Changes in Chemical Composition and Fatty Acid Profile of Milk and Cheese and Sensory Profile of Milk via Supplementation of Goats’ Diet with Marine Algae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Collection of Milk Samples
2.3. Cheese Processing and Collection of Cheese Samples
whey)/(440 − total solids of whey) × 100.
milk wt)
2.4. Chemical Analysis
2.5. Sensory Analysis of Milk
2.6. Statistical Analysis
3. Results
3.1. Milk, Cheese and Whey Composition
3.2. Milk and Cheese Fatty Acid Composition
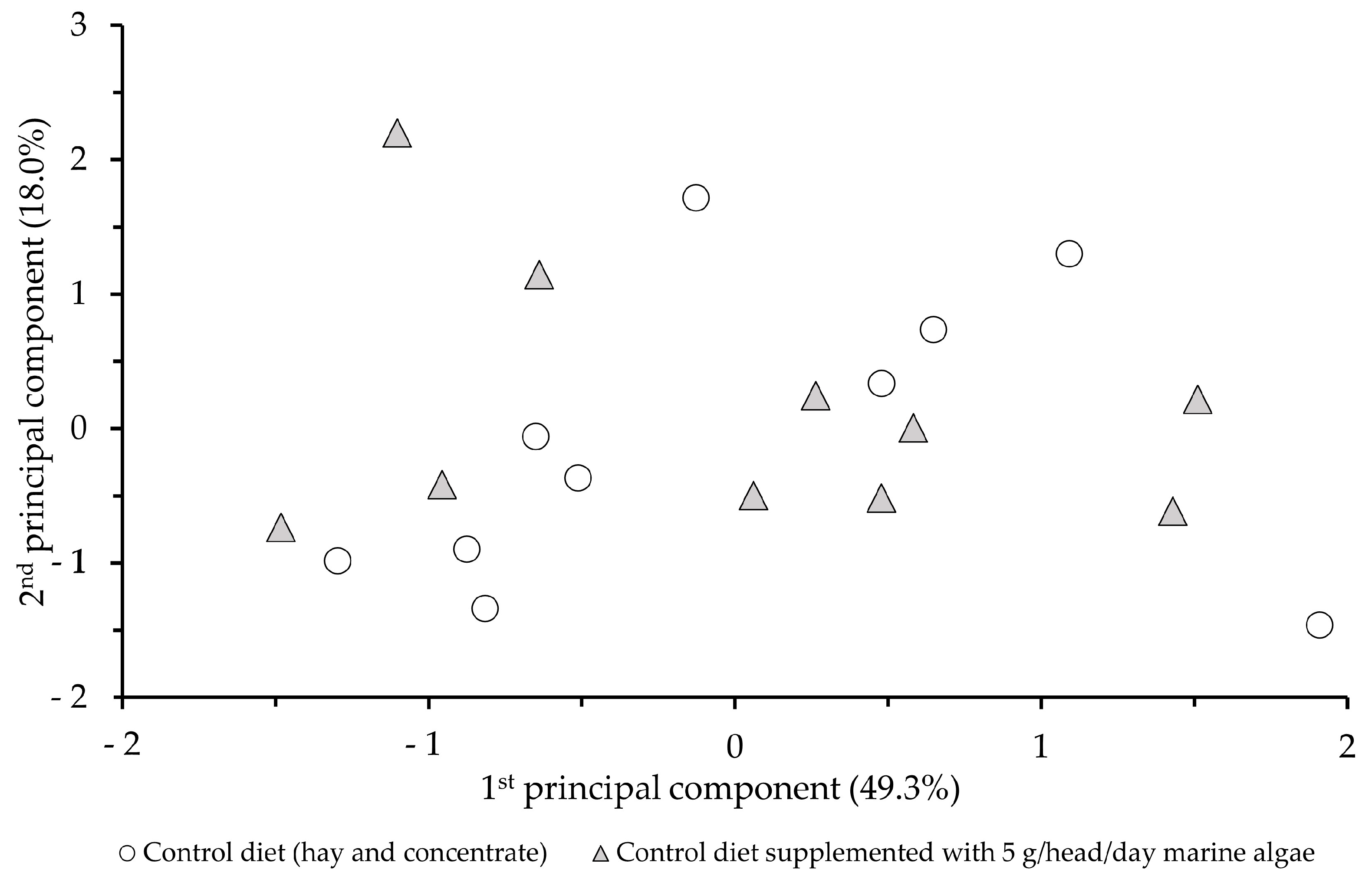
3.3. Sensory Profile of Milk
4. Discussion
4.1. Milk, Cheese, and Whey Composition
4.2. Milk and Cheese Fatty acid Composition
4.3. Sensory Profile of Milk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organisation of the United Nation). FAOSTAT—Statistical Databases. 2023. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 10 March 2023).
- Park, Y.W. Hypoallergenic and therapeutic significance of goat milk. Small Rumin. Res. 2007, 14, 151–159. [Google Scholar] [CrossRef]
- Haenlein, G.F.W. About the evolution of goat and sheep milk production. Small Rumin. Res. 2007, 68, 3–6. [Google Scholar] [CrossRef]
- Moate, P.J.; Williams, S.R.O.; Hannah, M.C.; Eckard, R.J.; Auldist, M.J.; Ribaux, B.E.; Jacobs, J.L.; Wales, W.J. Effects of feeding algal meal high in docosahexaenoic acid on feed intake, milk production, and methane emissions in dairy cows. J. Dairy Sci. 2013, 96, 3177–3188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiplakou, E.; Abdullah, M.A.M.; Alexandros, M.; Chatzikonstantinou, M.; Skliros, D.; Sotirakoglou, K.; Flemetakis, E.; Labrou, N.E.; Zervas, G. The effect of dietary Chlorella pyrenoidosa inclusion on goats milk chemical composition, fatty acids profile and enzymes activities related to oxidation. Livest. Sci. 2017, 197, 106–111. [Google Scholar] [CrossRef]
- Póti, P.; Pajor, F.; Bodnár, A.; Penksza, K.; Köles, P. Effect of micro-alga supplementation on goat and cow milk fatty acid composition. Chil. J. Agric. Res. 2015, 75, 259–263. [Google Scholar] [CrossRef]
- Li, D.; Bode, O.; Drummond, H.; Sinclair, A.J. Omega-3 (n-3) fatty acids. In Lipids for Functional Foods and Nutraceuticals; Gunstone, F.D., Ed.; Oily Press: Bridgwater, UK, 2003; pp. 225–262. [Google Scholar] [CrossRef]
- Kotue, T.C.; Djote, W.N.B.; Marlyne, M.; Pieme, A.C.; Kansci, G.; Fokou, E. Antisickling and Antioxidant Properties of Omega-3 Fatty Acids EPA/DHA. Nutr. Food Sci. Int. J. 2019, 9, 555752. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Toral, P.G.; Hervás, G.; Carreño, D.; Leskinen, H.; Belenguer, A.; Shingfield, J.K.; Frutos, P. In vitro response to EPA, DPA, and DHA: Comparison of effects on ruminal fermentation and biohydrogenation of 18-carbon fatty acids in cows and ewes. J. Dairy Sci. 2017, 100, 6187–6198. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- Pajor, F.; Egerszegi, I.; Steiber, O.; Bodnár, Á.; Póti, P. Effect of marine algae supplementation on the fatty acid profile of milk of dairy goats kept indoor and on pasture. J. Anim. Feed Sci. 2019, 28, 169–176. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Tsiplakou, E. The impact of the dietary supplementation level with Schizochytrium sp. on milk chemical composition and fatty acid profile, of both blood plasma and milk of goats. Small Rumin. Res. 2020, 193, 106252. [Google Scholar] [CrossRef]
- Boeckaert, C.; Vlaeminck, B.; Dijkstra, J.; Issa-Zacharia, A.; Van Nespen, T.; Van Straalen, W.; Fievez, V. Effect of dietary starch or micro algae supplementation on rumen fermentation and milk fatty acid composition of dairy cows. J. Dairy Sci. 2008, 91, 4714–4727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bichi, E.; Hervás, G.; Toral, P.G.; Loor, J.J.; Frutos, P. Milk fat depression induced by dietary marine algae in dairy ewes: Persistency of milk fatty acid composition and animal performance responses. J. Dairy Sci. 2013, 96, 524–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Białek, M.; Czauderna, M.; Białek, A. Partial replacement of rapeseed oil with fish oil, and dietary antioxidants supplementation affects concentrations of biohydrogenation products and conjugated fatty acids in rumen and selected lamb tissues. Anim. Feed Sci. Technol. 2018, 241, 63–74. [Google Scholar] [CrossRef]
- ISO. Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- Aouadi, B.; Zaukuu, J.-L.Z.; Vitális, F.; Bodor, Z.; Fehér, O.; Gillay, Z.; Bazar, G.; Kovacs, Z. Historical Evolution and Food Control Achievements of Near Infrared Spectroscopy, Electronic Nose, and Electronic Tongue—Critical Overview. Sensors 2020, 20, 5479. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Bodnár, A.; Egerszegi, I.; Kuchtik, J.; Penksza, K.; Póti, P.; Pajor, F. Effect of Grazing on Composition, Fatty Acid Profile and Nutritional of the Goat Milk and Cheese. J. Anim. Feed. Sci. 2021, 30, 320–328. [Google Scholar] [CrossRef]
- Maubois, J.L.; Mocquot, G. L’appréciation des rendements en fromagerie. Lait 1971, 51, 416–420. [Google Scholar] [CrossRef] [Green Version]
- Kalit, S.; Tudor Kalit, M.; Dolenčić Špehar, I.; Salajpal, K.; Samaržija, D.; Anušić, J.; Rako, A. The Influence of Milk Standardization on Chemical Composition, Fat and Protein Recovery, Yield and Sensory Properties of Croatian PGI Lički Škripavac Cheese. Foods 2021, 10, 690. [Google Scholar] [CrossRef]
- Hungarian Feed Codex. Laboratory methods and operations (in Hungarian). Magy. Közlöny 2003, 42, 3388–3436. [Google Scholar]
- MSZ 5874-8:1978; Determination of Nitrogen Content for Calculation of the Protein Content. Hungarian Standard: Budapest, Hungary, 1978.
- MSZ 5874–4:1980; Determination of the Dry Matter Content. Hungarian Standard: Budapest, Hungary, 1980.
- MSZ ISO 1443:2002; Raw Fat Content Determination by Diethyl Ether Extraction. Hungarian Standard: Budapest, Hungary, 2002.
- Kleyn, D.H. Determination of fat in raw and processed milks by Gerber method: Collaborative study. J. AOAC Int. 2001, 84, 1499–1508. [Google Scholar] [CrossRef] [Green Version]
- ISO 12966-2; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2011.
- Ulbright, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2006, 68, 88–113. [Google Scholar] [CrossRef] [Green Version]
- Kuchtík, J.; Králíčková, S.; Zapletal, D.; Węglarzy, K.; Šustová, K.; Skrzyżala, I. Changes in physico-chemical characteristics, somatic cell count and fatty acid profile of Brown Short-haired goat milk during lactation. Anim. Sci. Pap. Rep. 2015, 33, 71–83. [Google Scholar]
- Pajor, F.; Egerszegi, I.; Szucs, A.; Poti, P.; Bodnar, A. Effect of marine algae supplementation on somatic cell count, prevalence of udder pathogens, and fatty acid profile of dairy goats’ milk. Animals 2021, 11, 1097. [Google Scholar] [CrossRef]
- Moran, C.A.; Morlacchini, M.; Keegan, J.D.; Fusconi, G. The effect of dietary supplementation with Aurantiochytrium limacinum on lactating dairy cows in terms of animal health, productivity and milk composition. J. Anim. Physiol. Anim. Nutr. 2018, 102, 576–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulos, G.; Goulas, C.; Apostolaki, E.; Abril, R. Effects of dietary supplements of algae, containing polyunsaturated fatty acids, on milk yield and the composition of milk products in dairy ewes. J. Dairy Sci. 2002, 69, 357–365. [Google Scholar] [CrossRef]
- González-Morelo, K.J.; Correa, A.D.; Cabarcas, A.D.C.; Castillo, P.M.M.; Amador, B.L.O. Effect of Fat Content on the Properties of Colombian Queso Costeño Made from Goat Milk. Int. J. ChemTech Res. 2018, 11, 113–123. [Google Scholar] [CrossRef]
- European Commission. Commission Decision of 18 December 1996 laying down provisions for the implementation of Council Directive 96/16/EC on statistical surveys of milk and milk products (97/80/EC). Off. J. 1997, L24/26, 26–49. [Google Scholar]
- Pajor, F.; Steiber, O.; Tasi, J. Influence of extensive grazing on cheese composition, yield and fatty acids content of goats. Bulg. J. Agric. Sci. 2012, 18, 487–492. [Google Scholar]
- Fekadu, B.; Soryal, K.; Zeng, S.; Van Hekken, D.; Baha, B.; Villaquiran, M. Changes in goat milk composition during lactation and their effect on yield and quality of hard and semi-hard cheeses. Small Rumin. Res. 2005, 59, 55–63. [Google Scholar] [CrossRef]
- Pazzola, M.; Stocco, G.; Dettori, M.L.; Bittante, G.; Vacca, G.M. Effect of goat milk composition on cheesemaking traits and daily cheese production. J. Dairy Sci. 2019, 102, 3947–3955. [Google Scholar] [CrossRef] [PubMed]
- Vacca, G.M.; Stocco, G.; Dettori, L.M.; Summer, A.; Cipolat-Gotet, C.; Bittante, G.; Pazzola, M. Cheese yield, cheesemaking efficiency, and daily production of 6 breeds of goats. J. Dairy Sci. 2018, 101, 7817–7832. [Google Scholar] [CrossRef] [PubMed]
- Abbeddou, S.; Rischkowsky, B.; Richter, E.K.; Hess, H.D.; Kreuzer, M. Modification of milk fatty acid composition by feeding forages and agro-industrial byproducts from dry areas to Awassi sheep. J. Dairy Sci. 2011, 94, 4657–4668. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, T.; Zanten, K.; Satter, L. Effect of dietary fat source on fatty acid composition of cow’s milk. J. Sci. Food Agric. 1995, 69, 101–107. [Google Scholar] [CrossRef]
- Zhang, R.H.; Mustafa, A.F.; Zhao, X. Effects of feeding oilseeds rich in linoleic and linolenic fatty acids to lactating ewes on cheese yield and on fatty acid composition of milk and cheese. Anim. Feed. Sci. Technol. 2006, 127, 220–233. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhu, H.; Li, Y.; Hong, G. Continuous propionate production from whey permeate using a novel fibrous bed bioreactor. Biotechnol. Bioeng. 1994, 43, 1124–1130. [Google Scholar] [CrossRef]
- Heino, A.; Uusi-Rauva, J.; Rantamäki, P.; Vainen, O. Functional properties of native and cheese whey protein concentrate powders. Int. J. Dairy Technol. 2007, 60, 277–285. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Castro, N.; Morales-delaNuez, A.; Sánchez-Macías, D.; Assunção, P.; Capote, J.; Argüello, A. Farm and factory production of goat cheese whey results in distinct chemical composition. J. Dairy Sci. 2009, 92, 4792–4796. [Google Scholar] [CrossRef] [Green Version]
- Djordjevic, J.; Ledina, T.; Baltic, M.Z.; Trbovic, D.; Babic, M.; Bulajic, S. Fatty acid profile of milk. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012057. [Google Scholar] [CrossRef]
- Mumme, K.; Stonehouse, W. Effects of medium-chain triglycerides on weight loss and body composition: A meta-analysis of randomized controlled trials. J. Acad. Nutr. Diet. 2015, 115, 249–263. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Juárez, M.; de La Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Harfoot, C.G. Lipid metabolism in the rumen. In Lipid Metabolism in Ruminant Animals; Christie, W.W., Ed.; Pergamon Press: New York, NY, USA, 1981; pp. 21–55. [Google Scholar]
- Or-Rashid, M.M.; Kramer, J.K.G.; Wood, M.A.; McBride, B.W. Supplemental algal meal alters the ruminal trans-18:1 fatty acid and conjugated linoleic acid composition in cattle. J. Anim. Sci. 2008, 86, 187–196. [Google Scholar] [CrossRef]
- Kuhnt, K.; Kraft, J.; Moeckel, P.; Jahreis, G. Trans-11–18: 1 is effectively δ9-desaturated compared with Trans-12–18: 1 in humans. Br. J. Nutr. 2006, 95, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Kelley, N.; Hubbard, N.; Erickson, K. Conjugated linoleic acid isomers and cancer. J. Nutr. 2007, 137, 2599–2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lock, A.L.; Kraft, J.; Rice, B.H.; Bauman, D.E. Biosynthesis and biological activity of rumenic acid: A natural CLA isomer. In Trans Fatty Acids in Human Nutrition; Destaillats, F., Sébédio, J.L., Dionisi, F., Chardigny, J.M., Eds.; Oily Press: Bridgwater, UK, 2009; pp. 195–230. [Google Scholar] [CrossRef]
- Givens, D.I.; Shingfield, K.J. Optimizing dairy milk fatty acid composition. In Improving the Fat Content of Foods; Williams, C., Buttriss, J., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 252–280. [Google Scholar]
- Shingfield, K.J.; Bernard, L.; Leroux, C.; Chilliard, Y. Role of trans fatty acids in the nutritional regulation of mammary lipogenesis in ruminants. Animal 2010, 4, 1140–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.M.; Huang, M.C.; Saad, N.M.; Nathanielsz, P.W.; Brenna, J.T. Fetal baboons convert 18:3n-3 to 22:6n-3 in vivo. A stable isotope tracer study. J. Lipid Res. 2001, 42, 581–586. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Gómez-Cortés, P.; Frutos, P.; Juárez, M.; de La Fuente, M.A. Milk fatty acid profile and dairy sheep performance in response to diet supplementation with sunflower oil plus incremental levels of marine algae. J. Dairy Sci. 2010, 93, 1655–1667. [Google Scholar] [CrossRef] [Green Version]
- Zisis, F.; Kyriakaki, P.; Satolias, F.F.; Mavrommatis, A.; Simitzis, P.E.; Pappas, A.C.; Surai, P.F.; Tsiplakou, E. The Effect of Dietary Inclusion of Microalgae Schizochytrium spp. on Ewes’ Milk Quality and Oxidative Status. Foods 2022, 11, 2950. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Palmquist, D.L. Omega-3 fatty acids in metabolism, health and nutrition and for modified animal product foods. Prof. Anim. Sci. 2009, 25, 207–249. [Google Scholar] [CrossRef]
- Lu, L.; Hu, Z.; Hu, X.; Li, D.; Tian, S. Electronic tongue and electronic nose for food quality and safety. Food Res. Int. 2022, 162, 112214. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Ding, W.; Ma, L.J.; Jia, R. Discrimination and characterization of different intensities of goaty flavor in goat milk by means of an electronic nose. J. Dairy Sci. 2015, 98, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Items | Forage | Diet | |||
|---|---|---|---|---|---|
| Pasture | Concentrate | Marine Algae | Control 1 | Marine Algae 2 | |
| Daily intake g/day | 5210 | 600 | 5 | 5810 | 5815 |
| Daily dry matter (DM) intake g/day | 1589 | 540 | 4.645 | 2129 | 2133 |
| Chemical composition | |||||
| DM, g/kg forage | 305 | 900 | 929 | 366.36 | 366.84 |
| Crude protein, g/kg DM | 169 | 170 | 148 | 169.18 | 169.13 |
| Crude fat, g/kg DM | 37 | 26 | 482 | 33.84 | 34.80 |
| Crude fibre, g/kg DM | 239 | 65 | 23 | 194.56 | 194.18 |
| Crude ash, g/kg DM | 69 | 58 | 38 | 66.58 | 66.52 |
| Main FA, g/100g of fatty acids | |||||
| C12:0 | 0.33 | nd | 0.50 | 0.27 | 0.27 |
| C14:0 | 0.66 | 0.13 | 7.28 | 0.56 | 0.76 |
| C16:0 | 11.26 | 13.05 | 59.10 | 11.61 | 13.06 |
| C18:0 | 1.97 | 1.74 | 2.04 | 1.93 | 1.93 |
| C18:1n-9 | 21.19 | 26.23 | 0.71 | 22.17 | 21.53 |
| C18:2n-6 | 18.16 | 56.84 | 0.13 | 25.70 | 24.99 |
| C18:3n-3 | 38.95 | 1.82 | 0.10 | 31.71 | 30.68 |
| C22:6n-3 (DHA) | nd | nd | 30.10 | nd | 0.91 |
| Daily DHA intake, mg/g DM | nd | nd | 145.08 | nd | 0.316 |
| Daily DHA intake, mg/day | nd | nd | 673.91 | nd | 673.91 |
| Processing steps | Description |
|---|---|
| Heating of raw milk | Temperature: up to 65 °C for 10 min |
| Cooling | down to 36 °C |
| Addition of commercial rennet to milk | Hannilase powder commercial rennet (before adding: coagulant diluted by 20 °C water) and gentle stirring for 3 min. |
| Coagulation of milk | up to 40 min |
| Post-heating treatment | Temperature: up to 42 °C, duration 5 min |
| Scooping and draining of coagulated milk | size: 1 cm |
| Formation of cheeses blocks | Approx. 300 g of each |
| Whey removal | self-pressing (gravity method), duration: 24 h |
| Salt bath of cheese blocks | Salt concentration: 18%, salt temperature: 13 °C, duration: 6 h |
| Traits | Pre-Treatment | SEM | p-Value | Diet | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| C | MA | C | MA | |||||
| DMY, kg | 1.33 | 1.31 | 0.017 | 0.637 | 1.23 | 1.21 | 0.030 | 0.712 |
| Fat, % | 3.60 | 3.68 | 0.029 | 0.191 | 3.51 | 3.59 | 0.041 | 0.334 |
| Protein, % | 3.25 | 3.29 | 0.024 | 0.421 | 3.24 | 3.22 | 0.031 | 0.777 |
| Lactose, % | 4.45 | 4.43 | 0.017 | 0.589 | 4.47 | 4.41 | 0.023 | 0.102 |
| Total solids, % | 12.00 | 12.10 | 0.032 | 0.146 | 11.92 | 11.93 | 0.060 | 0.270 |
| Traits | Diet | SEM | p-Value | |
|---|---|---|---|---|
| C | MA | |||
| Actual cheese yield, kg/100 kg of milk | 12.74 | 12.03 | 0.682 | 0.982 |
| Moisture-adjusted cheese yield *, kg/100 kg of milk | 14.32 | 13.00 | 0.258 | 0.009 |
| MFFB, % 1 | 69.42 | 72.28 | 0.670 | 0.033 |
| Fat, % | 22.14 | 21.65 | 0.420 | 0.582 |
| Protein, % | 20.08 | 17.83 | 0.450 | 0.010 |
| Fat in TS, % | 48.37 | 49.23 | 1.176 | 0.731 |
| Total solids, % | 46.10 | 44.10 | 0.707 | 0.181 |
| Nutrient recovery rate | ||||
| - fat, % | 79.61 | 72.42 | 1.521 | 0.017 |
| - protein, % | 77.73 | 66.55 | 2.221 | 0.010 |
| Traits | Diet | SEM | p-Value | |
|---|---|---|---|---|
| C | MA | |||
| Fat, % | 0.60 | 1.04 | 0.056 | <0.001 |
| Protein, % | 0.85 | 1.20 | 0.017 | <0.001 |
| Lactose, % | 4.38 | 4.34 | 0.020 | 0.308 |
| Total solids, % | 6.37 | 7.15 | 0.104 | 0.389 |
| Fat in TS, % | 11.97 | 14.30 | 0.473 | 0.012 |
| Protein in TS, % | 13.01 | 16.87 | 0.490 | <0.001 |
| Lactose in TS, % | 66.92 | 60.96 | 0.875 | <0.001 |
| Fatty Acids | Milk | Cheese | ||||||
|---|---|---|---|---|---|---|---|---|
| C | MA | SEM | p-Value | C | MA | SEM | p-Value | |
| C4:0 | 1.76 | 1.72 | 0.071 | 0.647 | 2.50 | 2.00 | 0.111 | 0.320 |
| C6:0 | 1.31 | 1.38 | 0.121 | 0.585 | 2.99 | 2.92 | 0.126 | 0.799 |
| C8:0 | 1.61 | 1.75 | 0.163 | 0.388 | 2.56 | 2.97 | 0.103 | 0.059 |
| C10:0 | 4.88 | 6.14 | 0.560 | 0.046 | 5.11 | 7.42 | 0.327 | 0.002 |
| C12:0 | 1.87 | 2.53 | 0.138 | 0.000 | 2.46 | 3.42 | 0.088 | 0.000 |
| C14:0 | 6.53 | 8.77 | 0.351 | 0.000 | 7.62 | 9.97 | 0.182 | 0.000 |
| C14:1 | 0.04 | 0.09 | 0.005 | 0.000 | 0.05 | 0.11 | 0.004 | 0.000 |
| C16:0 | 24.54 | 30.06 | 0.555 | 0.000 | 24.13 | 28.36 | 0.246 | 0.000 |
| C16:1 | 0.57 | 0.63 | 0.029 | 0.048 | 0.55 | 0.58 | 0.014 | 0.255 |
| C18:0 | 19.79 | 14.92 | 0.496 | 0.000 | 16.44 | 11.94 | 0.339 | 0.000 |
| C18:1n-9 | 28.94 | 24.29 | 1.321 | 0.002 | 27.42 | 22.63 | 0.728 | 0.004 |
| C18:1t11 | 2.31 | 2.22 | 0.072 | 0.224 | 2.04 | 1.98 | 0.035 | 0.409 |
| rumenic acid | 0.62 | 0.88 | 0.024 | 0.000 | 0.57 | 0.77 | 0.015 | 0.000 |
| C18:2n-6 | 2.29 | 1.72 | 0.119 | 0.000 | 1.99 | 1.56 | 0.037 | 0.000 |
| C18:3n-3 | 0.96 | 0.84 | 0.029 | 0.002 | 0.90 | 0.78 | 0.016 | 0.001 |
| C20:3n-6 | 0.02 | 0.02 | 0.002 | 0.382 | 0.02 | 0.02 | 0.001 | 0.999 |
| C20:4n-6 | 0.17 | 0.18 | 0.006 | 0.751 | 0.15 | 0.15 | 0.004 | 0.681 |
| C20:5n-3 (EPA) | 0.08 | 0.08 | 0.003 | 0.377 | 0.07 | 0.07 | 0.002 | 0.164 |
| C22:5n-3 | 0.20 | 0.17 | 0.007 | 0.001 | 0.16 | 0.14 | 0.005 | 0.164 |
| C22:6n-3 (DHA) | 0.06 | 0.25 | 0.015 | 0.000 | 0.05 | 0.24 | 0.005 | 0.000 |
| odd FA | 1.81 | 1.80 | 0.048 | 0.894 | 1.77 | 1.75 | 0.015 | 0.523 |
| SFA | 64.69 | 69.60 | 1.358 | 0.002 | 68.00 | 73.19 | 0.785 | 0.004 |
| MUFA | 31.90 | 27.25 | 1.304 | 0.002 | 30.10 | 25.34 | 0.746 | 0.004 |
| PUFA | 4.41 | 4.14 | 0.138 | 0.073 | 3.90 | 3.74 | 0.062 | 0.192 |
| n-6 | 2.49 | 1.92 | 0.119 | 0.000 | 2.16 | 1.73 | 0.039 | 0.000 |
| n-3 | 1.30 | 1.35 | 0.039 | 0.293 | 1.18 | 1.23 | 0.021 | 0.112 |
| n-6/n-3 ratio | 1.92 | 1.43 | 0.103 | 0.000 | 1.84 | 1.41 | 0.025 | 0.000 |
| AI | 1.47 | 2.17 | 0.096 | 0.000 | 1.84 | 2.70 | 0.130 | 0.000 |
| Days 1 | Daily DHA, intake, mg/Day 2 | Average Milk Fat Content, g/100 g Milk | Average Milk DHA Content, g/100 g FA | Average Milk DHA Content, mg/100 g Milk | Milk Production, kg | DHA in Milk Yield, mg/Day | DHA Efficiency Ratio, % |
|---|---|---|---|---|---|---|---|
| Adaptation period, 42 d 3 | |||||||
| 7 | 673.91 | 3.68 | 0.08 | 2.94 | 1.23 | 36.21 | 5.37 |
| 14 | 673.91 | 3.71 | 0.10 | 3.71 | 1.22 | 45.26 | 6.72 |
| 21 | 673.91 | 3.75 | 0.13 | 4.88 | 1.21 | 58.99 | 8.75 |
| 28 | 673.91 | 3.78 | 0.16 | 6.05 | 1.23 | 74.39 | 11.04 |
| 35 | 673.91 | 3.81 | 0.19 | 7.24 | 1.22 | 88.32 | 13.10 |
| 42 | 673.91 | 3.85 | 0.26 | 10.01 | 1.22 | 122.12 | 18.12 |
| Experimental period 10 d 4 | |||||||
| 1–10 | 673.91 | 3.88 | 0.25 | 9.70 | 1.21 | 117.37 | 17.42 |
| Original Group Membership | Predicted Group Membership | ||
|---|---|---|---|
| C | MA | ||
| Original * | C | 100.0 | 0.0 |
| MA | 10.0 | 90.0 | |
| Cross-validated ** | C | 40.0 | 60.0 |
| MA | 50.0 | 50.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajor, F.; Várkonyi, D.; Dalmadi, I.; Pásztorné-Huszár, K.; Egerszegi, I.; Penksza, K.; Póti, P.; Bodnár, Á. Changes in Chemical Composition and Fatty Acid Profile of Milk and Cheese and Sensory Profile of Milk via Supplementation of Goats’ Diet with Marine Algae. Animals 2023, 13, 2152. https://doi.org/10.3390/ani13132152
Pajor F, Várkonyi D, Dalmadi I, Pásztorné-Huszár K, Egerszegi I, Penksza K, Póti P, Bodnár Á. Changes in Chemical Composition and Fatty Acid Profile of Milk and Cheese and Sensory Profile of Milk via Supplementation of Goats’ Diet with Marine Algae. Animals. 2023; 13(13):2152. https://doi.org/10.3390/ani13132152
Chicago/Turabian StylePajor, Ferenc, Dávid Várkonyi, István Dalmadi, Klára Pásztorné-Huszár, István Egerszegi, Károly Penksza, Péter Póti, and Ákos Bodnár. 2023. "Changes in Chemical Composition and Fatty Acid Profile of Milk and Cheese and Sensory Profile of Milk via Supplementation of Goats’ Diet with Marine Algae" Animals 13, no. 13: 2152. https://doi.org/10.3390/ani13132152
APA StylePajor, F., Várkonyi, D., Dalmadi, I., Pásztorné-Huszár, K., Egerszegi, I., Penksza, K., Póti, P., & Bodnár, Á. (2023). Changes in Chemical Composition and Fatty Acid Profile of Milk and Cheese and Sensory Profile of Milk via Supplementation of Goats’ Diet with Marine Algae. Animals, 13(13), 2152. https://doi.org/10.3390/ani13132152






