Simple Summary
The red squat lobster Pleuroncodes monodon, a key crustacean in the Humboldt Current System (HCS), plays an important role as predator and prey of several species of commercial interest. Off the Chilean coast, P. monodon presents benthic habits and constitutes the main resource of the industrial crustacean fishery. In contrast, off the coast of Peru, the red squat lobster exhibits pelagic habits, with knowledge gaps on traits of its ecology and no commercial fishery activities. Therefore, this review explores research conducted in the HCS, including ecological aspects and its use as a marine bioresource.
Abstract
This study focused on gathering available information on Pleuroncodes monodon, a widely distributed crustacean in the Humboldt Current System. Off the Chilean coast, this species presents benthic habits and constitutes the main resource of the industrial crustacean fishery; many studies have been carried out on its life cycle during the last century. In contrast, off the coast of Peru, this species exhibits mainly pelagic habits, with latent information gaps on aspects of its life history and no commercial fishery activities, such as catching, taking or harvesting from the marine environment. P. monodon is an ecologically important species, as a source of energy for its predators, which include invertebrates, birds, marine mammals and fish of commercial interest. Thus, P. monodon seems to play a key role in this ecosystem, mainly as an intermediate link between top predators and the first links in the food chain. In addition, this species presents various adaptation strategies to the changing oceanographic parameters of the areas it inhabits, even tolerating hypoxic environments and great depths in order to avoid being predated. Likewise, from an economic viewpoint, it has a high commercial value as a marine bioresource with great potential in the pharmaceutical and food industries. Considering this, more studies must be carried out to corroborate the biological, ecological, and fishing importance of this species in order to generate efficient management measures and ensure a sustainable fishery.
1. Introduction
Pleuroncodes monodon [1], commonly known as the “red squat lobster” belongs to the Munididae family and can be found from 15° N in Mexico to 41° S in Chile [2]. Its wide distribution and great abundance throughout South America has led it to be considered as the main resource of the industrial fishery of demersal crustaceans in Chile since the 1950s, as well as a resource with high exploitation potential in Peru [3]. The vast majority of studies regarding aspects of its life cycle have focused on Chile, with few investigations carried out in Peru, mainly due to the fact that it is not currently considered a fishery resource there [4,5].
As an abundant species with a great diversity of habitats, it seems that P. monodon plays an important role as the predator and prey of several species, many of which are of commercial interest [5]. In this context, what do we know and what do we need to find out about the red squat lobster Pleuroncodes monodon? This article has focused on reviewing available information regarding P. monodon in the Humboldt Current System (HCS), including biological and ecological aspects, as well as its current and potential use as a marine bioresource.
For the search and collection of information, an exhaustive, structured and systematized review of indexed scientific articles was carried out on the Web of Science (WoS), Scopus and Google Scholar platforms. In addition, technical reports from governmental institutions were sought. The information and data gathered was subsequently analyzed and summarized for its inclusion in the present review.
2. Humboldt Current System
The HCS is one of the most productive areas in the world in terms of the biomass of small pelagic fish that inhabit it [6,7]. The HCS withstands strong pressures from fishing activities, such as the anchoveta Engraulis ringens, which represents 10% of the world catch, and the Chilean jack mackerel Trachurus murphyi off the Chilean coast, which represented 59% of the catches made in the Eastern South Pacific from 1983 to 2019 [8,9].
This System is characterized by the presence of the Humboldt Current, which originates from the Antartic Circumpolar Current off the Chilean coast (45° S) towards the north of Peru and Ecuador (4° S), as part of the South Pacific Gyre induced by the Coriolis effect [10]. It encompasses a wide range of diverse ocean habitats and subterranean currents of mainly cold-water masses that flow towards the equator and then the poles [11].
The HCS is also one of the four Eastern Boundary Upwelling Systems. It presents high spatio-temporal variability in various parameters and has areas of high nutrient concentration. The HCS also has a shallow oxygen minimum zone (OMZ) [6,7], which is the fourth most important worldwide, among the six most widely recognized [7,12].
The combined effect of the surface winds coming from the southeast and the Earth’s rotation generate the upwelling of cold deep waters rich in nutrients, such as nitrates (NO3−), phosphates (PO43−) and silicates (SiO24+), which sustain the first link in the food chain [13], with a productivity of <300 g C/m2/yr [14]. These upwelling areas tend to be more continuous off the Peruvian coast and northern Chile, while in southern Chile they tend to occur seasonally [14]. In particular, the most important nuclei off the coast of Peru are located at 4–5° S, 7.8° S, 11–12° S and 14–15° S [15], characterized by the constant coastal winds towards the equator, which persist throughout the year [16]. In addition, the main pelagic fisheries in Chile, such as the Chilean jack mackerel and anchoveta, are also close to the most important upwelling zones, around 20–22° S, 32–34° S and 36–38° S [14].
However, the HCS is also subject to climate variability at different time scales [13]. In particular, during El Niño Southern Oscillation (ENSO) events, large-scale changes are generated in the atmospheric and oceanic circulation, which lead to an increase in surface temperatures and a deeper oxycline, nutricline, and thermocline [17,18]. As a consequence, the decrease in the contribution of nutrients to the euphotic zone generates a reduction in primary production [16] that can affect the bioenergetic condition of individuals and their fundamental physiological processes, such as growth and reproduction [19].
On the other hand, the high oxygen consumption due to the decomposition of organic matter leads to the development of an oxygen minimum zone (OMZ) [12]. In the water column, oxygen deficiency also benefits biochemical denitrification processes and anaerobic oxidation of ammonia [20]. This OMZ presents a greater depth off the northern coast of Peru, being shallower in the central-south zone [21]. Off the Chilean coast, the OMZ decreases its extension and vertical intensity as it approaches the south, covering a depth range of 100 to 450 m [22]. Likewise, off the Peruvian coast, its depth reaches the continental shelf and the slope, causing the release of Fe (II) from the sediments into the water column, contributing to the productivity of the area [23].
The OMZ also seems to modulate the vertical structure of the pelagic habitat, leading several species of zooplankton and nekton (mainly crustaceans, dominated by P. monodon and followed by euphausiids and copepods) to carry out daily vertical migrations to anoxic depths to feed [24]. In fact, Antezana [25] affirmed the presence of a community structure based on a divided habitat in which species avoid coexisting spatiotemporally in the OMZ by migrating to and from this zone at different times.
Considering that the coast of Peru goes from the parallels 0° to 18° S, and the coast of Chile extends from the parallels 17° to 56° S (Figure 1), it is also important to point out that the configuration of the continental shelf varies throughout these areas [26]. For example, off the Peruvian coast it tends to become narrower towards the south, while in the central zone, particularly in front of the district of Chimbote, department of Ancash (9°04′ S 78°35′ W), it becomes wider, reaching 70 nm and creating a favorable habitat for various pelagic resources [27]. On the other hand, off the coast of northern Chile, the continental shelf is narrow (~10 km) and becomes wider towards the south (~70 km) [28,29]. However, off the coast of Concepción (36–37° S) the Terrace of Itata can be found, which is the widest area of the continental shelf in central Chile, and where the highest values of primary productivity have been reported [26].
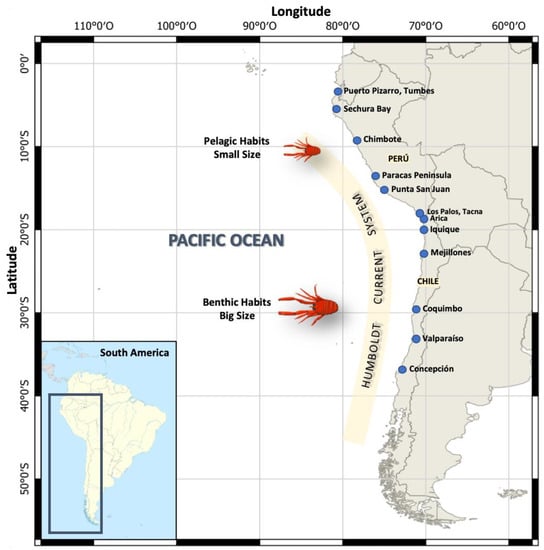
Figure 1.
Geographic distribution of Pleuroncodes monodon off the Chilean and Peruvian coasts along the Humboldt Current System. Adults with two morphotypes of body size and contrasting lifestyle habits, as follows: Peru (pelagic habits and small size) and Chile (benthic habits and big size).
3. Pleuroncodes monodon throughout the HCS
Within the Decapoda order, the Munididae family is comprised of 356 species worldwide [30]. These crustaceans have some very particular morphological characteristics that allow them to live from surface areas to the abyssal bottom, while maintaining their abundance, such as anatomical adaptations of their carapace and chelipeds [31]. Within this family, P. monodon is distributed throughout the HCS off of Peru and Chile. Studies carried out on its distribution off the coasts of northern and central Peru (5–14° S) have classified its habits as coastal-pelagic [32], while in central and southern Chile it has been described as benthodemersal and benthic, according to the stage of its life cycle [33] (Figure 1).
An extensive analysis of its distribution that encompassed the entire Peruvian coastline (4–18° S), indicated that its distribution is directly influenced by the biomass of macrozooplankton, the depth of the oxycline and the distance to the edge of the platform, concluding that the most favorable habitat for P. monodon is represented by cold coastal waters [4]. Between 11° and 14° S off of central Peru, its presence has been reported at depths between 70 and 150 m, swimming in the water column or settled on the seabed, mainly during daylight hours and towards nightfall [34]. This species has also been reported at a depth of ca. 300 m on the continental margin off of Bahía de Concepción, in central Chile, by Gallardo et al. [35].
Considering the above, the real distribution of this species within the water column comes into question. As stated by Sanfuentes [5], P. monodon performs vertical migrations conditioned by the oxygen levels in the environment. In addition, Palash [36] indicated that it is usually located mostly in surface waters at night, while during the day it descends towards the OMZ. This vertical displacement represents a pattern of behavior of species that ascend towards the surface at nightfall to feed and descend at dawn to conserve energy and avoid predation [25,36].
Likewise, from the hatching period to recruitment, P. monodon, as any other planktonic larvae, is subject to changes in marine currents and advection [5,37]. Off Chilean coasts, it has been observed in a pelagic phase during its larval stage, adopting a benthic-demersal habit during its juvenile and adult stages [33]. North of 21° S and off the Peruvian coast, mainly pelagic habits have been reported, in response to the shallower depth of the oxycline, which is contrary to the situation south of 22° S, where benthic habits reaching 50–350 m of depth have been reported [33].
Similarly, P. monodon is usually distributed bathymetrically according to its reproductive phase and the conditions of its habitat [37,38]. In particular, off the Chilean coast, it has been reported inhabiting depths of 200–300 m in the autumn, and later migrating to depths of 70–200 m in the winter [39]. However, its distribution varies according to its life cycle and environmental parameters [40].
For example, from 22° S to 38° S, at a depth of 50 to 199 m, between the continental shelf and slope, an assemblage of species has been reported, including P. monodon, the yellow squat lobster Cervimunida johni and the bigeye flounder [40]. Similarly, Melo et al. [41] reported P. monodon together with the sea snail Aenator fontainei, as species associated with low oxygen contents and higher temperatures, under the influence of shallow Equatorial Subsurface Water (<300 m). The topographic variations of the environment also influence the distribution of P. monodon [26], as observed in the northern Chilean fishing unit between Coquimbo and Valparaíso with a high presence of males and females (ovigerous and non-ovigerous) at a depth of 150–170 m [42]. Considering this, these authors pointed out that the breadth of the continental shelf off of Concepción (36–37° S), together with the oceanographic characteristics of the area, could favor the retention of adult P. monodon individuals as aggregations.
During the first stages of their life cycle, they usually inhabit coastal waters with a greater abundance of phytoplankton, varying their distribution in the water column according to the availability of food, while during their most advanced stages they migrate to the bottom to establish their benthic juvenile phase [43,44]. Thus, benthic populations of P. monodon are usually associated with low temperatures, between 11 °C and 12 °C and low oxygen in the environment, while pelagic populations tend to be distributed above the oxycline with average temperatures between 15 °C and 16 °C [40]. In particular, off of Concepción (36–37° S), it has been shown that larvae tend to present a horizontal migration from the coast towards the continental slope as they develop from zoea to post-larvae [42] with zoea I and zoea II aggregations between 50 and 100 m [26], as was observed during the release of larvae in Coquimbo, when juvenile cohorts were found from 100 to 250 m deep [40].
Regarding the relationship that P. monodon has demonstrated with the oxygen concentrations in the environment, during its zoea stage, it has proven to be an oxyconformer, becoming an oxyregulator at the megalopa stage; however, at the juvenile and adult stages, oxyregulation is highly developed [26].
Off the Peruvian coast (∼3–18° S), P. monodon overlaps its habitat with the dark dolphin Lagenorhynchus obscurus, the long-beaked common dolphin Delphinus capensis, the bottlenose dolphin Tursiops truncatus, the anchoveta Engraulis ringens, the Chilean silverside Odontesthes regia regia, Patagonian squid Doryteuthis gahi, Peruvian sea catfish Galeichthys peruvianus, Chilean jack mackerel Trachurus murphyi, Argonauta spp., Euphausia sp., and Humboldt squid Dosidicus gigas [44,45,46]. However, the magnitude of the habitat overlap is unknown, which limits the analysis and the implications that this entails. What has been reported represents a challenge to be resolved in the future in order to understand the dynamics of interspecific relationships among these species.
4. Prey or Predator?
In this section, the trophic role of P. monodon in the HCS has been explored by reviewing its importance as prey, predator, and its interaction with potential competitors. Within this ecosystem, it is usually the prey of a large number of predators, among which fish, birds and mammals stand out, many of which are of commercial interest (Table 1) (Figure 2 and Figure 3).

Table 1.
List of predators of P. monodon throughout the Humboldt Current System.
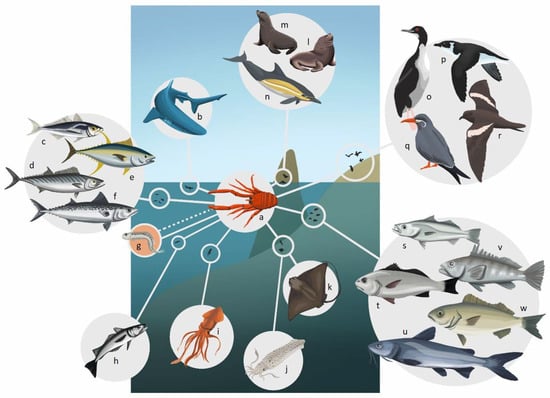
Figure 2.
Predators of P. monodon off the Peruvian coast. (a) P. monodon, (b) Prionace glauca, (c) Trachurus murphyi, (d) Sarda chilensis, (e) Thunus sp., (f) Scomber japonicus, (g) Engraulis ringens, (h) Merluccius gayi peruanus, (i) Dosidicus gigas, (j) Doryteuthis gahi, (k) Myliobatis chilensis, (l) Otaria byronia, (m) Arctocephalus australis, (n) Delphinus capensis, (o) Phalacrocorax bougainvillii, (p) Pelecanoides garnotii, (q) Larosterna inca, (r) Oceanodroma markhami, (s) Cynoscion analis, (t) Sciaena deliciosa, (u) Galeichthys peruvianus, (v) Paralabrax humeralis, (w) Isacia conceptionis. The dotted line on (g) Engraulis ringens represents the possibility of mutual predation of eggs and larvae between this species and P. monodon, as has been reported by Gutiérrez et al. [32].
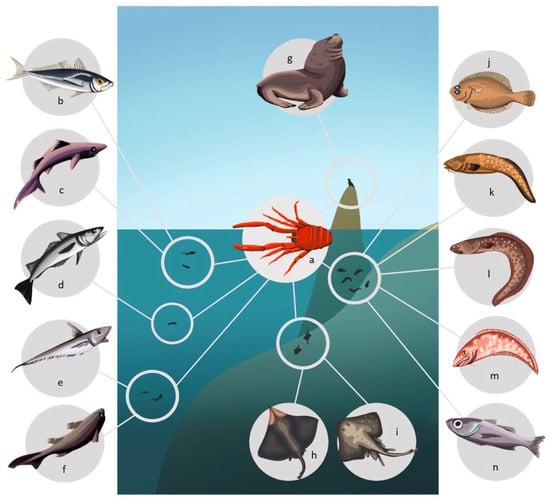
Figure 3.
Predators of P. monodon off the Chilean coast. (a) P. monodon, (b) Trachurus murphyi, (c) Centroscyllium granulatum, (d) Merluccius gayi gayi, (e) Coelorhynchus aconcagua, (f) Aculeola nigra, (g) Otaria byronia, (h) Zearaja chilensis, (i) Bathyraja sp., (j) Hippoglossina macrops, (k) Genypterus maculatus, (l) Genypterus blacodes, (m) Genypterus chilensis, (n) Epigonus crassicaudus.
Among its predators there are coastal, oceanic and benthic species. This coincides with the habits reported for this species on the HCS, where it usually presents benthic habits during most of its life cycle off of the coast of Chile [40], and mostly pelagic habits off of the coasts of Peru and northern Chile [32,76].
The first documented record of P. monodon as prey was made by Del Solar [52], who in a study carried out during 1930–1940 off the Peruvian coast, was able to detect its presence in the stomachs of various scombrids (such as the Eastern Pacific bonito Sarda chiliensis, tuna Thunnus spp. and the skipjack tuna Katsuwomus pelamis), which were in the area of influence of oceanic waters. Scombrids probably migrated to shore to feed or P. monodon migrated to ocean waters, which shows that P. monodon usually ventures into this area to feed, despite being a species that usually prefers coastal areas. As established by Del Solar [52], scombrids associated with oceanic waters can enter the coastal zone for short periods of time to have a greater source of food. Later, Konchina [77] defined this behavior as typical of facultative predators when studying the diet of the common hake Merluccius gayi and the jack mackerel Trachurus symmetricus, species that have different habitat preferences than the cold coastal waters typical of the HCS. However, recent studies have shown that the jack mackerel does not enter coastal waters due to the OMZ, which is expanding and limiting the volume of water for the entry of many other predators [78,79]; therefore, it is assumed that the Scombridae would not enter such an area, as previously established.
On the other hand, according to García-Godos et al. [73], ENSO events, by generating a shortage of anchoveta due to the incursion of warm waters in the coastal zone, favor a greater predation of P. monodon by other teleosts, as has been reported in the Peruvian rock seabass Paralabrax humeralis along the central coast of Peru [53]. Likewise, García-Godos et al. [66] reported that P. monodon was prey for the long-beaked common dolphin Delphinus capensis, along with other neritic and pelagic species, along the central and southern coasts of Peru (Table 1). Similarly, recent studies have shown that P. monodon is one of the most important prey for the Chilean jack mackerel Trachurus murphyi and chub mackerel Scomber japonicus along the entire Peruvian coastline (∼3–18° S) [53,63].
Off central Chile (25–36° S), it has been shown to be an important prey in the diet of the common hake Merluccius gayi [61,62].
P. monodon also constitutes an important prey for marine mammals and birds, as has been demonstrated in the Paracas Peninsula and in the Reserva Guanera Punta San Juan in Peru [71,73,80]. In northern Chile, in Arica (18°34′ S), Iquique (20°48′ S) and Mejillones (23°04′ S) P. monodon has been reported as the most important prey of the South American sea lion Otaria byronia [69] and as the dominant prey south of Iquique during the ENSO events of 1997–1998 and 2009–2010, which positively influenced its abundance [67].
Regarding the factors that influence its predation, a study developed by Kiko et al. [34], reported that P. monodon is vulnerable to predation due to its limited swimming capacity and reddish coloration, which stands out to its predators. Other factors that influence its predation include the presence of light equivalent to 1% that extends through the water column to a depth of 40 m and the oxycline manifested between 10 and 30 m of depth [75]. Therefore, it has been reported that to avoid predation P. monodon migrates towards the ocean bottom between hypoxic and anoxic environments during the day, reaching the continental slope, in further support that P. monodon is not strictly a coastal pelagic species [34].
Another possible strategy against predation is that P. monodon has been observed in video recordings crawling or partially buried in the substrate and inhabiting caves in large groups off the coast of central Chile (25–36° S) [81].
According to a report by Pauly and Christensen [82], P. monodon occupies a trophic position of 2.6, feeding mainly on primary producers, bacteria and debris, and classified as an opportunistic omnivore according to Lovrich and Thiel [83]. However, in a recent study its trophic position was identified as 3.6 and 3.8 according to its location off the southern and northern coasts of Peru, respectively [84]. Likewise, within their pelagic habitats, they have been identified as large filter feeders of large phytoplankton cells and small zooplankton [34], making this species a very important intermediate link between pelagic primary productivity and the lower layers of the water column [83]. Even though its benthic habitats are unknown, in Sechura Bay (5.6° S) it was observed feeding on debris through sediment resuspension [85]. Off the central zone of Chile, it was reported that it can prey on foraminifera, amphipods, zoeas, crustacean eggs, diatoms, organic matter and fish scales [86].
Likewise, it has been postulated that P. monodon can predate on the first stages of commercial species, among which the anchoveta is included, with which it shares a similar spatio-temporal distribution [4,87] (Figure 2). Along the Peruvian coast, both of these species most likely compete for food, including the possibility of mutual predation of eggs and larvae, which could potentially be negative [32,88]. However, compared to Peru, along the Chilean coast the anchoveta has a more extensive feeding area and is not as coastal as P. monodon [83].
Even though the predators of P. monodon include species that inhabit the water column in shallow and deep areas (Figure 2 and Figure 3), the magnitude of their predation and the impact they may have on its abundance is still unknown. Thus, taking into account that P. monodon represents an important link between lower and higher trophic levels in the trophic web, the potential effects that its increase or decrease in abundance could have on its predators or potential competitors must be considered.
In this regard, it has been postulated that P. monodon can predate on the first stages of commercial species, among which the anchoveta is included, with which it shares a similar spatio-temporal distribution [4,87]. Along the Peruvian coast, both of these species most likely compete for food, including the possibility of mutual predation of eggs and larvae (Figure 2), which could potentially be negative [32,88]. However, compared to Peru, along the Chilean coast the anchoveta has a more extensive feeding area and is not as coastal as P. monodon [84].
Currently, isotopic niche studies are currently underway (P. Espinoza; personal communication, July 2023); however, the available information on the trophic niche of P. monodon in the HCS are still scarce. Espinoza et al. [84] recommended analyzing the dietary habits of P. monodon in order to understand how the role of its pelagic or benthic phases varies, as well as that of other species with which it is involved. A recent study off the coast of Coquimbo and Concepción in Chile showed that juveniles are eminently filter feeders; this evidence was supported using trophic biomarkers from phytoplankton, small copepods and detritus [29]. However, further studies analyzing the dietary habits of P. monodon are still essential in order to understand current trophic relationships and thus implement fishery management plans with an ecosystem approach [89].
5. Reproduction and Bioenergetics
P. monodon is characterized by having a prolonged reproductive cycle with multiple annual offspring [90]. Off the coast of Concepción (Chile), it has been estimated that the reproductive potential of this species varies from 1808 to 33,966 eggs per laying [39,91], where individuals reach the adult stage after five larval stages [33].
Regarding the size of females that initially reach sexual maturity, Flores et al. [46] found LC50 values of ∼21.0 mm in the north and south of Chile, according to the functional criteria (histology), while according to physiological criteria, the LC50 values were 24.1 mm and 25.4 mm in the north and south, respectively. On the other hand, the same authors demonstrated a synchronous gonadal development among females of the population; however, the total number of offspring produced during the reproductive season is yet unknown.
Regarding the laying of eggs, a study carried out by Guzmán-Rivas et al. [92] in Chile proposed that this species could present an r-strategy (Please, refer to Jeschke et al. [93] and Reynolds [94] on r/k strategies and the concept of fast and slow life histories) during the summer and a k-strategy during the winter. Therefore, the authors pointed out that both the temperature and the availability of food in the environment influence their reproductive period. In Chile, the presence of ovigerous females was reported from February to December, with a greater abundance between May and October [39]. Similarly, between ∼36° S and ∼30° S peaks of ovigerous females in the benthic phase have been observed in the winter, coinciding with periods of high oxygen levels in the water column [40,95]. This characteristic coincides with the behavior of P. monodon populations in Costa Rica, where the reproductive period begins when the water temperature decreases and there is an upwelling event [91]. According to Pörtner and Gutt [96], this adaptation to the oceanographic conditions of the environment during the reproductive period allows P. monodon to maximize its fertility levels. However, a study carried out by Yannicelli and Castro [33] with ovigerous females collected on the south-central Chilean continental shelf showed that in the summer this species could experience better temperature conditions and food availability for hatching eggs, significantly improving development, growth and survival time. A study carried out along the Peruvian coast, from the coastal zone to approximately 100 nautical miles offshore, indicated that the highest percentages of females in a state of advanced maturity (stages 2 and 3) and with developed embryos (stage 4) was found during spring, indicating that the period of egg release or larval hatching takes place from this time of the year.
Considering the above, as a reproductive strategy, the larvae resort to the high capacity they have to tolerate certain periods of hypoxia, which allows them to disperse towards areas with more favorable oceanographic parameters until they reach the juvenile stage [33]. In Peru, the presence of P. monodon has been associated with intense upwelling events from Puerto Pizarro, Tumbes (03° S), to Los Palos, Tacna (18° S), favoring their reproductive period [97], as well as the availability of food by providing a constant source of nutrients that sustain primary productivity [98].
Tam et al. [99] indicated that the presence of a significant variation in the environmental conditions of the habitat could severely affect the reproductive period of P. monodon. For example, during the ENSO event of 1997–1998, there was a population decrease and a displacement towards the south, with its abundance later increased as a result of the expansion of the cold coastal waters [4]. On the other hand, events such as La Niña, are beneficial for the population of P. monodon because this species takes advantage of the cooling of the water masses to reproduce [4].
Likewise, oceanographic events indirectly affect the interannual and seasonal variability of the biochemical composition of P. monodon eggs. This has been evidenced by eggs presenting a higher concentration of lipids and a lower concentration of proteins during winter and vice versa during summer, resulting in a much higher energy content in the winter season [92]. This bioenergetic variability could also be influenced by the size and production of eggs, which can vary along a latitudinal gradient [100]. Similarly, it was reported that, in the winter, female bodies showed a higher percentage of lipids [101], saturated and polyunsaturated fatty acids [102], and their embryos presented increased concentrations of saturated and essential fatty acids (C18:2n6cis, C18:3n6 and C22:6n3) [101]. This is beneficial during hatching because it increases the probability of larvae survival in an environment with adverse variables [103]. These bioenergetic compounds, in the form of lipids and fats, favorably influence the nutrition, reproduction and growth of individuals [29,104].
It has been shown that the larvae that hatch from larger eggs in the winter are greater in size, have a higher dry weight and higher concentration of lipids and essential fatty acids [105]. This seems to be a reproductive adaptation strategy to ensure a high-energy reserve during seasons of lower temperature and less food availability [105]. Therefore, understanding the variations in the reproductive characteristics of this species and taking into account the fact that females must be managed in a particular way, it is important to establish better fishing models to guarantee the sustainable exploitation of P. monodon [100,106].
Regarding the mating behavior of this species, Espinoza-Fuenzalida et al. [95] showed that the mating behavior of P. monodon, which does not entail the need to molt, is beneficial, reducing energy costs during its reproductive period and the risk of being predated by its congeners or other species [107]. It is also advantageous for this species to produce more offspring in a shorter period of time [95]. Thus, identifying the reproductive biology of this species is essential to generate an efficient population management.
6. Genetic Aspects
The main morphological characteristics of P. monodon larvae were described by Fagetti and Campodonico [108] in order to distinguish them from their congener, Pleuroncodes planipes. However, Bianchi [109] stated that both species corresponded to P. monodon. Considering this, molecular analyzes (COI and 16S) were carried out, which confirmed that they belong to the same species [91].
Regarding its development, similar to other resident species, P. monodon consists of a population with a high gene flow throughout the HCS [110]. Kilada and Acuña [111] found differences in the growth parameters and sexual maturity of individuals collected off the northern and southern coasts of Chile. However, the authors attributed these spatial differences to the phenotypic response to habitat conditions during its benthic phase, with the oxycline representing the main oceanographic factor that could be exerting the most influence. To corroborate this, mitochondrial DNA studies indicated that the haplotypes of individuals with a pelagic life cycle and individuals with a benthic life cycle do not present genetic differentiation, sharing a common demographic history and a recent population expansion [110].
7. Fisheries
In the ‘90s this species became abundant off the coast of Peru, reaching values of up to 3.4 million tons [32]. Changes in ecosystem dynamics, such as the decrease of its predators may have generated the population increase of P. monodon, especially considering that the catches of sardines, one of its predators, decreased notably during that decade [112]. In Peru, P. monodon is not yet commercially exploited; however, the Ministry of Production (PRODUCE) has been promoting the extractive activity of this species since 2013 [4,113]. Even so, Instituto del Mar del Perú (IMARPE) [54] reported that from 1998 to 2007 this species was landed as bycatch for anchovy and other pelagic resources. Consequently, it is currently possible to investigate various parameters of its life cycle, taking into account that the population has not yet been exploited as a fishing resource as in [91].
Off the Chilean coast, P. monodon is a target species of trawling (23–39° S), comprising the Northern Fishery Unit (NFU) from the XV to the IV Region [3,7] and the Southern Fishery Unit (SFU) from the V to the VIII Region [29,114,115] as a fully exploited resource and under a recovering fishery regime, due to the high levels of overfishing that occurred between 1992 and 2000 [116,117]. The state of the resource is analyzed annually, and a potentially sustainable quota is established in relation to the stock assessments that have been carried out since 1993 [118]. Likewise, it has a closure per year (from January to March) and is subject to the measure of the maximum catch limit per shipowner [119], which has been established as a precautionary measure to prevent overfishing, and it has been effective in curbing overfishing. Between January and March, the fishery does not operate because the individuals are growing and molting. The percentage of landings of this species as a companion fauna of other target species is also regulated [120]. P. monodon, together with other demersal crustaceans, such as the Chilean nylon shrimp and yellow squat lobster, economically support artisanal and industrial fishing in Chile through the export of frozen and processed products [119]. However, it should be noted that P. monodon fishery activities lead to possible incidental catches of other species of economic importance, such as the Chilean common hake, the armed box crab, and the Patagonian grenadier Macruronus magellanicus [118].
However, in central Chile, it has been shown that when some predators, such as the Chilean common hake, Chilean jack mackerel and small pelagic fish are caught, a top-down effect is generated that is beneficial for P. monodon [121]. Considering this, the authors suggest considering trophic interactions when evaluating stocks and managing fisheries, bearing in mind that predation has a direct effect on the population dynamics of other commercially important resources.
Hernáez and Wehrtmann [91] found differences in egg production between exploited and unexploited populations of P. monodon; various intrinsic and extrinsic factors most likely cause this variability. Considering that the Chilean population is in the exploitation phase, while the Peruvian population is not, comparative studies could be carried out in order to clarify whether there are significant differences in the egg production of this species throughout the HCS.
8. Implications as a Marine Bioresource
P. monodon has recently been called “future interesting food”, classifying it as a potentially edible species even better than other commercial species due to its concentrations of EPA, DHA, ARA and micronutrients such as Cu, Fe, Mn and Zn [122]. However, in the same mentioned study, 1.15 ± 0.59 µg/g wet weight of cadmium in the edible tissue was also recorded, which far exceeds the maximum permissible limit established at 0.5 µg/g wet weight for crustacean muscle, according to the Peruvian National Fisheries Health Agency [123]. Thus, the potential risk to human health should not be ignored, as well as the mechanisms of bioaccumulation and biomagnification, which should be resolved as soon as possible considering their carcinogenic nature and liver damage and kidney disease potential [124]. On the other hand, in Chile, where it is an exploited commercial resource, P. monodon is used as fresh-frozen tail and is exported mainly to the United States, Germany and Japan [125].
This species is not only important from the fishing point of view it can also be highly used as a raw material. From an industrial point of view, there are efforts aimed at extracting pigments (such as carotenoids and astaxanthin) and enzymes (such as digestive proteases) for the production and ripening of cheeses [126,127]. In fact, studies carried out in Peru have already proven that P. monodon offers a high potential for obtaining astaxanthin and fatty acids (Omega 3), it is thus recommended to be included in rainbow trout diets [128]. Consequently, it is an important species for food production; it can be included as a partial or total substitute for fishmeal, having proven to have greater digestibility and greater efficiency of feed conversion and growth in prawn farming [127,129].
Another usable compound of P. monodon in Chile is chitosan, a biopolymer derived from chitin obtained from its exoskeleton [130,131]. This compound has been shown to be effective as an antibacterial agent against problematic pathogens for aquaculture such as Vibrio alginolyticus, Vibrio parahaemolyticus and Lactococcus garviae, due to its cationic properties, causing a membrane alteration effect [132,133,134].
The application of chitosan has also been found to remove heavy metals such as chromium, copper, mercury and lead from water [135,136]. Similarly, it has been used in wastewater treatment, generating clarification by destabilizing the oil/water emulsion [137]. The success of chitosan derives from its physicochemical characteristics, which give it chemical stability, high reactivity and excellent chelating behavior [135]. Currently, there is no industrial chain in Peru, linked to the fishing sector for the extraction of chitosan, only there some laboratory studies related to the extraction of bioactive lipids from P. monodon [138,139], despite the fact that there are studies in other parts of the world that show its relevance as a marine bioresource [140].
In the biomedical industry, chitosan derived from P. monodon shows excellent results in the recovery of skin tissues damaged by burns, ulcers and injuries, due to its anti-hemorrhagic and antimicrobial properties [136]. It can also be used as a biopharmaceutical product for immunological, antitumor and hemostatic treatments, among others [141].
9. Conclusions and Future Perspectives
P. monodon presents various ecological clues (i.e., a high physiological flexibility: “oxygen and thermal tolerance”) and biochemical attributes (i.e., rich in essential fatty acids, bioactive compounds, chitosan, and carotenoids pigments) that allow could be an excellent candidate for the biotechnological industry. Particularly, this species has a high commercial value as a marine bioresource with great potential in the pharmaceutical and food industries.
In turn, P. monodon plays a key role within the HCS. It is highly tolerant to variations in the oceanographic parameters of its habitat, presenting various adaptation strategies that allow it to continue developing and reproducing. From an ecological point of view, this species seems to be channeling energy from the lower links of the trophic chain and fulfilling an important role as a common prey for organisms of various taxa.
Comparative studies between the Chilean and Peruvian populations of P. monodon could provide interesting insights about this species, taking into account that only one of them is under pressure from industrial fishery activities. Even though Chile has carried out a vast number of studies, there are still information gaps that must be resolved, including the displacement patterns and connectivity according to the life stage of the individuals, the total number of offspring produced during the reproductive period, and that subsequently recruit to adult populations.
Likewise, taking into account that in Peru there is a growing interest in establishing the extractive activity of P. monodon for its use as a marine bioresource, it is imminent that studies be carried out to clarify aspects of its life cycle and the inter- species relationships with other commercially important organisms. In this way, efficient population management could be ensured from an ecosystem approach.
Author Contributions
Conceptualization, A.L.Y.-P., Á.U. and P.E.; methodology, A.L.Y.-P.; formal analysis, A.L.Y.-P., M.Q.-M., F.G.-R., Á.U. and P.E.; investigation, A.L.Y.-P., Á.U. and P.E.; resources, Á.U.; data curation, A.L.Y.-P.; writing—original draft preparation, A.L.Y.-P., Á.U. and P.E.; writing—review and editing, A.L.Y.-P., M.Q.-M., F.G.-R., Á.U. and P.E.; visualization, A.L.Y.-P.; supervision, Á.U. and P.E.; project administration, Á.U. and P.E.; funding acquisition, Á.U. and P.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agencia Nacional de Investigación y Desarrollo de Chile-ANID, grant number BMBF 180034. The APC was funded by FA DI-UCSC and Universidad Científica del Sur.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Special thanks to Christine Harrower for correcting the English and improving this manuscript. We would also like to thank Leonardo Sánchez Laguna for the illustrations, and the two anonymous reviewers for their valuable suggestions to this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Milne-Edwards, H. Histoire Naturelle des Crustace’s, Comprenant l’Anatomie, la Physiologie et la Classification de ces Animaux; Encyclopedique de Roret: Paris, France, 1837; pp. 1–532. [Google Scholar]
- Hendrickx, M.E.; Harvey, A.W. Checklist of anomuran crabs (Crustacea: Decapoda) from the Eastern Tropical Pacific. Belg. J. Zool. 1999, 129, 363–389. [Google Scholar]
- Queirolo, D.; Erzini, K.; Hurtado, C.F.; Gaete, E.; Soriguer, M.C. Species composition and bycatches of a new crustacean trawl in Chile. Fish. Res. 2011, 110, 149–159. [Google Scholar] [CrossRef]
- Santivañez, M.; Herbozo, G.; Gutiérrez, M.; Bertrand, A. The spatio-temporal relationship of red squat lobster (Pleuroncodes monodon) with the superficial water masses of the Peruvian Sea between 1998 and 2016. In Proceedings of the IEEE/OES Acoustics in Underwater Geosciences Symposium (RIO Acoustics), Rio de Janeiro, Brasil, 25–27 July 2017. [Google Scholar] [CrossRef]
- Sanfuentes, F. Recopilación de Antecedentes Biológico-Pesqueros del Langostino Enano (Pleuroncodes sp.) en Chile y Perú; CIAM (Centro de Investigación Aplicada del Mar) Publisher: Iquique, Chile, 2017; Volume 1, pp. 1–22. Available online: http://www.ciamchile.cl/wp-content/uploads/2015/03/Informe-Antecedentes-Langostino-Enano.pdf (accessed on 5 May 2023).
- Chavez, F.; Bertrand, A.; Guevara-Carrasco, R.; Soler, P.; Csirke, J. The northern Humboldt Current System: Brief history, present status and a view towards the future. Prog. Oceanogr. 2008, 79, 95–105. [Google Scholar] [CrossRef]
- Montecino, V.; Lange, C.B. The Humboldt Current System: Ecosystem components and processes, fisheries, and sediment studies. Prog. Oceanogr. 2009, 83, 65–79. [Google Scholar] [CrossRef]
- Espino, M.; Yamashiro, C. La variabilidad climática y las pesquerías en el Pacifico suroriental. Lat. Am. J. Aquat. Res. 2012, 40, 705–721. [Google Scholar] [CrossRef]
- Yañez, E.; Aranis, A.; Caballero, L.; Silva, C. Capturas totales permisible de jurel (Trachurus murphyi) en el Pacífico Suroriental. Rev. Versión Difer. 2020, 32, 68–69. [Google Scholar]
- Gutiérrez, D.; Akester, M.; Naranjo, L. Productivity and Sustainable Management of the Humboldt Current Large Marine Ecosystem under climate change. Environ. Dev. 2016, 17, 126–144. [Google Scholar] [CrossRef]
- Tomczak, M.; Godfrey, J.S. Regional Oceanography: An. Introduction, 2nd ed.; Daya Publishing House: Delhi, India, 2003; pp. 1–390. [Google Scholar]
- Fuenzalida, R.; Schneider, W.; Garcés-Vargas, J.; Bravo, L.; Lange, C. Vertical and horizontal extension of the oxygen minimum zone in the eastern South Pacific Ocean. Deep Sea Res. Part. II Top. Stud. Oceanogr. 2009, 56, 992–1003. [Google Scholar] [CrossRef]
- Echevin, V.; Goubanova, K.; Belmadani, A.; Dewitte, B. Sensitivity of the Humboldt Current system to global warming: A downscaling experiment of the IPSL-CM4 model. Clim. Dyn. 2011, 38, 761–774. [Google Scholar] [CrossRef]
- Kämpf, J.; Chapman, P. The Peruvian-Chilean Coastal Upwelling System. In Upwelling Systems of the World, 1st ed.; Kämpf, J., Chapman, P., Eds.; Springer: Cham, Switzerland, 2016; pp. 161–201. [Google Scholar] [CrossRef]
- Morón, O. Características del ambiente marino frente a la costa peruana. Bol. Inst. Mar. Peru 2000, 19, 179–204. [Google Scholar]
- Briceño-Zuluaga, F.J.; Sifeddine, A.; Caquineau, S.; Cardich, J.; Salvatteci, R.; Gutierrez, D.; Ortlieb, L.; Velazco, F.; Boucher, H.; Machado, C. Terrigenous material supply to the Peruvian central continental shelf (Pisco, 14° S) during the last 1000 years: Paleoclimatic implications. Clim. Past. 2016, 12, 787–798. [Google Scholar] [CrossRef]
- Karstensen, J.; Ulloa, O. Peru-Chile Current System. In Encyclopedia of Ocean. Sciences, 2nd ed.; Steele, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 385–392. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Quipúzcoa, L.; Enríquez, E. Oxygen deficiency and benthic communities in the Peruvian upper continental margin. Gayana Oceanol. 2006, 70, 29–36. [Google Scholar] [CrossRef]
- Meza, E. Impacto de las Variaciones Ambientales Climatológicas en las Larvas de Anchoveta Engraulis ringens(Jenyns, 1842) y Sardina Sardinops sagax (Jenyns, 1842) Usando un Modelo de Balance Energético Dinámico. Master’s Thesis, Universidad Peruana Cayetano Heredia, Lima, Peru, 2016. [Google Scholar]
- Farías, L.; Besoain, V.; García-Loyola, S. Presence of nitrous oxide hotspots in the coastal upwelling area off central Chile: An analysis of temporal variability based on ten years of a biogeochemical time series. Environ. Res. Lett. 2015, 10, 044017. [Google Scholar] [CrossRef]
- Pizarro, O. Dynamics of seasonal and interannual variability of the Peru-Chile Undercurrent. Geophys. Res. Lett. 2002, 29, 22-1–22-4. [Google Scholar] [CrossRef]
- Quiroga Jamett, E.J. La Influencia de la Zona de Mínimo Oxígeno Sobre el Macrobentos Sublitoral en el Sistema de Corrientes Humboldt. Doctoral Dissertation, Universidad de Concepción, Concepción, Chile, 2005. [Google Scholar]
- Scholz, F.; Hensen, C.; Noffke, A.; Rohde, A.; Liebetrau, V.; Wallmann, K. Early diagenesis of redox-sensitive trace metals in the Peru upwelling area—Response to ENSO-related oxygen fluctuations in the water column. Geochim. Cosmochim. Acta 2011, 75, 7257–7276. [Google Scholar] [CrossRef]
- Kiko, R.; Hauss, H. On the Estimation of Zooplankton-Mediated Active Fluxes in Oxygen Minimum Zone Regions. Front. Mar. Sci. 2019, 6, 741. [Google Scholar] [CrossRef]
- Antezana, T. Species-specific patterns of diel migration into the Oxygen Minimum Zone by euphausiids in the Humboldt Current Ecosystem. Prog. Oceanogr. 2009, 83, 228–236. [Google Scholar] [CrossRef]
- Yannicelli, B.; Castro, L.; Parada, C.; Schneider, W.; Colas, F.; Donoso, D. Distribution of Pleuroncodes monodon larvae over the continental shelf of south-central Chile: Field and modeling evidence for partial local retention and transport. Prog. Oceanogr. 2012, 92, 206–227. [Google Scholar] [CrossRef]
- Morales, M.; Cerpa, L.; Cornejo, T.; Girón, I.; Chacaltana, C.; Valdivia, W. Geología de la plataforma continental del Perú: Paralelos 03°30′ y 14°00′ latitud sur. Boletín Ingemmet Ser. D Estud. Reg. 2020, 32, 118. [Google Scholar]
- Thiel, M.; Macaya, E.C.; Acuña, E.; Arntz, W.E.; Bastias, H. The Humboldt Current System of Northern and Central Chile. Oceanogr. Mar. Biol. 2007, 45, 195–345. [Google Scholar] [CrossRef]
- Guzmán-Rivas, F.; Quispe-Machaca, M.; Queirolo, D.; Ahumada, M.; Urzúa, Á. Latitudinal changes in the lipid content and fatty acid profiles of juvenile female red squat lobsters (Pleuroncodes monodon) in breeding areas of the Humboldt Current System. PLoS ONE 2021, 16, e0253314. [Google Scholar] [CrossRef]
- Baba, K.; Macpherson, E.; Poore, G.C.B.; Ahyong, S.T.; Bermudez, A.; Cabezas, P.; Lin, C.W.; Nizinski, M.; Rodrigues, C.; Schnabel, K.E. Catalogue of squat lobsters of the world (Crustacea: Decapoda: Anomura—Families Chirostylidae, Galatheidae and Kiwaidae). Zootaxa 2008, 1905, 1–220. [Google Scholar] [CrossRef]
- Baba, K. Deep-sea chirostylid and galatheid crustaceans (Decapoda: Anomura) from the Indo-West Pacific, with a list of species. Galathea Rep. 2005, 20, 1–317. [Google Scholar]
- Gutiérrez, M.; Ramirez, A.; Bertrand, S.; Morón, O.; Bertrand, A. Ecological niches and areas of overlap of the squat lobster ‘munida’ (Pleuroncodes monodon) and anchoveta (Engraulis ringens) off Peru. Prog. Oceanogr. 2008, 79, 256–263. [Google Scholar] [CrossRef]
- Yannicelli, B.; Castro, L. Ecophysiological constraints on the larvae of Pleuroncodes monodon and the implications for its reproductive strategy in poorly oxygenated waters of the Chile-Peru undercurrent. J. Plankton Res. 2013, 35, 566–581. [Google Scholar] [CrossRef]
- Kiko, R.; Hauss, H.; Dengler, M.; Sommer, S.; Melzner, F. The squat lobster Pleuroncodes monodon tolerates anoxic “dead zone” conditions off Peru. Mar. Biol. 2015, 162, 1913–1921. [Google Scholar] [CrossRef]
- Gallardo, V.A.; Palma, M.; Carrasco, F.D.; Gutiérrez, D.; Levin, L.A.; Cañete, J.I. Macrobenthic zonation caused by the oxygen minimum zone on the shelf and slope off central Chile. Deep Sea Res. Part. II Top. Stud. Oceanogr. 2004, 51, 2475–2490. [Google Scholar] [CrossRef]
- Palash, S.A. Fine-Scale Vertical Distribution of Zooplankton in the Oxygen Minimum Zone off Peru. Master’s Thesis, GEOMAR Centro Helmholtz de Investigación Oceánica de Kiel, Kiel, Germany, 2019. [Google Scholar]
- Rivera, J.; Santander, E. Variabilidad estacional de la distribución y abundancia de larvas de langostino colorado en la zona norte de Chile (Decapoda, Anomura, Galatheidae). Investig. Mar. 2005, 33, 3–23. [Google Scholar] [CrossRef]
- Parada, C.; Yannicelli, B.; Hormazábal, S.; Vásquez, S.; Porobic, J.; Ernst, B.; Gatica, C.; Arteaga, M.; Montecinos, A.; Nuñez, S.; et al. Variabilidad ambiental y recursos pesqueros en el Pacífico suroriental: Estado de la investigación y desafíos para el manejo pesquero. Lat. Am. J. Aquat. Res. 2013, 41, 1–28. [Google Scholar] [CrossRef]
- Palma, G.S.; Arana, E.P. Aspectos reproductivos del langostino colorado (Pleuroncodes monodon H. Milne Edwards, 1837), frente a la costa de Concepción, Chile. Investig. Mar. 1997, 25, 203–221. [Google Scholar] [CrossRef]
- Gallardo, M.A.; González López, A.E.; Ramos, M.; Mujica, A.; Muñoz, P.; Sellanes, J.; Yannicelli, B. Reproductive patterns in demersal crustaceans from the upper boundary of the OMZ off north-central Chile. Cont. Shelf. Res. 2017, 141, 26–37. [Google Scholar] [CrossRef]
- Melo, T.; Silva, N.; Muñoz, P.; Díaz-Naaveas, J.; Sellanes, J.; Bravo, A.; Lamilla, J.; Sepúlveda, J.; Vögler, R.; Guerrero, Y.; et al. Caracterización del Fondo Marino Entre la III y X Regiones (Informe Final FIP-IT/2005); Pontificia Universidad Católica de Valparaíso: Valparaíso, Chile, 2007. [Google Scholar]
- Quispe-Machaca, M.; Guzmán-Rivas, F.; Urzúa, A. Estado de condición nutricional en morfotipos contrastantes del langostino colorado Pleuroncodes monodon en ambientes contrastantes a lo largo del Ecosistema de Humboldt. In Proceedings of the XLI Congreso de Ciencias del Mar. Las Ciencias del Mar en Tiempos de Cambio, Concepción, Chile, 23–27 May 2022. [Google Scholar]
- Palma, G.S. Distribución y abundancia de larvas de langostino colorado Pleuroncodes monodon frente a la costa de Concepción, Chile. Investig. Mar. 1994, 22, 13–29. [Google Scholar] [CrossRef]
- Gallardo, V.; Cañete, I.; Enriquez, S.; Roa, R.; Acuñay, A.; Baltazar, M. Biología del langostino colorado Pleuroncodes monodon H. Milne Edwards, 1837 y especies afines (Crustacea, Decapoda, Anomura, Galatheidae): Sinopsis. In Elementos Básicos para la Gestión de los Recursos vivos Marinos Costeros de la Región del Biobío, 1st ed.; Faranda, F., Parra, O., Eds.; Programa EULA: Monografías Científicas; Universidad de Concepción: Concepción, Chile, 1993; Volume 2, pp. 67–113. [Google Scholar]
- Manrique, M.; Mayaute, L. Hábitos Alimentarios de las Rayas Pseudobatos planiceps (Guzmán, 1880), Hypanus dipterurus (Jordan and Gilbert, 1880) y Myliobatis chilensis (Philippi, 1892) en Pisco. Bachelor’s Thesis, Universidad Nacional San Luis Gonzaga, Ica, Peru, 2016. [Google Scholar]
- Flores, A.; Brown, D.I.; Queirolo, D.; Ahumada, M. Gonadal development of female red squat lobsters (Pleuroncodes monodon H Milne Edwards, 1837). Fish. Res. 2020, 225, 105508. [Google Scholar] [CrossRef]
- Córdova, F.A. Análisis de la Dieta del Tiburón azul Prionace glauca (Linnaeus, 1758) en la Zona Norte del Perú Durante el 2015. Bachelor’s Thesis, Universidad Nacional Agraria La Molina, Lima, Peru, 2018. [Google Scholar]
- Arancibia, H.; Meléndez, C.R. Alimentación de peces concurrentes en la pesquería de Pleuroncodes monodonMilne Edwards. Investig. Pesq. 1987, 34, 113–128. [Google Scholar]
- Caramantín, H.; López, E. Análisis del régimen alimentario de la cachema Cynoscion analis (Yenyns). Biotempo. 2000, 4, 35–40. [Google Scholar] [CrossRef]
- Espinoza, P.; Bertrand, A. Revisiting Peruvian anchovy (Engraulis ringens) trophodynamics provides a new vision of the Humboldt Current system. Prog. Oceanogr. 2008, 79, 215–227. [Google Scholar] [CrossRef]
- Elliot, W.; Paredes, F. Estructura especiológica del subsistema costero, Prospección 9512–9601. Inf. Inst. Mar. Peru 1996, 121, 14–26. [Google Scholar]
- Del Solar, E. Ensayo sobre la ecología de la anchoveta. Bol. Comp. Adm. del Guano 1942, 18, 1–23. [Google Scholar]
- Alegre, A.; Bertrand, A.; Espino, M.; Espinoza, P.; Dioses, T.; Ñiquen, M.; Navarro, I.; Simier, M.; Ménard, F. Diet diversity of jack and chub mackerels and ecosystem changes in the northern Humboldt Current system: A long-term study. Prog. Oceanogr. 2015, 137, 299–313. [Google Scholar] [CrossRef]
- Instituto del Mar del Perú. Anuario Científico Tecnológico; IMARPE: Lima, Peru, 2007; Volume 7, p. 128. [Google Scholar]
- Roque, A.E. Hábitat Trófico y Relaciones Alimenticias de Peces Costeros en el Norte del Ecosistema de la Corriente de Humboldt. Master’s Thesis, Universidad Peruana Cayetano Heredia, Lima, Peru, 2017. [Google Scholar]
- Castañeda, J.; Carbajal, W.; Galán, J.; Gutiérrez, M. Bioecología del bagre Galeichthys peruvianus en el mar del Perú. Inf. Inst. Mar. Peru 2007, 34, 295–307. [Google Scholar]
- Villarroel, J.C.; Acuña, E. Alimentación y relaciones predador-presa en el lenguado de ojos grandes Hippoglossina macrops Steindachner, 1876 Pisces Paralichthyidae de la zona norte de Chile. Rev. Biol. Mar. Oceanogr. 1999, 342, 145–154. [Google Scholar]
- Acuña, E.; Andrade, M.; Cubillos, L.; Arancibia, H.; Moraga, J.; Mujica, A.; Berríos, M.; Lancelotti, D.; Villarroel, J.C.; Haye, P.; et al. Determinación de Zonas y épocas de Reclutamiento de Camarón Nailon, Langostino Amarillo y Langostino Colorado en las Regiones III y IV.; Universidad Católica del Norte: Antofagasta, Chile, 2007. [Google Scholar]
- Bahamonde, N.; Zavala, P. Contenidos gástricos de Genypterus maculatus (Tschudi) y Genypterus blacodes(Schneider) capturados en Chile entre 31° y 37° S (Teleostomi, Ophidiidae). Bol. Mus. Nac. Hist. Nat. 1981, 38, 53–59. [Google Scholar]
- Chong, J.; Sepúlveda, K.; Ibáñez, C.M. Variación temporal en la dieta del congrio colorado, Genypterus chilensis (Guichenot, 1881) frente al litoral de Talcahuano, Chile (36°32′ S–36°45′ S). Rev. Biol. Mar. Oceanogr. 2006, 41, 195–202. [Google Scholar] [CrossRef]
- Cubillos, L.A.; Alarcón, C.; Arancibia, H. Selectividad por tamaño de las presas en merluza común (Merluccius gayi gayi), zona centro-sur de Chile (1992–1997). Investig. Mar. 2007, 35, 55–69. [Google Scholar] [CrossRef]
- Acuña, E.; Alarcón, R.; Arancibia, H.; Cortés, A.; Cid, L.; Cubillos, L. Evaluación Directa de Langostino Colorado y Langostino Amarillo Entre la II y VIII Regiones, año 2012; Universidad Católica del Norte: Antofagasta, Chile, 2012. [Google Scholar]
- Alegre, A.; Espinoza, P.; Espino, M. Ecología trófica del jurel Trachurus murphyi en el Perú entre 1977–2011. Rev. Peru Biol. 2013, 20, 75–82. [Google Scholar] [CrossRef]
- Alegre, A.; Ménard, F.; Tafur, R.; Espinoza, P.; Arguelles, J.; Maehara, V.; Flores, O.; Simier, M.; Bertrand, A. Comprehensive model of jumbo squid Dosidicus gigas trophic ecology in the northern Humboldt Current System. PLoS ONE 2014, 9, e85919. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, R. Ecología trófica de Octopus mimus Gould, 1852; Doryteuthis gahi (d’Orbigny, 1835) y Dosidicus gigas (d’Orbigny, 1835) (Cephalopoda) durante 2016. Bol. Inst. Mar. Peru 2019, 34, 165–197. [Google Scholar]
- García-Godos, I.; Van Waerebeek, K.; Reyes, J.C.; Alfaro-Shigueto, J.; Arias-Schreiber, M. Prey occurrence in the stomach contents of four small cetacean species in Peru. Lat. Am. J. Aquat. Mamm. 2007, 6, 171–183. [Google Scholar] [CrossRef]
- Zavalaga, C.B.; Paredes, R.; Arias-Schreiber, M. Dieta del lobo fino (Arctocephalus australis) y del lobo chusco (Otaria byronia) en la costa sur del Perú en febrero de 1998. Inf. Prog. Inst. Mar. Peru 1998, 79, 3–16. [Google Scholar]
- Paredes, R.; Arias-Schreiber, M. Dieta del Lobo Fino (Arctocephalus australis) y Lobo Chusco (Otaria byronia) en la Costa Peruana Durante Mayo y Junio de 1999; Subdirección de Investigaciones en Mamíferos Marinos; Instituto del Mar del Perú: Callao, Perú, 1999. [Google Scholar]
- Sielfeld, W.; Barraza, J.; Amado, N. Patrones locales de alimentación del león marino sudamericano Otaria byronia: El caso de Punta Patache, Norte de Chile. Rev. Biol. Mar. Oceanogr. 2018, 53, 307–319. [Google Scholar] [CrossRef]
- Sarmiento-Devia, R.; Sepúlveda, M.; Pavez, G.; Valdés, J.; Canto, A.; Orellana, M.; Oliva, D. Diet composition of an opportunistic predator from an upwelling area in the Southeastern Pacific. Austral. Ecol. 2020, 45, 1145–1155. [Google Scholar] [CrossRef]
- Ayala, F.; Cardeña, M.; Cárdenas-Alaya, S. Registro preliminar de microplásticos en fecas del león marino sudamericano (Otaria byronia [de Blainville 1820]) recolectadas en Punta San Juan, Perú. Rev. Int. Contam. Ambient. 2021, 37, 273–279. [Google Scholar] [CrossRef]
- Jahncke, J.; García-Godos, A.; Goya Sueyoshi, E. Dieta del guanay Leucocarbo bougainvilli, del piquero peruano Sula variegata y otras aves de la costa peruana. Abril y mayo de 1997. Inf. Inst. Mar. Peru 1997, 126, 75–86. [Google Scholar]
- García-Godos, I.; Goya, E.; Jahncke, J. The diet of Markham’s Storm Petrel Oceanodroma markhami on the central coast off Perú. Mar. Ornithol. 2002, 30, 77–83. [Google Scholar]
- Quispe-Machaca, M.A. Estudio del Espectro Alimenticio del Guanay Phalacrocorax bougainvillii (lesson, 1937) y su Variabilidad Durante las Etapas Pre-Reproductiva y Post-Reproductiva 2013–2014 en Punta San Juan Marcona. Bachelor’s Thesis, Universidad Nacional de San Agustín de Arequipa, Arequipa, Peru, 2015. [Google Scholar]
- García-Godos, I.; Goya, E. Diet of the Peruvian Diving Petrel Pelecanoides garnotii at La Vieja Island, Peru, 1997-2000: Potential fishery interactions and conservation implications. Mar. Ornithol. 2006, 34, 33–41. [Google Scholar]
- Bertrand, A.; Gerlotto, F.; Bertrand, S.; Gutiérrez, M.; Alza, L.; Chipollini, A.; Díaz, E.; Espinoza, P.; Ledesma, J.; Quesquén, R.; et al. Schooling behavior and environmental forcing in relation to anchoveta distribution: An analysis across multiple spatial scales. Prog. Oceanogr. 2008, 79, 264–277. [Google Scholar] [CrossRef]
- Konchina, Y.V. The feeding niche of the hake Merluccius gayi (Merluccidae), and the jack mackerel, Trachurus symmetricus (Carangidae) in the trophic system of the Peruvian coastal upwelling. J. Ichthyol. 1983, 23, 87–98. [Google Scholar]
- Bertrand, A.; Barbieri, M.A.; Gerlotto, F.; Leiva, F.; Córdova, J. Determinism and plasticity of fish schooling behaviour as exemplified by the South Pacific jack mackerel Trachurus murphyi. Mar. Ecol. Prog. Ser. 2006, 311, 145–156. [Google Scholar] [CrossRef]
- Bertrand, A.; Habasque, J.; Hattab, T.; Hintzen, N.T.; Oliveros-Ramos, R.; Gutiérrez, M.; Demarc, H.; Gerlotto, F. 3-D habitat suitability of jack mackerel Trachurus murphyi in the Southeastern Pacific, a comprehensive study. Prog. Oceanogr. 2016, 146, 199–211. [Google Scholar] [CrossRef]
- Arias-Schreiber, M. Informe sobre el estado de conocimiento y conservación de los mamíferos marinos en el Perú. Inf. Prog. Inst. Mar. Perú 1996, 38, 3–30. [Google Scholar]
- Ahumada, M.; Queirolo, D.; Acuña, E.; Gaete, E. Caracterización de agregaciones de langostino colorado (Pleuroncodes monodon) y langostino amarillo (Cervimunida johni) mediante un sistema de filmación remolcado. Lat. Am. J. Aquat. Res. 2013, 41, 199–208. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V. Primary production required to sustain global fisheries. Nature 1995, 374, 255–257. [Google Scholar] [CrossRef]
- Lovrich, G.; Thiel, M. Ecology, physiology, feeding and trophic role of squat lobsters. In The Biology of Squat Lobsters, 1st ed.; Poore, G.C.B., Ahyong, S.T., Taylor, J., Eds.; CSIRO Publishing: Clayton, Australia, 2011; pp. 183–221. [Google Scholar]
- Espinoza, P.; Lorrain, A.; Ménard, F.; Cherel, Y.; Tremblay-Boyer, L.; Argüelles, J.; Tafur, R.; Bertrand, S.; Tremblay, Y.; Ayón, P.; et al. Trophic structure in the northern Humboldt Current system: New perspectives from stable isotope analysis. Mar. Biol. 2017, 164, 1–15. [Google Scholar] [CrossRef]
- Kluger, L.; Taylor, M.H.; Mendo, J.; Tam, J.; Wolff, M. Carrying capacity simulations as a tool for ecosystem-based management of a scallop aquaculture system. Ecol. Model. 2015, 331, 44–55. [Google Scholar] [CrossRef]
- Gallardo, V.A.; Bustos, E.; Acuña, A.; Díaz, L.; Erbs, V.; Meléndez, R.; Oviedo, L. Relaciones Ecológicas de las Comunidades Bentónicas y Bentodemersales de la Plataforma Continental de Chile Central; Universidad de Concepción: Concepción, Chile, 1980. [Google Scholar]
- Espinoza, P. Trophic Dynamics in the Northern Humboldt Current System: Insights from Stable Isotopes and Stomach Content Analyses. Doctoral Dissertation, Université de Bretagne Occidentale, Brest, France, 2014. [Google Scholar]
- Escobedo, R. Índice de Abundancia para Munida (Pleuroncodes monodon) a Partir de Datos de su Captura Incidental en la Pesca Industrial de la Anchoveta Peruana (Engraulis ringens) Desde 1997 a 2014. Master’s Thesis, Universidad Peruana Cayetano Heredia, Lima, Peru, 2018. [Google Scholar]
- Cowan, J.H.; Rice, J.C.; Walters, C.J.; Hilborn, R.; Essington, T.E.; Day, J.W.; Boswell, K.M. Challenges for Implementing an Ecosystem Approach to Fisheries Management. Mar. Coast. Fish. 2012, 4, 496–510. [Google Scholar] [CrossRef]
- Thiel, M.; Espinoza-Fuenzalida, N.; Acuna, E.; Rivadeneira, M. Annual brood number and breeding periodicity of squat lobsters (Decapoda: Anomura: Galatheidae) from the continental shelf of the SE Pacific—Implications for fisheries management. Fish. Res. 2012, 130, 28–37. [Google Scholar] [CrossRef]
- Hernáez, P.; Wehrtmann, I. Sexual maturity and egg production in an unexploited population of the red squat lobster Pleuroncodes monodon (Decapoda, Galatheidae) from Central America. Fish. Res. 2011, 107, 276–282. [Google Scholar] [CrossRef]
- Guzmán-Rivas, F.; Bascur, M.; Olavarria, L.; Mora, S.; Riera, R.; Urzúa, Á. Seasonal and interannual changes in reproductive parameters and eggs biochemical composition of the fishery resource Pleuroncodes monodon(Decapoda: Munididae) from the Humboldt Current System. Fish. Res. 2020, 221, 105404. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Kokko, H. The roles of body size and phylogeny in fast and slow life histories. Evol. Ecol. 2009, 23, 867–878. [Google Scholar] [CrossRef]
- Reynolds, J.D. Life histories and extinction risk. In Macroecology: Concepts and Consequences; Blackburn, T.M., Gaston, K.J., Eds.; Blackwell: Oxford, UK, 2003; pp. 195–217. [Google Scholar]
- Espinoza-Fuenzalida, N.L.; Acuña, E.; Hinojosa, I.A.; Thiel, M. Reproductive biology of two species of squat lobsters—Female receptivity and interbrood intervals. J. Crust. Biol. 2012, 32, 565–574. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Gutt, J. Impacts of Climate Variability and Change on (Marine) Animals: Physiological Underpinnings and Evolutionary Consequences. Integr. Comp. Biol. 2016, 56, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Franco-Meléndez, M. Comportamiento reproductivo y variación de la proporción sexual de Pleuroncodes monodon (Crustacea: Galatheidae) en la costa peruana. Cienc. Mar. 2012, 38, 441–457. [Google Scholar] [CrossRef]
- Mann, K.H.; Lazier, J.R.N. Dynamics of Marine Ecosystems: Biological-Physical Interactions in the Oceans; Blackwell Scientific Publications: Boston, MA, USA, 1991. [Google Scholar]
- Tam, J.; Taylor, M.H.; Blaskovic, V.; Espinoza, P.; Ballón, R.M.; Díaz, E.; Wosnitza-Mendo, C.; Argüelles, J.; Purca, S.; Ayón, P.; et al. Trophic modeling of the Northern Humboldt Ecosystem, Part I: Comparing trophic linkages under La Niña and El Niño conditions. Prog. Oceanogr. 2008, 79, 352–365. [Google Scholar] [CrossRef]
- Guzmán-Rivas, F.; Olavarría, L.; Urzúa, A. Seasonal variation in reproductive parameters of the squat lobster Pleuroncodes monodon from a South Pacific population. Invertebr. Reprod. Dev. 2016, 60, 137–144. [Google Scholar] [CrossRef]
- Bascur, M.; Guzmán-Rivas, F.; Mora, S.; Urzúa, Á. Seasonal changes in the biochemical composition of females and offspring of red squat lobster, Pleuroncodes monodon (Decapoda, Munididae), from the Southeastern Pacific. Mar. Ecol. 2017, 38, e12419. [Google Scholar] [CrossRef]
- Bascur, M.; Guzmán, F.; Mora, S.; Espinoza, P.; Urzúa, Á. Temporal variation in the fatty acid composition of ovigerous females and embryos of the squat lobster Pleuroncodes monodon (Decapoda, Munididae). J. Mar. Biol. Assoc. United Kingd. 2018, 98, 1977–1990. [Google Scholar] [CrossRef]
- Espinoza, C.; Guzmán-Rivas, F.; Bascur, M.; Urzúa, Á. Effect of starvation on the nutritional condition of early zoea larvae of the red squat lobster Pleuroncodes monodon (Decapoda, Munididae). Invertebr. Reprod. Dev. 2016, 60, 152–160. [Google Scholar] [CrossRef]
- Guzmán-Rivas, F.; Quispe, M.; Urzúa, Á. Contrasting nursery habitats promote variations in the bioenergetic condition of juvenile female red squat lobsters (Pleuroncodes monodon) of the Southern Pacific Ocean. PeerJ. 2022, 10, e13393. [Google Scholar] [CrossRef]
- Seguel, V.; Guzmán-Rivas, F.; Bascur, M.; Riera, R.; Urzúa, Á. Temporal variation in larval biochemical condition at hatching of the red squat lobster Pleuroncodes monodon (Decapoda: Munididae) from Humboldt Current System. Invertebr. Reprod. Dev. 2019, 63, 282–293. [Google Scholar] [CrossRef]
- Guzmán-Rivas, F.; Quispe-Machaca, M.; Olavarría, L.; Zilleruelo, M.; Urzúa, Á. Inter-sexual comparison of body biomass, proximate biochemical composition, and fatty acid profiles of new juvenile squat lobsters (Pleuroncodes monodon) in the Southeast Pacific Ocean. Mar. Ecol. 2022, 43, e12690. [Google Scholar] [CrossRef]
- Karas, P.; Gorny, M.; Alarcon, R. Experimental studies on the feeding ecology of Munida subrugosa (White, 1847) (Decapoda: Anomura: Galatheidae) from the Magellan region, Southern Chile. Sci. Mar. 2007, 71, 187–190. [Google Scholar] [CrossRef]
- Fagetti, E.; Campodonico, I. Larval development of the red crab Pleuroncodes monodon (Decapoda Anomura: Galatheidae) under laboratory conditions. Mar. Biol. 1971, 8, 70–71. [Google Scholar] [CrossRef]
- Bianchi, G. Demersal assemblages of the continental shelf and slope edge between the Gulf of Mexico and the Gulf of Papagayo (Costa Rica). Mar. Ecol. Prog. Ser. 1991, 73, 121–140. [Google Scholar] [CrossRef]
- Haye, P.A.; Salinas, P.; Acuña, E.; Poulin, E. Heterochronic phenotypic plasticity with lack of genetic differentiation in the southeastern Pacific squat lobster Pleuroncodes monodon. Evol. Dev. 2010, 12, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Kilada, R.; Acuña, E. Direct age determination by growth band counts of three commercially important crustacean species in Chile. Fish. Res. 2015, 170, 134–143. [Google Scholar] [CrossRef]
- Cárdenas-Quintana, G.; Franco-Melendez, M.; Salcedo-Rodríguez, J.; Ulloa-Espejo, D.; Pellón-Farfán, J. La sardina peruana, Sardinops sagax: Análisis histórico de la pesquería (1978-2005). Cienc. Mar. 2015, 41, 203–216. [Google Scholar] [CrossRef]
- Ministerio de la Producción (PRODUCE). Comisión Técnica de Trabajo Encargada de Realizar el Análisis y la Recopilación de Información Científica y Tecnológica Acerca del Recurso Camaroncito rojo. Resolución Ministerial N° 249-2009-PRODUCE Ministerio de Economy Publisher; Lima, Perú. Available online: https://www2.produce.gob.pe/dispositivos/publicaciones/2009/octubre/rm459-2009-produce.pdf (accessed on 2 March 2023).
- Subsecretaría de Pesca y Acuicultura (SUBPESCA). Control. Cuota de Captura Langostino Colorado (Pleuroncodes monodon) Regiones II y IV y Langostino Colorado Regiones V a VIII, Año 2018; Ministerio de Economía, Fomento y Turismo: Valparaíso, Chile, 2018. [Google Scholar]
- Instituto de Fomento Pesquero (IFOP). Programa de Seguimiento de las Principales Pesquerías Pelágicas de la Zona Centro sur de Chile, V-XI Regiones, año 2018; Boletín de Difusión: Valparaíso, Chile, 2018. [Google Scholar]
- Arana, P. Recursos Pesqueros del mar de Chile; Ediciones Universitarias de Valparaíso: Valparaíso, Chile, 2012. [Google Scholar]
- Subsecretaría de Pesca y Acuicultura (SUBPESCA). Decreto Exento N°241. Ministerio de Economía, Fomento y Turismo; Ministerio de Economía, Fomento y Turismo: Valparaíso, Chile, 2019. [Google Scholar]
- Montero, J.T.; Flores, A.; Queirolo, D.; Farias, A.; Wiff, R.; Lima, M.; Rivera-Rebella, C.; Ahumada, M. Potential effects of bycatch from the squat lobster fisheries in central Chile on the benthic ecosystem: A survey data approach. Mar. Freshw. Res. 2020, 71, 1281–1293. [Google Scholar] [CrossRef]
- Subsecretaría de Pesca y Acuicultura (SUBPESCA). Estado de Situación de las Principales Pesquerías Chilenas (State of Affairs of the Main Chilean fisheries); Ministerio de Economía, Fomento y Turismo: Valparaíso, Chile, 2015. [Google Scholar]
- Subsecretaría de Pesca y Acuicultura (SUBPESCA). Decreto Exento N°170. Ministerio de Economía, Fomento y Turismo; SUBPESCA Ministerio de Economía Publisher: Valparaíso, Chile, 2019. [Google Scholar]
- Neira, S.; Arancibia, H. Trophic interactions and community structure in the upwelling system off Central Chile (33–39° S). J. Exp. Mar. Biol. Ecol. 2004, 2, 349–366. [Google Scholar] [CrossRef]
- Loaiza, I.; De Troch, M.; De Boeck, G. Marine species as safe source of LC-PUFA and micronutrients: Insights in new promising marine food in Peru. Food Chem. 2020, 321, 126724. [Google Scholar] [CrossRef]
- Organismo Nacional de Sanidad Pesquera (SANIPES). Indicadores Sanitarios y de Inocuidad para los Productos Pesqueros y Acuícolas para Mercado Nacional y de Exportación; Ministerio de la Producción: Lima, Perú, 2016. [Google Scholar]
- Castro-González, M.I.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Andrés, A.; Gonzalez, E.; Notte-Cuello, E.; Chacana, M.; Lemus-Mondaca, R. Mathematical modelling on the drying process of yellow squat lobster (Cervimunida jhoni) fishery waste for animal feed. Anim. Feed Sci. Technol. 2009, 151, 268–279. [Google Scholar] [CrossRef]
- Aurioles-Gamboa, D.; Balart, E.F.; Castro-Aguirre, J.L. La langostilla: Biología, ecología y aprovechamiento. In Recomendaciones para la Explotación y Aprovechamiento de la Langostilla, 1st ed.; Aurioles-Gamboa, D., Balart, E.F., Eds.; Centro de Investigaciones Biológicas del Noroeste, S.C.: La Paz, Mexico, 1995; pp. 221–223. [Google Scholar]
- Balart, E.F. Recurso Langostilla. In Estudio del Potencial Pesquero y Acuícola de Baja California Sur, 1st ed.; Casas-Valdez, M., Ponce-Díaz, G., Eds.; Centro de Investigaciones Biológicas del Noroeste, S.C.: La Paz, Mexico, 1996. [Google Scholar]
- Morante, F. Obtención de Astaxantina de la Munida Pleuroncodes monodon (Crustacea, Decapoda, Anomura), Empleando el CO2 Supercrítico para su Aplicación en Acuicultura. Bachelor’s Thesis, Universidad Nacional Federico Villareal, Lima, Peru, 2021. [Google Scholar]
- Goytortúa-Bores, E.; Civera-Cerecedo, R.; Rocha-Meza, S.; Green-Yee, A. Partial replacement of red crab (Pleuroncodes planipes) meal for fish meal in practical diets for the white shrimp Litopenaeus vannamei. Effects on growth and in vivo digestibility. Aquaculture 2006, 256, 414–422. [Google Scholar] [CrossRef]
- Cárdenas, G.; Anaya, P.; von Plessing, C.; Rojas, C.; Sepúlveda, J. Chitosan composite films. Biomedical applications. J. Mater. Sci. Mater. Med. 2008, 19, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Taboada, E.; Cabrera, G.; Cárdenas, G. Retention capacity of chitosan for copper and mercury ions. J. Chil. Chem. Soc. 2003, 48, 7–12. [Google Scholar] [CrossRef]
- Chaiyakosa, S.; Charernjiratragul, W.; Umsakul, K.; Vuddhakul, V. Comparing the efficiency of chitosan with chlorine for reducing Vibrio parahaemolyticus in shrimp. Food Control. 2007, 18, 1031–1035. [Google Scholar] [CrossRef]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Dima, J.B.; Sequeiros, C.; Zaritzky, N.E. Hexavalent chromium removal in contaminated water using reticulated chitosan micro/nanoparticles from seafood processing wastes. Chemosphere 2015, 141, 100–111. [Google Scholar] [CrossRef]
- Calderón, N.E. Obtención y Caracterización de Nanopartículas de Quitosano por el Método de Gelación Iónica. Bachelor’s Thesis, Universidad Nacional de San Agustín de Arequipa, Arequipa, Peru, 2015. [Google Scholar]
- Ahmad, A.L.; Sumathi, S.; Hameed, B.H. Coagulation of residue oil and suspended solid in palm oil mill effluent by chitosan, alum and PAC. Chem. Eng. J. 2006, 118, 99–105. [Google Scholar] [CrossRef]
- Montufar, P.; Churacutipa Mamani, M.; Barriga-Sánchez, M. Use of oily extract of Pleuroncodes monodon as pigment in the feed of the rainbow trout (Oncorhynchus mykiss). Rev. Inv. Vet. Perú. 2021, 32, e20376. [Google Scholar] [CrossRef]
- Barriga-Sánchez, M.; Sanchez-Gonzales, G.; Varas Condori, M.A.; Sanjinez Alvites, M.N.; Galdos de Valenzuela, M.E. Extraction of bioactive lipids from Pleuroncodes monodon using organic solvents and supercritical CO2. Grasas Aceites. 2023, 74, e492. [Google Scholar] [CrossRef]
- Diez, M.J. Squat Lobster Fisheries. In Fisheries and Aquaculture; Lovrich, G., Thiel, M., Eds.; Oxford Academic: New York, NY, USA, 2021; Volume 9, pp. 117–136. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).