossRef]
- Morelli, S.; Diakou, A.; Di Cesare, A.; Colombo, M.; Traversa, D. Canine and feline parasitology: Analogies, differences, and relevance for human health. Clin. Microbiol. Rev. 2021, 34, e0026620. [Google Scholar] [CrossRef]
- Baneth, G.; Samish, M.; Shkap, V. Life cycle of Hepatozoon canis (Apicomplexa: Adeleorina: Hepatozoidae) in the tick Rhipicephalus sanguineus and domestic dog (Canis familiaris). J. Parasitol. 2007, 93, 283–299. [Google Scholar] [CrossRef]
- Ewing, S.A.; Mathew, J.S.; Panciera, R.J. Transmission of Hepatozoon americanum (Apicomplexa: Adeleorina) by ixodids (Acari: Ixodidae). J. Med. Entomol. 2002, 39, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Inoue, M.; Taura, Y.; Nakama, S.; Abe, H.; Fujisaki, K. Detection of Hepatozoon canis oocyst from ticks collected from the infected dogs. J. Vet. Med. Sci. 1995, 57, 111–112. [Google Scholar] [CrossRef]
- Forlano, M.; Scofield, A.; Elisei, C.; Fernandes, K.R.; Ewing, S.A.; Massard, C.L. Diagnosis of Hepatozoon spp. in Amblyomma ovale and its experimental transmission in domestic dogs in Brazil. Vet. Parasitol. 2005, 134, 1–7. [Google Scholar] [CrossRef]
- Giannelli, A.; Lia, R.P.; Annoscia, G.; Buonavoglia, C.; Lorusso, E.; Dantas-Torres, F.; Baneth, G.; Otranto, D. Rhipicephalus turanicus, a new vector of Hepatozoon canis. Parasitology 2017, 144, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Tanczos, B.; Fernandez de Mera, I.G.; de la Fuente, J.; Hofmann-Lehmann, R.; Farkas, R. High prevalence of Hepatozoon-infection among shepherd dogs in a region considered to be free of Rhipicephalus sanguineus. Vet. Parasitol. 2013, 196, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Mathew, J.S.; Shkap, V.; Macintire, D.K.; Barta, J.R.; Ewing, S.A. Canine hepatozoonosis: Two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol. 2003, 19, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G. Perspectives on canine and feline hepatozoonosis. Vet. Parasitol. 2011, 181, 3–11. [Google Scholar] [CrossRef]
- Johnson, E.M.; Panciera, R.J.; Allen, K.E.; Sheets, M.E.; Beal, J.D.; Ewing, S.A.; Little, S.E. Alternate pathway of infection with Hepatozoon americanum and the epidemiologic importance of predation. J. Vet. Intern. Med. 2009, 23, 1315–1318. [Google Scholar] [CrossRef]
- Schafer, I.; Muller, E.; Nijhof, A.M.; Aupperle-Lellbach, H.; Loesenbeck, G.; Cramer, S.; Naucke, T.J. First evidence of vertical Hepatozoon canis transmission in dogs in Europe. Parasit. Vectors 2022, 15, 296. [Google Scholar] [CrossRef]
- Barati, A.; Razmi, G.R. A parasitologic and molecular survey of Hepatozoon canis infection in stray dogs in northeastern Iran. J. Parasitol. 2018, 104, 413–417. [Google Scholar] [CrossRef]
- Tadesse, H.; Grillini, M.; Simonato, G.; Mondin, A.; Dotto, G.; Frangipane di Regalbono, A.; Kumsa, B.; Cassini, R.; Menandro, M.L. Epidemiological survey on tick-borne pathogens with zoonotic potential in dog populations of southern Ethiopia. Trop. Med. Infect. Dis. 2023, 8, 102. [Google Scholar] [CrossRef]
- Allen, K.E.; Johnson, E.M.; Little, S.E. Hepatozoon spp. infections in the United States. Vet. Clin. North. Am. Small Anim. Pr. 2011, 41, 1221–1238. [Google Scholar] [CrossRef]
- Andersson, M.O.; Tolf, C.; Tamba, P.; Stefanache, M.; Waldenstrom, J.; Dobler, G.; Chitimia-Dobler, L. Canine tick-borne diseases in pet dogs from Romania. Parasit Vectors 2017, 10, 155. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Weigl, S.; Latrofa, M.S.; Stanneck, D.; Decaprariis, D.; Capelli, G.; Baneth, G. Diagnosis of Hepatozoon canis in young dogs by cytology and PCR. Parasit. Vectors 2011, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewska, E.J.; Dwuznik, D.; Koczwarska, J.; Stanczak, L.; Opalinska, P.; Krokowska-Paluszak, M.; Wierzbicka, A.; Gorecki, G.; Bajer, A. The red fox (Vulpes vulpes), a possible reservoir of Babesia vulpes, B. canis and Hepatozoon canis and its association with the tick Dermacentor reticulatus occurrence. Ticks Tick. Borne Dis. 2021, 12, 101551. [Google Scholar] [CrossRef]
- Ortuno, M.; Nachum-Biala, Y.; Garcia-Bocanegra, I.; Resa, M.; Berriatua, E.; Baneth, G. An epidemiological study in wild carnivores from Spanish Mediterranean ecosystems reveals association between Leishmania infantum, Babesia spp. and Hepatozoon spp. infection and new hosts for Hepatozoon martis, Hepatozoon canis and Sarcocystis spp. Transbound. Emerg. Dis. 2022, 69, 2110–2125. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Cortes, H.C.; Eyal, O.; Reis, A.; Lopes, A.P.; Vila-Vicosa, M.J.; Rodrigues, P.A.; Baneth, G. Molecular and histopathological detection of Hepatozoon canis in red foxes (Vulpes vulpes) from Portugal. Parasit. Vectors 2014, 7, 113. [Google Scholar] [CrossRef]
- Gabrielli, S.; Kumlien, S.; Calderini, P.; Brozzi, A.; Iori, A.; Cancrini, G. The first report of Hepatozoon canis identified in Vulpes vulpes and ticks from Italy. Vector Borne Zoonotic Dis. 2010, 10, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Cassini, R.; Zanutto, S.; Frangipane di Regalbono, A.; Gabrielli, S.; Calderini, P.; Moretti, A.; Tampieri, M.P.; Pietrobelli, M. Canine piroplasmosis in Italy: Epidemiological aspects in vertebrate and invertebrate hosts. Vet. Parasitol. 2009, 165, 30–35. [Google Scholar] [CrossRef]
- Helm, C.S.; Samson-Himmelstjerna, G.V.; Liesner, J.M.; Kohn, B.; Muller, E.; Schaper, R.; Pachnicke, S.; Schulze, C.; Krucken, J. Identical 18S rRNA haplotypes of Hepatozoon canis in dogs and foxes in Brandenburg, Germany. Ticks Tick. Borne Dis. 2020, 11, 101520. [Google Scholar] [CrossRef] [PubMed]
- Margalit Levi, M.; Nachum-Biala, Y.; King, R.; Baneth, G. A survey of Babesia spp. and Hepatozoon spp. in wild canids in Israel. Parasit. Vectors 2018, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Farkas, R.; Solymosi, N.; Takacs, N.; Hornyak, A.; Hornok, S.; Nachum-Biala, Y.; Baneth, G. First molecular evidence of Hepatozoon canis infection in red foxes and golden jackals from Hungary. Parasit. Vectors 2014, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Shkap, V.; Presentey, B.Z.; Pipano, E. Hepatozoon canis: The prevalence of antibodies and gametocytes in dogs in Israel. Vet. Res. Commun. 1996, 20, 41–46. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Shi, Z.; Song, C.; Zheng, X.; Zhang, Y.; Hao, Y.; Dong, H.; Wei, L.; El-Mahallawy, H.S.; et al. Molecular detection of vector-borne agents in dogs from ten provinces of China. Parasit. Vectors 2015, 8, 501. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.P.; Xie, G.C.; Xue, Z.Q.; Yu, J.J.; Jian, R.; Du, L.Y.; Li, Y.N. Molecular detection of Hepatozoon canis in dogs and ticks in Shaanxi province, China. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101514. [Google Scholar] [CrossRef]
- Poolsawat, N.; Nooroong, P.; Junsiri, W.; Watthanadirek-Wijidwong, A.; Srionrod, N.; Sangchuai, S.; Minsakorn, S.; Tazawa, K.; Anuracpreeda, P. Ehrlichia canis: Molecular characterization and genetic diversity based on the p28 and trp36 genes. Res. Vet. Sci. 2023, 155, 88–102. [Google Scholar] [CrossRef]
- Skotarczak, B. Canine ehrlichiosis. Ann. Agric. Env. Med. 2003, 10, 137–141. [Google Scholar]
- Dordio, A.M.; Beck, R.; Nunes, T.; Pereira da Fonseca, I.; Gomes, J. Molecular survey of vector-borne diseases in two groups of domestic dogs from Lisbon, Portugal. Parasit. Vectors 2021, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Harrus, S.; Waner, T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): An overview. Vet. J. 2011, 187, 292–296. [Google Scholar] [CrossRef]
- Komnenou, A.A.; Mylonakis, M.E.; Kouti, V.; Tendoma, L.; Leontides, L.; Skountzou, E.; Dessiris, A.; Koutinas, A.F.; Ofri, R. Ocular manifestations of natural canine monocytic ehrlichiosis (Ehrlichia canis): A retrospective study of 90 cases. Vet. Ophthalmol. 2007, 10, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef]
- Harrus, S.; Kass, P.H.; Klement, E.; Waner, T. Canine monocytic ehrlichiosis: A retrospective study of 100 cases, and an epidemiological investigation of prognostic indicators for the disease. Vet. Rec. 1997, 141, 360–363. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Capri, A.; Solano-Gallego, L.; Lombardo, G.; Torina, A.; Masucci, M. Prevalence of antibodies against Rickettsia conorii, Babesia canis, Ehrlichia canis, and Anaplasma phagocytophilum antigens in dogs from the Stretto di Messina area (Italy). Ticks Tick. Borne Dis. 2012, 3, 315–318. [Google Scholar] [CrossRef]
- Mircean, V.; Dumitrache, M.O.; Gyorke, A.; Pantchev, N.; Jodies, R.; Mihalca, A.D.; Cozma, V. Seroprevalence and geographic distribution of Dirofilaria immitis and tick-borne infections (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, and Ehrlichia canis) in dogs from Romania. Vector Borne Zoonotic Dis. 2012, 12, 595–604. [Google Scholar] [CrossRef]
- Sukara, R.; Andric, N.; Andric, J.F.; Mihaljica, D.; Veinovic, G.; Rankovic, V.; Tomanovic, S. Autochthonous infection with Ehrlichia canis and Hepatozoon canis in dogs from Serbia. Vet. Med. Sci. 2023, 9, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Manoj, R.R.S.; Iatta, R.; Latrofa, M.S.; Capozzi, L.; Raman, M.; Colella, V.; Otranto, D. Canine vector-borne pathogens from dogs and ticks from Tamil Nadu, India. Acta Trop. 2020, 203, 105308. [Google Scholar] [CrossRef]
- Suh, G.H.; Ahn, K.S.; Ahn, J.H.; Kim, H.J.; Leutenegger, C.; Shin, S. Serological and molecular prevalence of canine vector-borne diseases (CVBDs) in Korea. Parasit. Vectors 2017, 10, 146. [Google Scholar] [CrossRef]
- Iatta, R.; Sazmand, A.; Nguyen, V.L.; Nemati, F.; Ayaz, M.M.; Bahiraei, Z.; Zafari, S.; Giannico, A.; Greco, G.; Dantas-Torres, F.; et al. Vector-borne pathogens in dogs of different regions of Iran and Pakistan. Parasitol. Res. 2021, 120, 4219–4228. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Wang, D.; Li, W.; Beugnet, F.; Zhou, J. Epidemiological survey of ticks and tick-borne pathogens in pet dogs in south-eastern China. Parasite 2017, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Wang, L.; Lei, Y.; Ren, Y.; Cai, K.; Zhang, J.; Zhang, Z.; Yu, W.; Peng, Y.; Cai, X.; et al. Molecular detection and genetic variability of Ehrlichia canis in pet dogs in Xinjiang, China. Vet. World 2020, 13, 916–922. [Google Scholar]
- Wong, S.S.; Teng, J.L.; Poon, R.W.; Choi, G.K.; Chan, K.H.; Yeung, M.L.; Hui, J.J.; Yuen, K.Y. Comparative evaluation of a point-of-care immunochromatographic test SNAP 4Dx with molecular detection tests for vector-borne canine pathogens in Hong Kong. Vector Borne Zoonotic Dis. 2011, 11, 1269–1277. [Google Scholar] [CrossRef]
- Muguiro, D.H.; Nekouei, O.; Lee, K.Y.; Hill, F.; Barrs, V.R. Prevalence of Babesia and Ehrlichia in owned dogs with suspected tick-borne infection in Hong Kong, and risk factors associated with Babesia gibsoni. Prev. Vet. Med. 2023, 214, 105908. [Google Scholar] [CrossRef]
- Gary, A.T.; Richmond, H.L.; Tasker, S.; Hackett, T.B.; Lappin, M.R. Survival of Mycoplasma haemofelis and 'Candidatus Mycoplasma haemominutum' in blood of cats used for transfusions. J. Feline Med. Surg. 2006, 8, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Willi, B.; Novacco, M.; Meli, M.; Wolf-Jackel, G.; Boretti, F.; Wengi, N.; Lutz, H.; Hofmann-Lehmann, R. Haemotropic mycoplasmas of cats and dogs: Transmission, diagnosis, prevalence and importance in Europe. Schweiz. Arch. Tierheilkd. 2010, 152, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Wengi, N.; Willi, B.; Boretti, F.S.; Cattori, V.; Riond, B.; Meli, M.L.; Reusch, C.E.; Lutz, H.; Hofmann-Lehmann, R. Real-time PCR-based prevalence study, infection follow-up and molecular characterization of canine hemotropic mycoplasmas. Vet. Microbiol. 2008, 126, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Novacco, M.; Meli, M.L.; Gentilini, F.; Marsilio, F.; Ceci, C.; Pennisi, M.G.; Lombardo, G.; Lloret, A.; Santos, L.; Carrapico, T.; et al. Prevalence and geographical distribution of canine hemotropic mycoplasma infections in Mediterranean countries and analysis of risk factors for infection. Vet. Microbiol. 2010, 142, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Inpankaew, T.; Hii, S.F.; Chimnoi, W.; Traub, R.J. Canine vector-borne pathogens in semi-domesticated dogs residing in northern Cambodia. Parasit. Vectors 2016, 9, 253. [Google Scholar] [CrossRef]
- Aktas, M.; Ozubek, S. Molecular survey of haemoplasmas in shelter dogs and associations with Rhipicephalus sanguineus sensu lato. Med. Vet. Entomol. 2017, 31, 457–461. [Google Scholar] [CrossRef]
- Cortese, L.; Beall, M.; Buono, F.; Buch, J.; Pacifico, L.; Neola, B.; Palatucci, A.T.; Tyrrell, P.; Fioretti, A.; Breitschwerdt, E.B.; et al. Distribution and risk factors of canine haemotropic mycoplasmas in hunting dogs from southern Italy. Vet. Microbiol. 2020, 251, 108910. [Google Scholar] [CrossRef]
- Hetzel, N.J.; Barker, E.N.; Helps, C.R.; Tasker, S.; Arteaga, A.; Barrs, V.R.; Beatty, J. Prevalence of canine haemotropic mycoplasma infections in Sydney, Australia. Vet. Rec. 2012, 171, 126. [Google Scholar] [CrossRef]
- Zheng, W.Q.; Chen, H.Y.; Liu, M.M.; Adjou Moumouni, P.F.; Efstratiou, A.; Liu, Z.B.; Xuan, X.N. First evidence of Mycoplasma haemocanis in China. Trop. Biomed. 2017, 34, 983–990. [Google Scholar]
- Zhuang, Q.J.; Zhang, H.J.; Lin, R.Q.; Sun, M.F.; Liang, X.J.; Qin, X.W.; Pu, W.J.; Zhu, X.Q. The occurrence of the feline “Candidatus Mycoplasma haemominutum” in dog in China confirmed by sequence-based analysis of ribosomal DNA. Trop. Anim. Health Prod. 2009, 41, 689–692. [Google Scholar] [CrossRef]
- Obara, H.; Fujihara, M.; Watanabe, Y.; Ono, H.K.; Harasawa, R. A feline hemoplasma, ‘Candidatus Mycoplasma haemominutum’, detected in dog in Japan. J. Vet. Med. Sci. 2011, 73, 841–843. [Google Scholar] [CrossRef]
- Shi, H.; Li, B.; Li, J.; Chen, S.; Wang, L.; Bai, Z.; Zhu, L.; Yan, B.; Yao, L. Molecular detection of haemophilic pathogens reveals evidence of Candidatus Mycoplasma haemobos in dogs and parasitic ticks in central China. BMC Vet. Res. 2022, 18, 254. [Google Scholar] [CrossRef]
- Parola, P.; Roux, V.; Camicas, J.L.; Baradji, I.; Brouqui, P.; Raoult, D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 707–708. [Google Scholar] [CrossRef]
- Inokuma, H.; Okuda, M.; Ohno, K.; Shimoda, K.; Onishi, T. Analysis of the 18S rRNA gene sequence of a Hepatozoon detected in two Japanese dogs. Vet. Parasitol. 2002, 106, 265–271. [Google Scholar] [CrossRef]
- Varanat, M.; Maggi, R.G.; Linder, K.E.; Breitschwerdt, E.B. Molecular prevalence of Bartonella, Babesia, and hemotropic Mycoplasma sp. in dogs with splenic disease. J. Vet. Intern. Med. 2011, 25, 1284–1291. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nicholas, K.B.; Nicholas, H.B.J.; Deerfield, D.W.I. GeneDoc: Analysis and vsualization of genetic variation. Embnew News 1997, 4, 14. Available online: http://nrbsc.org/gfx/genedoc/ (accessed on 5 April 2023).
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Dalimi, A.; Jameie, F.; Mohammadiha, A.; Barati, M.; Molaei, S. Molecular detection of Hepatozoon canis in dogs of Ardabil Province, northwest of Iran. Arch. Razi Inst. 2017, 72, 197–201. [Google Scholar] [PubMed]
- Ahmad, A.S.; Saeed, M.A.; Rashid, I.; Ashraf, K.; Shehzad, W.; Traub, R.J.; Baneth, G.; Jabbar, A. Molecular characterization of Hepatozoon canis from farm dogs in Pakistan. Parasitol. Res. 2018, 117, 1131–1138. [Google Scholar] [CrossRef]
- Demoner, L.C.; Magro, N.M.; da Silva, M.R.L.; de Paula Antunes, J.M.A.; Calabuig, C.I.P.; O’Dwyer, L.H. Hepatozoon spp. infections in wild rodents in an area of endemic canine hepatozoonosis in southeastern Brazil. Ticks Tick. Borne Dis. 2016, 7, 859–864. [Google Scholar] [CrossRef]
- Alho, A.M.; Lima, C.; Latrofa, M.S.; Colella, V.; Ravagnan, S.; Capelli, G.; Madeira de Carvalho, L.; Cardoso, L.; Otranto, D. Molecular detection of vector-borne pathogens in dogs and cats from Qatar. Parasit. Vectors 2017, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Singla, L.D.; Sumbria, D.; Mandhotra, A.; Bal, M.S.; Kaur, P. Critical analysis of vector-borne infections in dogs: Babesia vogeli, Babesia gibsoni, Ehrlichia canis and Hepatozoon canis in Punjab, India. Acta Parasitol. 2016, 61, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Poolsawat, N.; Tazawa, K.; Junsiri, W.; Watthanadirek, A.; Srionrod, N.; Chawengkirttikul, R.; Anuracpreeda, P. Molecular discrimination and genetic diversity of three common tick-borne pathogens in dogs in Thailand. Parasitology 2022, 149, 65–75. [Google Scholar] [CrossRef]
- Gruenberger, I.; Liebich, A.V.; Ajibade, T.O.; Obebe, O.O.; Ogbonna, N.F.; Wortha, L.N.; Unterkofler, M.S.; Fuehrer, H.P.; Ayinmode, A.B. Vector-borne pathogens in guard dogs in Ibadan, Nigeria. Pathogens 2023, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Stich, R.W.; Blagburn, B.L.; Bowman, D.D.; Carpenter, C.; Cortinas, M.R.; Ewing, S.A.; Foley, D.; Foley, J.E.; Gaff, H.; Hickling, G.J.; et al. Quantitative factors proposed to influence the prevalence of canine tick-borne disease agents in the United States. Parasit. Vectors 2014, 7, 417. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, X.Y.; Liu, J.Z. Ticks (Acari: Ixodoidea) in China: Geographical distribution, host diversity, and specificity. Arch. Insect Biochem. Physiol. 2019, 102, e21544. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Song, M.; Liu, H.; Wang, B.; Wang, S.; Wang, Z.; Ma, H.; Li, Z.; Zeng, Z.; Qian, J.; et al. Molecular detection and characterization of zoonotic and veterinary pathogens in ticks from northeastern China. Front. Microbiol. 2016, 7, 1913. [Google Scholar] [CrossRef]
- Arrais, R.C.; Paula, R.C.; Martins, T.F.; Nieri-Bastos, F.A.; Marcili, A.; Labruna, M.B. Survey of ticks and tick-borne agents in maned wolves (Chrysocyon brachyurus) from a natural landscape in Brazil. Ticks Tick. Borne Dis. 2021, 12, 101639. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Veneziano, V.; D'Alessio, N.; Di Prisco, F.; Lucibelli, M.G.; Borriello, G.; Cerrone, A.; Dantas-Torres, F.; Latrofa, M.S.; Otranto, D.; et al. Molecular survey of Ehrlichia canis and Coxiella burnetii infections in wild mammals of southern Italy. Parasitol. Res. 2016, 115, 4427–4431. [Google Scholar] [CrossRef]
- Aziz, M.U.; Hussain, S.; Song, B.; Ghauri, H.N.; Zeb, J.; Sparagano, O.A. Ehrlichiosis in dogs: A comprehensive review about the pathogen and its vectors with emphasis on south and east Asian countries. Vet. Sci. 2023, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Kelly, P.J.; Zhang, J.; Luo, Q.; Yang, Y.; Mao, Y.; Yang, Z.; Li, J.; Wu, H.; Wang, C. Molecular detection of Anaplasma spp. and Ehrlichia spp. in ruminants from twelve provinces of China. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 9183861. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Liu, Z.; Liu, J.; Yang, J.; Li, Q.; Li, Y.; Luo, J.; Yin, H. Molecular survey of Anaplasma and Ehrlichia of red deer and sika deer in Gansu, China in 2013. Transbound. Emerg. Dis. 2016, 63, e228–e236. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Aguilar, A.A.; Barbachano-Guerrero, A.; Angulo, D.F.; Jarquin-Diaz, V.H. Phylogeography and population differentiation in Hepatozoon canis (Apicomplexa: Hepatozoidae) reveal expansion and gene flow in world populations. Parasit. Vectors 2021, 14, 467. [Google Scholar] [CrossRef]
- Hodzic, A.; Georges, I.; Postl, M.; Duscher, G.G.; Jeschke, D.; Szentiks, C.A.; Ansorge, H.; Heddergott, M. Molecular survey of tick-borne pathogens reveals a high prevalence and low genetic variability of Hepatozoon canis in free-ranging grey wolves (Canis lupus) in Germany. Ticks Tick. Borne Dis. 2020, 11, 101389. [Google Scholar] [CrossRef]
- Rollins, R.E.; Schaper, S.; Kahlhofer, C.; Frangoulidis, D.; Strauss, A.F.T.; Cardinale, M.; Springer, A.; Strube, C.; Bakkes, D.K.; Becker, N.S.; et al. Ticks (Acari: Ixodidae) on birds migrating to the island of Ponza, Italy, and the tick-borne pathogens they carry. Ticks Tick. Borne Dis. 2021, 12, 101590. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).