The Relationship between Signs of Medical Conditions and Cognitive Decline in Senior Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Questionnaire
- (1)
- Demographic information about the dog—breed, weight, height, age, body condition score (BCS), and how recently it had been examined by a veterinarian;
- (2)
- Assessment of the dog’s cognitive health—CCAS scale [25] comprising 17 items which assessed six domains of behaviour (disorientation, sleep–wake cycles, social interactions, learning and memory, activity level, and anxiety). The scale utilises a four-point Likert scale; never (0), once a month (1), once a week (2), almost every day (3), reporting behaviour over the previous six months. If a participant was unsure how to respond, they were asked to select ‘Never’ as opposed to leaving it blank as directed by the original CCAS;
- (3)
- An assessment of the subject’s general health—37 questions regarding behaviours that reflect the pathology of different body systems, hereafter referred to as ‘general health questions (GHQs),’ and 12 questions regarding diagnoses made by a veterinarian in the preceding year, hereafter referred to as ‘diagnoses questions’ (see Supplementary Information). Questionnaires published in the literature concerning the assessment of health in older dogs [34,35,36] were used as a basis for the GHQs. Items were phrased in colloquial English to ensure that they were not ambiguous. The GHQs utilised a four-point Likert scale describing the degree of change. The diagnoses questions had binary yes/no response options.
2.3. Data Analysis
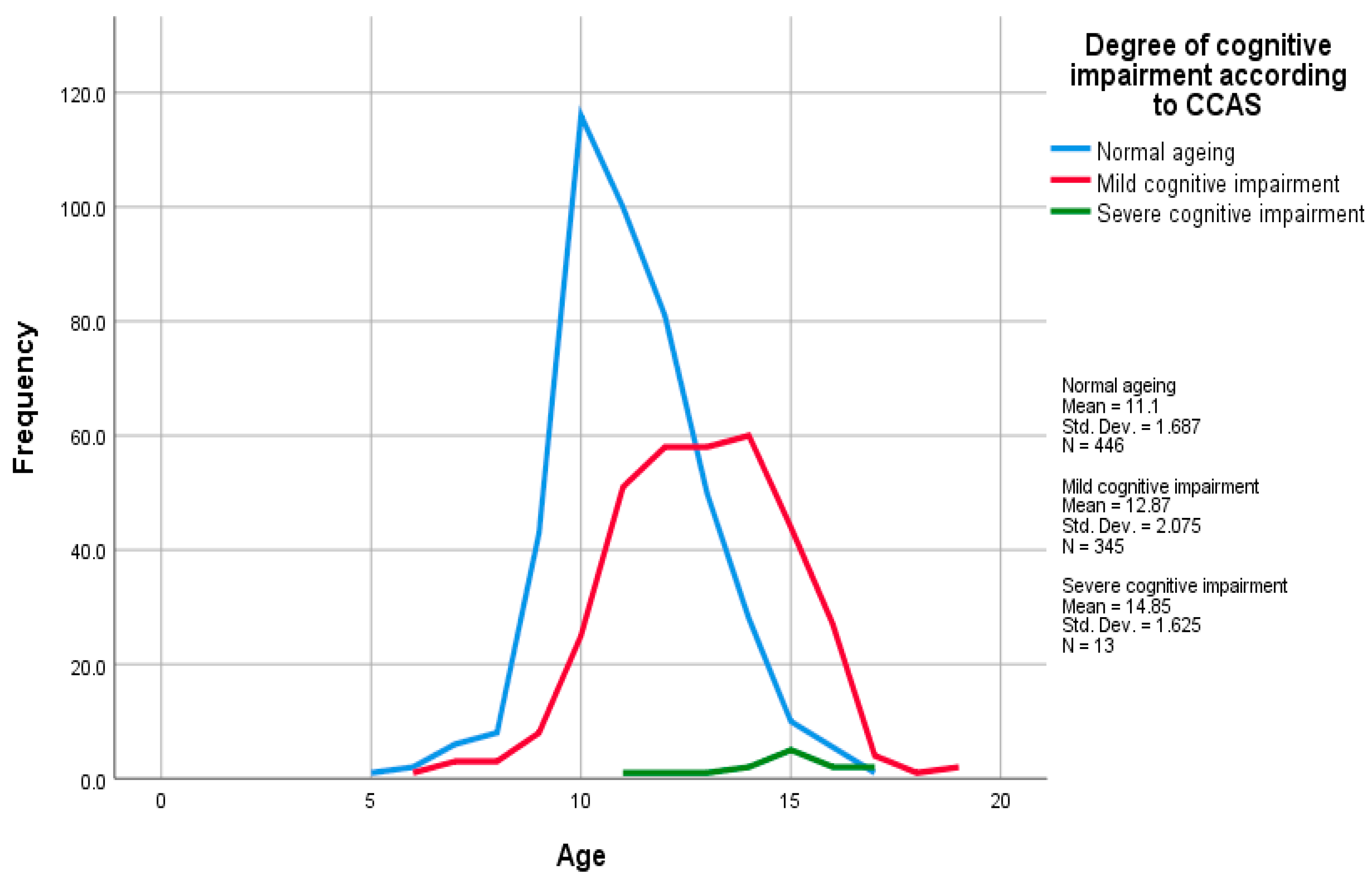
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewey, C.W.; Davies, E.S.; Xie, H.; Wakshlag, J.J. Canine Cognitive Dysfunction: Pathophysiology, Diagnosis, and Treatment. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 477. [Google Scholar] [CrossRef] [PubMed]
- Salvin, H.E.; McGreevy, P.D.; Sachdev, P.S.; Valenzuela, M.J. The canine cognitive dysfunction rating scale (CCDR): A data-driven and ecologically relevant assessment tool. Vet. J. 2011, 188, 331–336. [Google Scholar] [CrossRef]
- Azkona, G.; García-Belenguer, S.; Chacón, G.; Rosado, B.; León, M.; Palacio, J. Prevalence and risk factors of behavioural changes associated with age-related cognitive impairment in geriatric dogs. J. Small Anim. Pract. 2009, 50, 87–91. [Google Scholar] [CrossRef]
- Osella, M.C.; Re, G.; Odore, R.; Girardi, C.; Badino, P.; Barbero, R.; Bergamasco, L. Canine cognitive dysfunction syndrome: Prevalence, clinical signs and treatment with a neuroprotective nutraceutical. Appl. Anim. Behav. Sci. 2007, 105, 297–310. [Google Scholar] [CrossRef]
- Neilson, J.C.; Hart, B.L.; Cliff, K.D.; Ruehl, W.W. Prevalence of behavioral changes associated with age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc. 2001, 218, 1787. [Google Scholar] [CrossRef] [PubMed]
- Schutt, T.; Toft, N.; Berendt, M. Cognitive Function, Progression of Age-related Behavioral Changes, Biomarkers, and Survival in Dogs More than 8 Years Old. J. Vet. Intern. Med. 2015, 29, 1569–1577. [Google Scholar] [CrossRef]
- Madari, A.; Farbakova, J.; Katina, S.; Smolek, T.; Novak, P.; Weissova, T.; Novak, M.; Zilka, N. Assessment of severity and progression of canine cognitive dysfunction syndrome using the CAnine DEmentia Scale (CADES). Appl. Anim. Behav. Sci. 2015, 171, 138. [Google Scholar] [CrossRef]
- Salvin, H.E.; McGreevy, P.D.; Sachdev, P.S.; Valenzuela, M.J. Under diagnosis of canine cognitive dysfunction: A cross-sectional survey of older companion dogs. Vet. J. 2010, 184, 277–281. [Google Scholar] [CrossRef]
- Landsberg, G.; Araujo, J.A. Behavior problems in geriatric pets. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 675–698. [Google Scholar] [CrossRef]
- Spitznagel, M.B.; Jacobson, D.M.; Cox, M.D.; Carlson, M.D. Predicting caregiver burden in general veterinary clients: Contribution of companion animal clinical signs and problem behaviors. Vet. J. 2018, 236, 23–30. [Google Scholar] [CrossRef]
- Christiansen, S.B.; Kristensen, A.T.; Sandøe, P.; Lassen, J. Looking after chronically ill dogs: Impacts on the caregiver’s life. Anthrozoös 2013, 26, 519–533. [Google Scholar] [CrossRef]
- Spitznagel, M.B.; Cox, M.D.; Jacobson, D.M.; Albers, A.L.; Carlson, M.D. Assessment of caregiver burden and associations with psychosocial function, veterinary service use, and factors related to treatment plan adherence among owners of dogs and cats. J. Am. Vet. Med. Assoc. 2019, 254, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Pegram, C.; Gray, C.; Packer, R.M.A.; Richards, Y.; Church, D.B.; Brodbelt, D.C.; O’Neill, D.G. Proportion and risk factors for death by euthanasia in dogs in the UK. Sci. Rep. 2021, 11, 9145. [Google Scholar] [CrossRef]
- Su, M.-Y.; Tapp, P.D.; Vu, L.; Chen, Y.-F.; Chu, Y.; Muggenburg, B.; Chiou, J.-Y.; Chen, C.; Wang, J.; Bracco, C.; et al. A longitudinal study of brain morphometrics using serial magnetic resonance imaging analysis in a canine model of aging. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Scarpante, E.; Cherubini, G.B.; Stefani, A.; Taeymans, O. Magnetic resonance imaging features of leukoaraiosis in elderly dogs. Vet. Radiol. Ultrasound 2017, 58, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, D.; Yayoshi, N.; Fujita, Y.; Fujita, M.; Orima, H. Measurement of interthalamic adhesion thickness as a criteria for brain atrophy in dogs with and without cognitive dysfunction (dementia). Vet. Radiol. Ultrasound 2005, 46, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Gavin, P.R. Growth of clinical veterinary magnetic resonance imaging. Vet. Radiol. Ultrasound 2011, 52, S2–S4. [Google Scholar] [CrossRef]
- Cummings, B.J.; Head, E.; Afagh, A.J.; Milgram, N.W.; Cotman, C.W. β-Amyloid Accumulation Correlates with Cognitive Dysfunction in the Aged Canine. Neurobiol. Learn. Mem. 1996, 66, 11–23. [Google Scholar] [CrossRef]
- Colle, M.A.; Hauw, J.J.; Crespeau, F.; Uchihara, T.; Akiyama, H.; Checler, F.; Pageat, P.; Duykaerts, C. Vascular and parenchymal Aβ deposition in the aging dog: Correlation with behavior. Neurobiol. Aging 2000, 21, 695–704. [Google Scholar] [CrossRef]
- Piotti, P.; Albertini, M.; Pirrone, F. Peripheral Concentration of Amyloid-β, TAU Protein, and Neurofilament Light Chain as Markers of Cognitive Dysfunction Syndrome in Senior Dogs: A Meta-analysis. Adv. Small Anim. Care 2022, 3, 23–38. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Hunthausen, W.; Ackerman, L. The effects of aging on behavior in senior pets. In Handbook of Behavior Problems of the Dog and Cat, 2nd ed.; Landsberg, G.M., Hunthausen, W., Ackerman, L., Eds.; Saunders: Philidelphia, PA, USA, 2003; pp. 269–280. [Google Scholar]
- Landsberg, G.M.; Nichol, J.; Araujo, J.A. Cognitive Dysfunction Syndrome:A Disease of Canine and Feline Brain Aging: A Disease of Canine and Feline Brain Aging. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 749–768. [Google Scholar] [CrossRef] [PubMed]
- Szabó, D.; Miklósi, Á.; Kubinyi, E. Owner reported sensory impairments affect behavioural signs associated with cognitive decline in dogs. Behav. Process. 2018, 157, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Wallis, L.J.; Szabo, D.; Erdelyi-Belle, B.; Kubinyi, E. Demographic Change Across the Lifespan of Pet Dogs and Their Impact on Health Status. Front. Vet. Sci. 2018, 5, 200. [Google Scholar] [CrossRef] [PubMed]
- Le Brech, S.; Amat, M.; Temple, D.; Manteca, X. Evaluation of Two Practical Tools to Assess Cognitive Impairment in Aged Dogs. Animal 2022, 12, 3538. [Google Scholar] [CrossRef] [PubMed]
- Bognár, Z.; Piotti, P.; Szabó, D.; Le Nézet, L.; Kubinyi, E. A novel behavioural approach to assess responsiveness to auditory and visual stimuli before cognitive testing in family dogs. Appl. Anim. Behav. Sci. 2020, 228, 105016. [Google Scholar] [CrossRef]
- Piotti, P.; Szabó, D.; Bognár, Z.; Egerer, A.; Hulsbosch, P.; Carson, R.; Kubinyi, E. Effect of age on discrimination learning, reversal learning, and cognitive bias in family dogs. Learn. Behav. 2018, 46, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Piotti, P.; Piseddu, A.; Aguzzoli, E.; Sommese, A.; Kubinyi, E. Two valid and reliable tests for monitoring age-related memory performance and neophobia differences in dogs. Sci. Rep. 2022, 12, 16175. [Google Scholar] [CrossRef] [PubMed]
- Kubinyi, E.; Iotchev, I.B. A Preliminary Study toward a Rapid Assessment of Age-Related Behavioral Differences in Family Dogs. Animals 2020, 10, 1222. [Google Scholar] [CrossRef]
- Barnett, K.P.; Mercer, S.W.P.; Norbury, M.M.; Watt, G.P.; Wyke, S.P.; Guthrie, B.P. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Bartges, J.; Boynton, B.; Vogt, A.H.; Krauter, E.; Lambrecht, K.; Svec, R.; Thompson, S. AAHA Canine Life Stage Guidelines. J. Am. Anim. Hosp. Assoc. 2012, 48, 1–11. [Google Scholar] [CrossRef]
- Galis, F.; Van Der Sluijs, I.; Van Dooren, T.J.M.; Metz, J.A.J.; Nussbaumer, M. Do large dogs die young? J. Exp. Zool. Part B Mol. Dev. Evol. 2007, 308, 119–126. [Google Scholar] [CrossRef]
- Greer, K.A.; Canterberry, S.C.; Murphy, K.E. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res. Vet. Sci. 2007, 82, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Bellows, J.; Colitz, C.M.H.; Daristotle, L.; Ingram, D.K.; Lepine, A.; Marks, S.L.; Sanderson, S.L.; Tomlinson, J.; Zhang, J. Defining healthy aging in older dogs and differentiating healthy aging from disease. J. Am. Vet. Med. Assoc. 2015, 246, 77. [Google Scholar] [CrossRef] [PubMed]
- Banzato, T.; Franzo, G.; Di Maggio, R.; Nicoletto, E.; Burti, S.; Cesari, M.; Canevelli, M. A Frailty Index based on clinical data to quantify mortality risk in dogs. Sci. Rep. 2019, 9, 16749. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Kuehn, N.; Landsberg, G.; Lascelles, B.; Marks, S.; Schaedler, J.; Tuzio, H. AAHA senior care guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2005, 41, 81–91. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Neyman, J.; Pearson, E.S. On the use and interpretation of certain test criteria for purposes of statistical inference part i. Biometrika 1928, 20, 175–240. [Google Scholar] [CrossRef]
- Freeman, L.; Becvarova, I.; Cave, N.; MacKay, C.; Nguyen, P.; Rama, B.; Takashima, G.; Tiffin, R.; van Beukelen, P.; Yathiraj, S. WSAVA Nutritional Assessment Guidelines. J. Feline Med. Surg. 2011, 13, 516–525. [Google Scholar] [CrossRef]
- Pett, M.A.; Lackey, N.R.; Sullivan, J.J. Making Sense of Factor Analysis: The Use of Factor Analysis for Instrument Development in Health Care Research; SAGE Publications Inc.: Los Angeles, CA, USA, 2003. [Google Scholar]
- Hutcheson, G.; Sofroniou, N. The Multivariate Social Scientist: Introductory Statistics Using Generalized Linear Models; Sage Publications: London, UK, 1999. [Google Scholar]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Cattell, R.B. The Scree Test for the Number of Factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using IBM SPSS Statistics, 5th ed.; Sage: Los Angeles, CA, USA, 2018. [Google Scholar]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 2nd ed.; Guilford Press: New York, NY, USA; London, UK, 2005. [Google Scholar]
- Katina, S.; Farbakova, J.; Madari, A.; Novak, M.; Zilka, N. Risk factors for canine cognitive dysfunction syndrome in Slovakia. Acta Vet. Scand. 2016, 58, 17. [Google Scholar] [CrossRef] [PubMed]
- Buller, K.; Ballantyne, K.C. Living with and loving a pet with behavioral problems: Pet owners’ experiences. J. Vet. Behav. 2020, 37, 41–47. [Google Scholar] [CrossRef]
- Mondino, A.; Wagner, G.; Russell, K.; Lobaton, E.; Griffith, E.; Gruen, M.; Lascelles, B.D.X.; Olby, N.J. Static posturography as a novel measure of the effects of aging on postural control in dogs. PLoS ONE 2022, 17, e0268390. [Google Scholar] [CrossRef] [PubMed]
- Mondino, A.; Khan, M.; Case, B.; Giovagnoli, S.; Thomson, A.; Lascelles, B.D.X.; Gruen, M.; Olby, N. Activity patterns are associated with fractional lifespan, memory, and gait speed in aged dogs. Sci. Rep. 2023, 13, 2588. [Google Scholar] [CrossRef]
- O’Neill, D.G.; James, H.; Brodbelt, D.C.; Church, D.B.; Pegram, C. Prevalence of commonly diagnosed disorders in UK dogs under primary veterinary care: Results and applications. BMC Vet. Res. 2021, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Bisset, E.S.; Howlett, S.E. The biology of frailty in humans and animals: Understanding frailty and promoting translation. Aging Med. 2019, 2, 27–34. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Ma, L.; Chan, P. Understanding the Physiological Links Between Physical Frailty and Cognitive Decline. Aging Dis. 2020, 11, 405–418. [Google Scholar] [CrossRef]
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding how we age: Insights into inflammaging. Longev. Healthspan 2013, 2, 8. [Google Scholar] [CrossRef]
- Halil, M.; Cemal Kizilarslanoglu, M.; Emin Kuyumcu, M.; Yesil, Y.; Cruz Jentoft, A. Cognitive aspects of frailty: Mechanisms behind the link between frailty and cognitive impairment. J. Nutr. Health Aging 2015, 19, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Hua, J.; Hoummady, S.; Muller, C.; Pouchelon, J.L.; Blondot, M.; Gilbert, C.; Desquilbet, L. Assessment of frailty in aged dogs. Am. J. Vet. Res. 2016, 77, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J. Ageing, Immunosenescence and Inflammageing in the Dog and Cat. J. Comp. Pathol. 2010, 142, S60–S69. [Google Scholar] [CrossRef]
- Lin, T.; Liu, G.A.; Perez, E.; Rainer, R.D.; Febo, M.; Cruz-Almeida, Y.; Ebner, N.C. Systemic Inflammation Mediates Age-Related Cognitive Deficits. Front. Aging Neurosci. 2018, 10, 236. [Google Scholar] [CrossRef]
- Shen, X.-N.; Lu, Y.; Tan, C.T.Y.; Liu, L.-Y.; Yu, J.-T.; Feng, L.; Larbi, A. Identification of inflammatory and vascular markers associated with mild cognitive impairment. Aging 2019, 11, 2403–2419. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Cunningham, C.; Zotova, E.; Woolford, J.; Dean, C.; Kerr, S.; Culliford, D.; Perry, V.H. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009, 73, 768. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Lindquist, K.; Penninx, B.W.; Simonsick, E.M.; Pahor, M.; Kritchevsky, S.; Launer, L.; Kuller, L.; Rubin, S.; Harris, T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 2003, 61, 76. [Google Scholar] [CrossRef]
- Sparks Stein, P.; Steffen, M.J.; Smith, C.; Jicha, G.; Ebersole, J.L.; Abner, E.; Dawson, D. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimer’s Dement. 2012, 8, 196–203. [Google Scholar] [CrossRef]
- Moore, S.A. Managing Neuropathic Pain in Dogs. Front. Vet. Sci. 2016, 3, 12. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic Pain: A Maladaptive Response of the Nervous System to Damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef]
- Apkarian, A.; Baliki, M.; Geha, P. Towards a theory of chronic pain. Prog. Neurobiol. 2008, 87, 81–97. [Google Scholar] [CrossRef]
- Lopes Fagundes, A.L.; Hewison, L.; McPeake, K.J.; Zulch, H.; Mills, D.S. Noise Sensitivities in Dogs: An Exploration of Signs in Dogs with and without Musculoskeletal Pain Using Qualitative Content Analysis. Front. Vet. Sci. 2018, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, A.M.; Mills, D.S.; Zulch, H. Clinical indicators of occult musculoskeletal pain in aggressive dogs. Vet. Rec. 2015, 176, 465. [Google Scholar] [CrossRef]
- Mills, S.D.; Demontigny-Bédard, I.; Gruen, M.; Klinck, P.M.; McPeake, J.K.; Barcelos, M.A.; Hewison, L.; Van Haevermaet, H.; Denenberg, S.; Hauser, H.; et al. Pain and Problem Behavior in Cats and Dogs. Animals 2020, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Piotti, P.; Albertini, M.; Lavesi, E.; Ferri, A.; Pirrone, F. Physiotherapy improves dogs’ quality of life measured with the Milan pet quality of life scale: Is pain involved? Vet. Sci. 2022, 9, 335. [Google Scholar] [CrossRef]
- Hart, R.; Wade, J.; Martelli, M. Cognitive impairment in patients with chronic pain: The significance of stress. Curr. Pain Headache Rep. 2003, 7, 116–126. [Google Scholar] [CrossRef]
- Quaranta, N.; Coppola, F.; Casulli, M.; Barulli, O.; Lanza, F.; Tortelli, R.; Capozzo, R.; Leo, A.; Tursi, M.; Grasso, A.; et al. The Prevalence of Peripheral and Central Hearing Impairment and Its Relation to Cognition in Older Adults. Audiol. Neurotol. 2015, 19, 10–14. [Google Scholar] [CrossRef]
- Panza, F.; Solfrizzi, V.; Logroscino, G. Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat. Rev. Neurol. 2015, 11, 166–175. [Google Scholar] [CrossRef]
- Wayne, R.V.; Johnsrude, I.S. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res. Rev. 2015, 23, 154–166. [Google Scholar] [CrossRef]
- Macdonald, S.W.S.; Keller, C.J.C.; Brewster, P.W.H.; Dixon, R.A. Contrasting Olfaction, Vision, and Audition as Predictors of Cognitive Change and Impairment in Non-Demented Older Adults. Neuropsychology 2018, 32, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Fefer, G.; Khan, M.Z.; Panek, W.K.; Case, B.; Gruen, M.E.; Olby, N.J. Relationship between hearing, cognitive function, and quality of life in aging companion dogs. J. Vet. Intern. Med. 2022, 36, 1708–1718. [Google Scholar] [CrossRef]
- Urfer, S.R.; Greer, K.; Wolf, N.S. Age-related cataract in dogs: A biomarker for life span and its relation to body size. Age 2011, 33, 451–460. [Google Scholar] [CrossRef]
- Behrend, E.; Holford, A.; Lathan, P.; Rucinsky, R.; Schulman, R. 2018 AAHA Diabetes Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2018, 54, 1–21. [Google Scholar] [CrossRef]
- Beaver, B.V.; Haug, L.I. Canine behaviors associated with hypothyroidism. J. Am. Anim. Hosp. Assoc. 2003, 39, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Knopman, D.S.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; Baertlein, L.; Boeve, B.F.; Tangalos, E.G.; Ivnik, R.J.; Mielke, M.M.; et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimer’s Dement. 2014, 10, 18–26. [Google Scholar] [CrossRef]
- Kaltsatou, A. The Impact of Inflammation on Cognitive Impairment in Chronic Kidney Disease Patients. J. Clin. Exp. Nephrol. 2016, 1, 20. [Google Scholar] [CrossRef]
- Ferrante, A.W., Jr. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. J. Intern. Med. 2007, 262, 408–414. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, H.; Simental-Mendía, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and Inflammation: Epidemiology, Risk Factors, and Markers of Inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef]
- McVinnie, D.S. Obesity and pain. Br. J. Pain 2013, 7, 163–170. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Deo, M.S.; Kerse, N.; Vandal, A.C.; Jarrett, P. Dermatological disease in the older age group: A cross-sectional study in aged care facilities. BMJ Open 2015, 5, e009941. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, N.A.; Eisenschenk, M.; Harvey, R.G.; Nuttall, T. Skin Diseases of the Dog and Cat; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
| Factor | ||||
|---|---|---|---|---|
| 1 Musculoskeletal–Neurological | 2 Digestive | 3 Metabolic | 4 Dermatological | |
| Struggles in/out of the car | 0.741 | −0.028 | −0.030 | 0.160 |
| Tires during exercise | 0.746 | −0.052 | −0.045 | 0.157 |
| Assistance on stairs | 0.680 | 0.156 | −0.079 | −0.034 |
| Activity levels | 0.735 | −0.109 | 0.022 | 0.051 |
| Assistance to stand | 0.611 | 0.288 | −0.036 | −0.137 |
| Foot scuffing | 0.630 | 0.037 | −0.132 | 0.127 |
| Hearing | 0.599 | −0.088 | 0.191 | −0.166 |
| Sight | 0.470 | −0.038 | 0.290 | −0.101 |
| Play | 0.606 | −0.133 | 0.157 | −0.144 |
| Lameness | 0.598 | −0.070 | −0.076 | 0.316 |
| Faecal incontinence | 0.412 | 0.207 | −0.060 | −0.373 |
| Assistance feeding | −0.070 | 0.880 | 0.032 | 0.014 |
| Decreased appetite | −0.032 | 0.831 | 0.043 | 0.120 |
| Polyuria | −0.035 | 0.033 | 0.872 | 0.071 |
| Polydipsia | 0.032 | 0.046 | 0.820 | 0.075 |
| Pruritic | 0.072 | 0.005 | 0.008 | 0.759 |
| Licks body | 0.045 | 0.140 | 0.129 | 0.702 |
| Eigenvalues | 5.09 | 1.55 | 1.35 | 1.27 |
| % of variance | 29.93 | 9.12 | 7.97 | 7.44 |
| α | 0.85 | 0.68 | 0.76 | 0.55 |
| n | df | Pearson Chi-Square | p-Value | Cramer’s V | |
|---|---|---|---|---|---|
| Cognitive state: normal vs. impaired | |||||
| Body condition | 804 | 2 | 21.25 | <0.001 | 0.163 |
| Dental disease | 710 | 1 | 18.87 | <0.001 | 0.163 |
| Gastrointestinal disease | 710 | 1 | 2.33 | 0.127 | 0.057 |
| Dermatological disease | 710 | 1 | 0.855 | 0.355 | 0.035 |
| Hypothyroidism | 710 | 1 | 0.143 | 0.705 | 0.014 |
| Hyperadrenocorticism | 710 | 1 | 1.657 | 0.198 | 0.048 |
| Chronic kidney disease | 710 | 1 | 2.831 | 0.092 | 0.063 |
| Epilepsy | 710 | 1 | 0.011 | 0.918 | 0.04 |
| Hepatic disease | 710 | 1 | 2.019 | 0.155 | 0.053 |
| Cardiovascular disease | 710 | 1 | 4.183 | 0.041 | 0.077 |
| Cancer | 710 | 1 | 0.270 | 0.603 | 0.020 |
| Cognitive state: normal vs. mild vs. severe impairment | |||||
| Musculoskeletal disease | 710 | 2 | 28.55 | <0.001 | 0.201 |
| Breed category | 804 | 2 | 0.537 | 0.764 | 0.026 |
| Predictor | Estimate (±S.E.) | p |
|---|---|---|
| Factor 1 (Musculoskeletal–neurological) | 1.84 (0.15) | 0.001 |
| Factor 2 (Digestive) | 0.25 (0.12) | 0.040 |
| Factor 3 (Metabolic) | 0.51 (0.12) | 0.001 |
| Factor 4 (Dermatological) | 0.47 (0.08) | 0.001 |
| Age (years) | 0.29 (0.03) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrightson, R.; Albertini, M.; Pirrone, F.; McPeake, K.; Piotti, P. The Relationship between Signs of Medical Conditions and Cognitive Decline in Senior Dogs. Animals 2023, 13, 2203. https://doi.org/10.3390/ani13132203
Wrightson R, Albertini M, Pirrone F, McPeake K, Piotti P. The Relationship between Signs of Medical Conditions and Cognitive Decline in Senior Dogs. Animals. 2023; 13(13):2203. https://doi.org/10.3390/ani13132203
Chicago/Turabian StyleWrightson, Rosalind, Mariangela Albertini, Federica Pirrone, Kevin McPeake, and Patrizia Piotti. 2023. "The Relationship between Signs of Medical Conditions and Cognitive Decline in Senior Dogs" Animals 13, no. 13: 2203. https://doi.org/10.3390/ani13132203
APA StyleWrightson, R., Albertini, M., Pirrone, F., McPeake, K., & Piotti, P. (2023). The Relationship between Signs of Medical Conditions and Cognitive Decline in Senior Dogs. Animals, 13(13), 2203. https://doi.org/10.3390/ani13132203






