Characterization of Leishmania Parasites Isolated from Naturally Infected Mammals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Culture and DNA Extraction
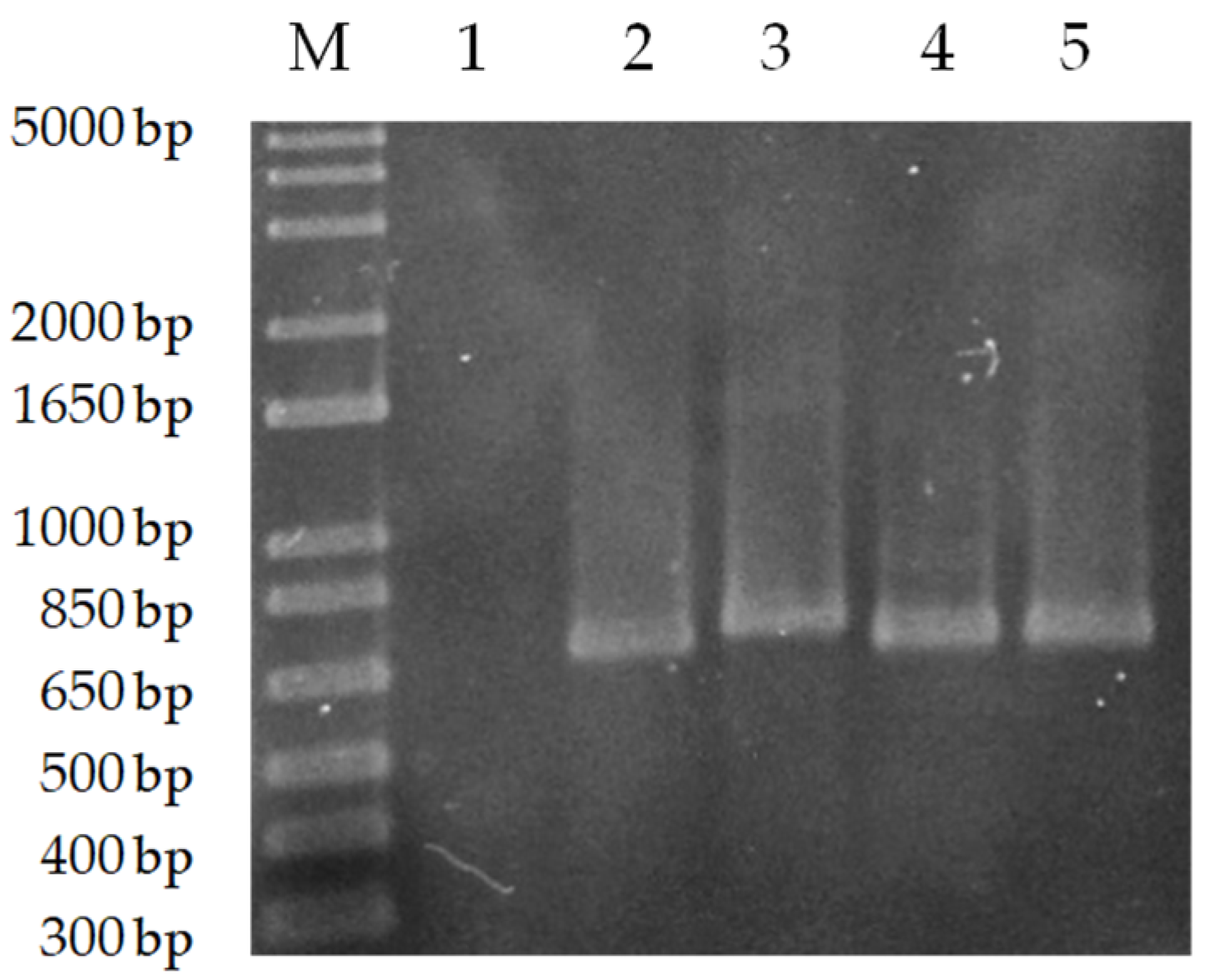
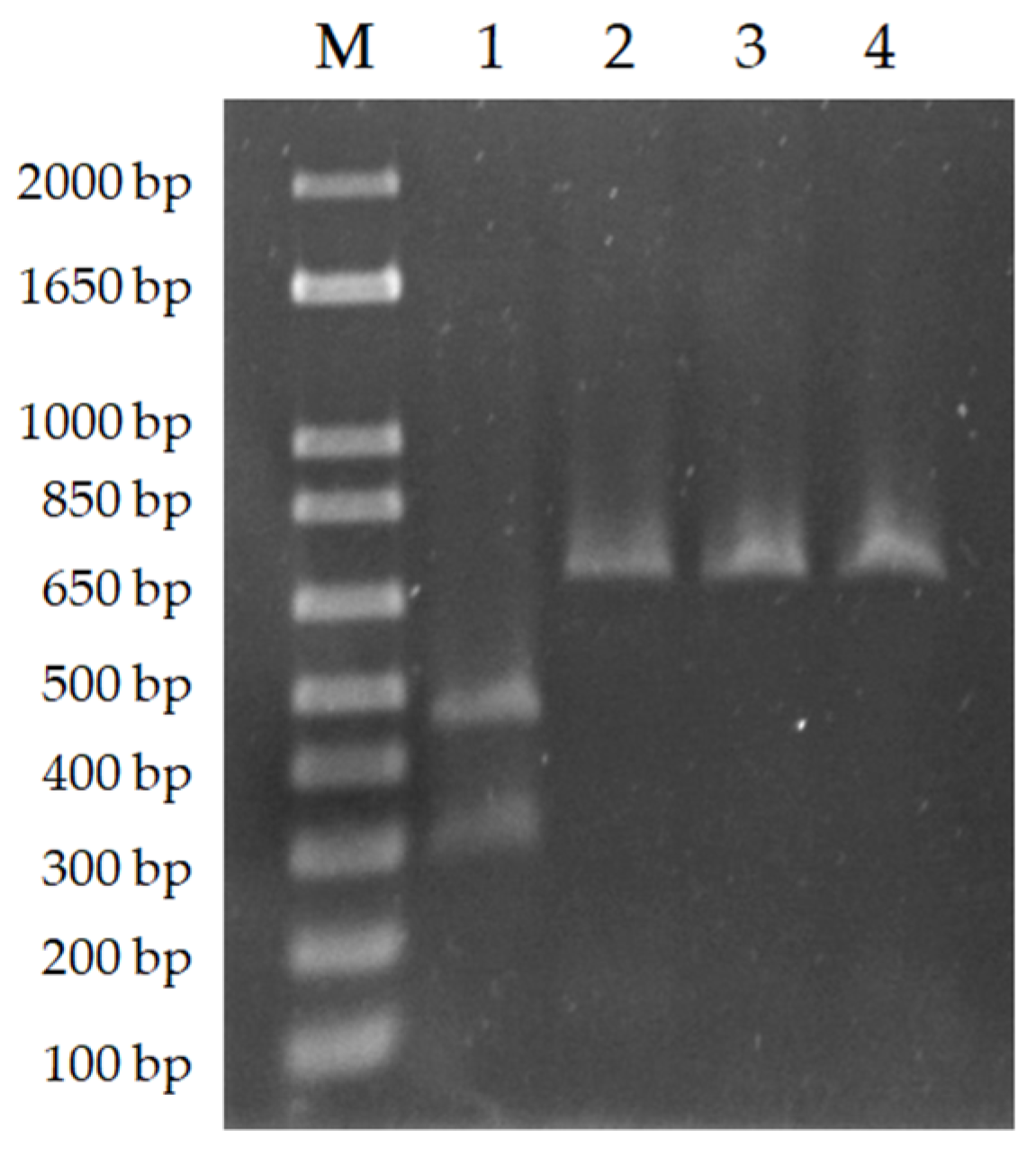
2.2. PCR-RFLP
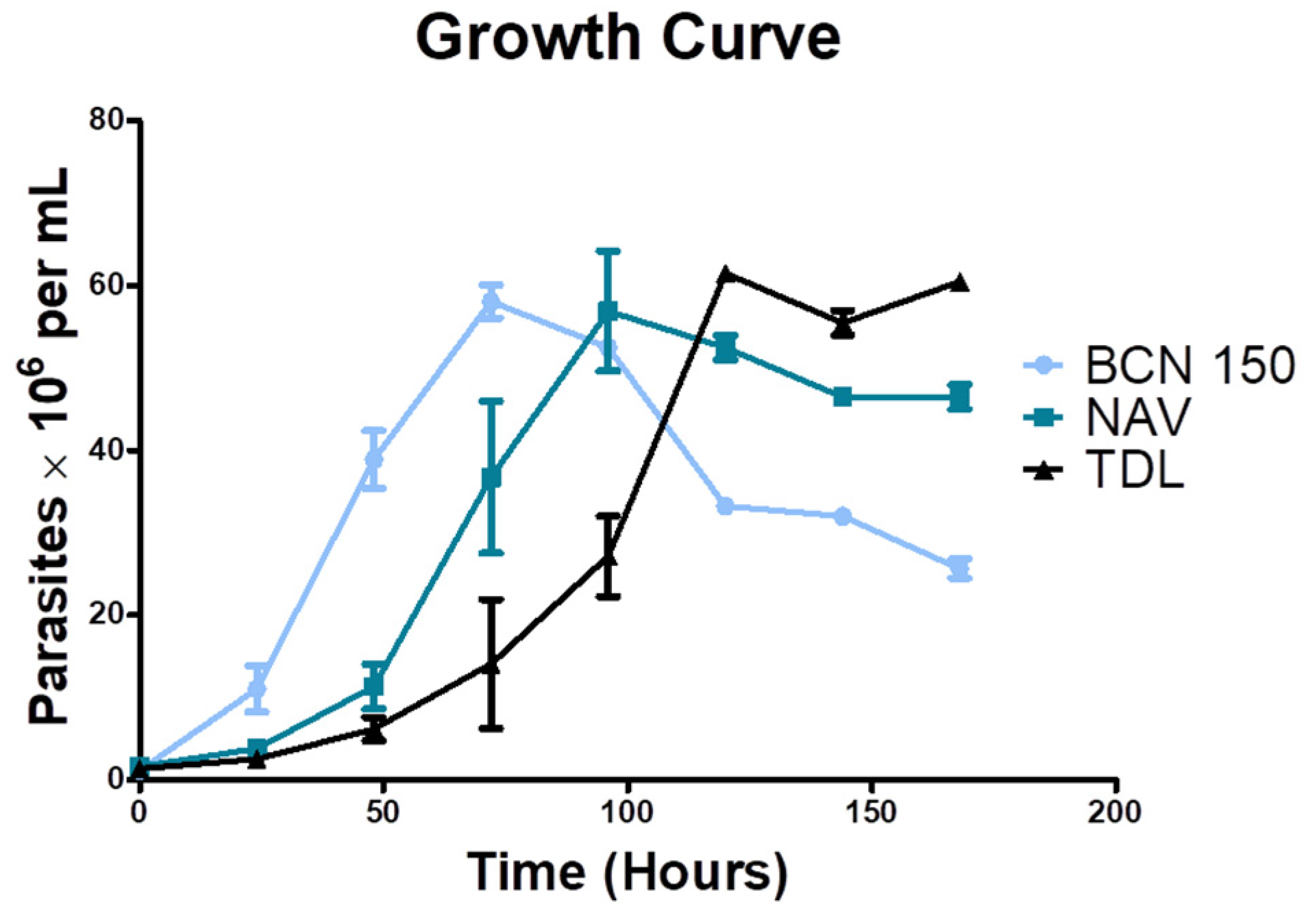
2.3. In Vitro Growth Curve of Promastigote Parasites
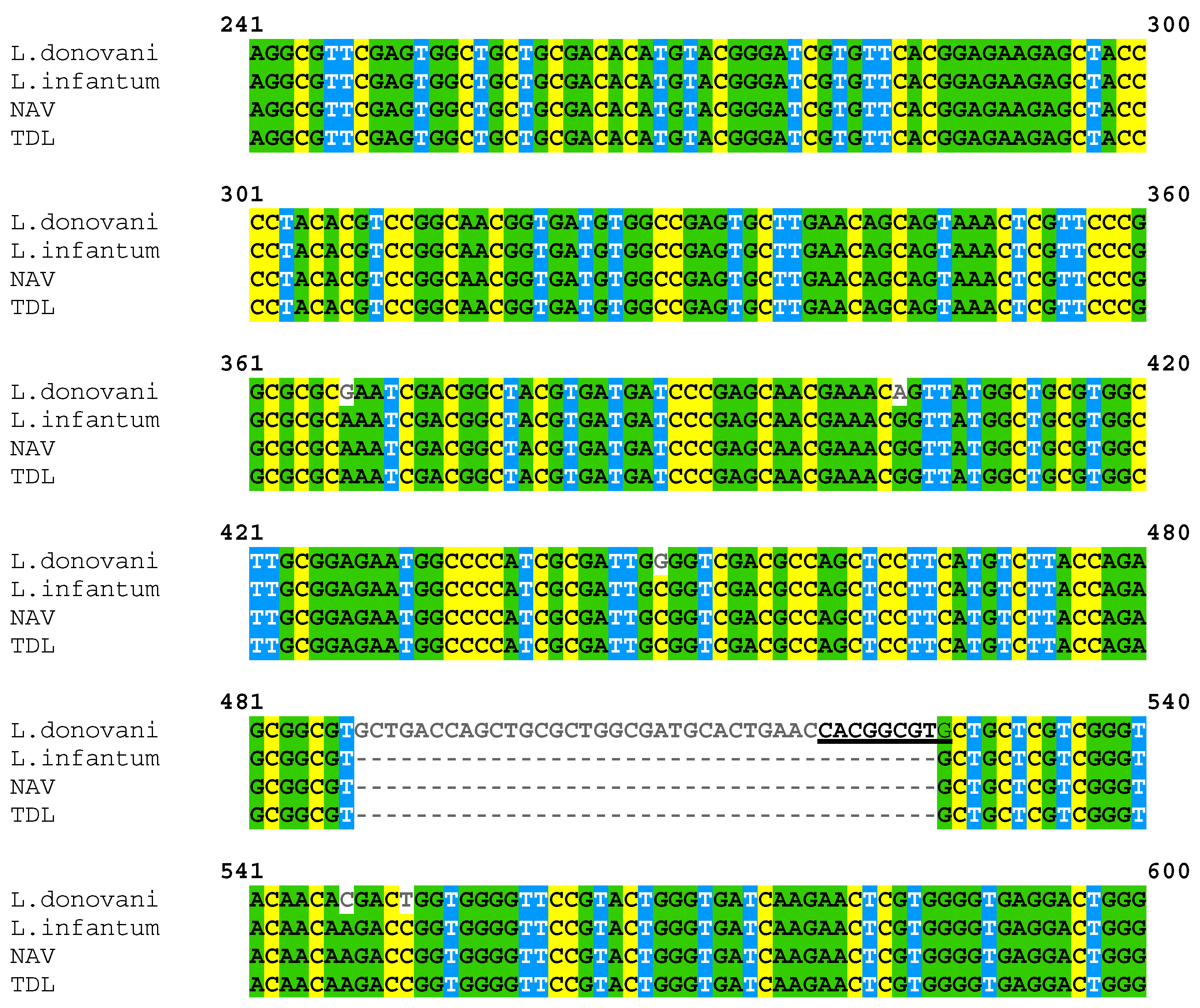
2.4. Nucleotide Sequences Alignment
2.5. In Vitro Sensitivity of Promastigotes to Drugs
2.6. RNA Extraction and Quantitative Reverse Transcription-PCR (RT-qPCR) Gene Expression Analysis
2.7. In Vitro Infection of Bone Marrow-Derived Macrophages
2.8. Treatment Response of Intracellular Amastigotes
2.9. Statistical Analysis
3. Results
3.1. PCR-RFLP Allowed the Specific Species Identification of NAV and TDL Isolates
3.2. PCR Product Sequencing Confirmed That Isolates Were L. infantum
3.3. Isolates Showed a Slower Growth Rate
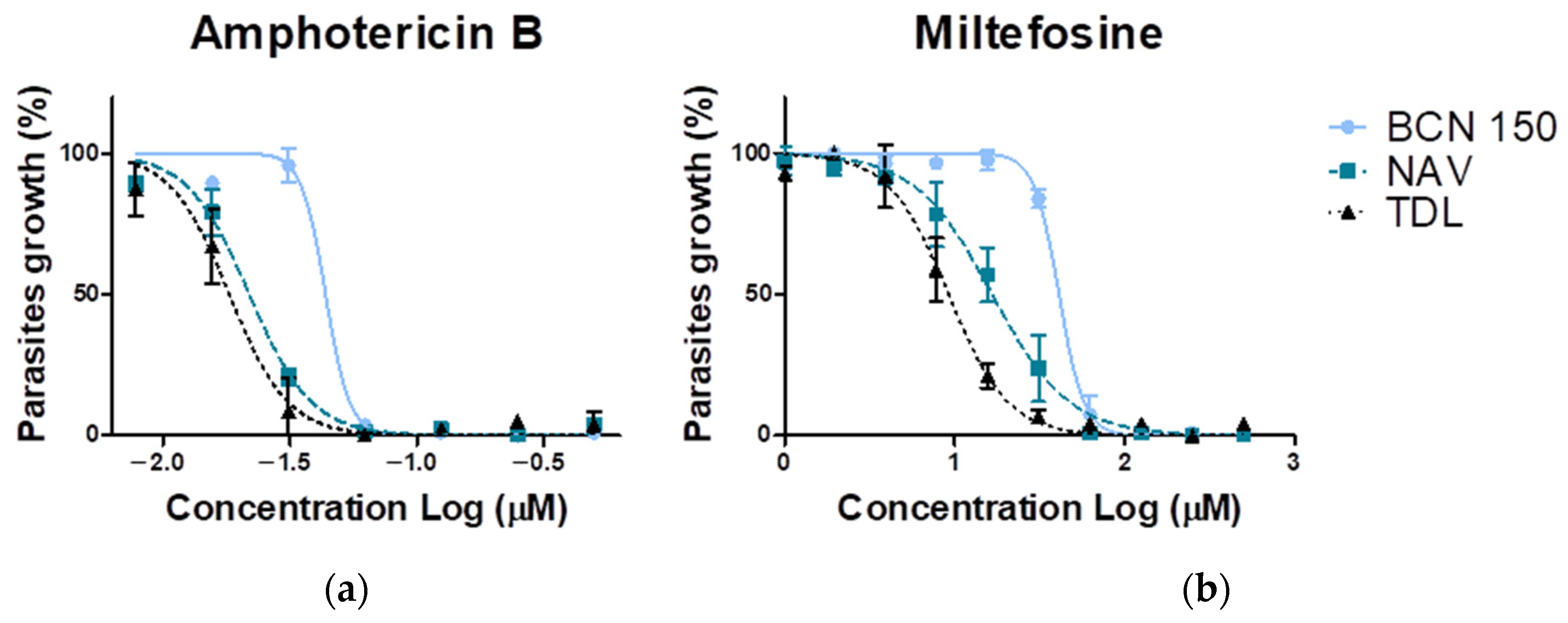
3.4. NAV and TDL Promastigote Parasites Were More Susceptible to Miltefosine and Amphotericin B
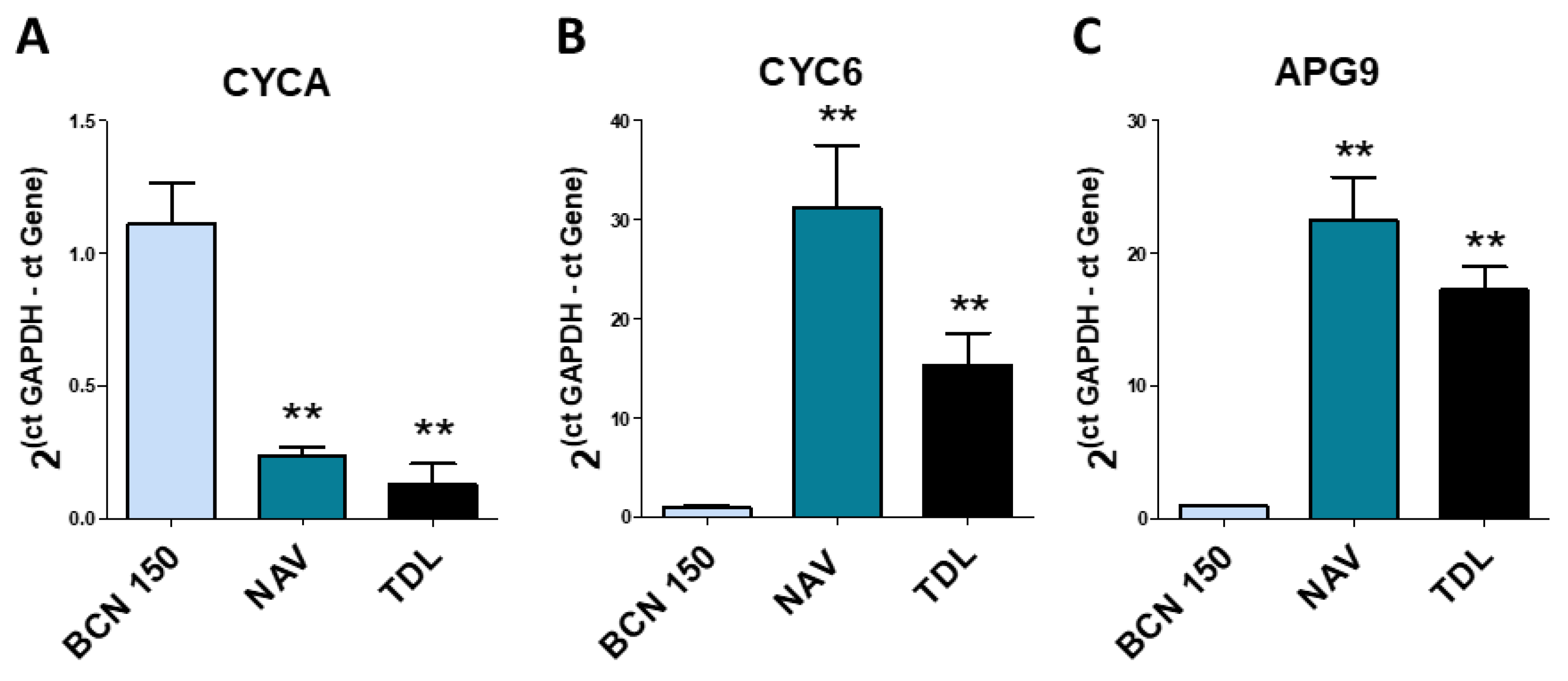
3.5. The Expression of Several Genes Was Different in the Promastigote Form of the Parasites
3.6. Both Isolates Exhibited a High In Vitro Infection Capacity
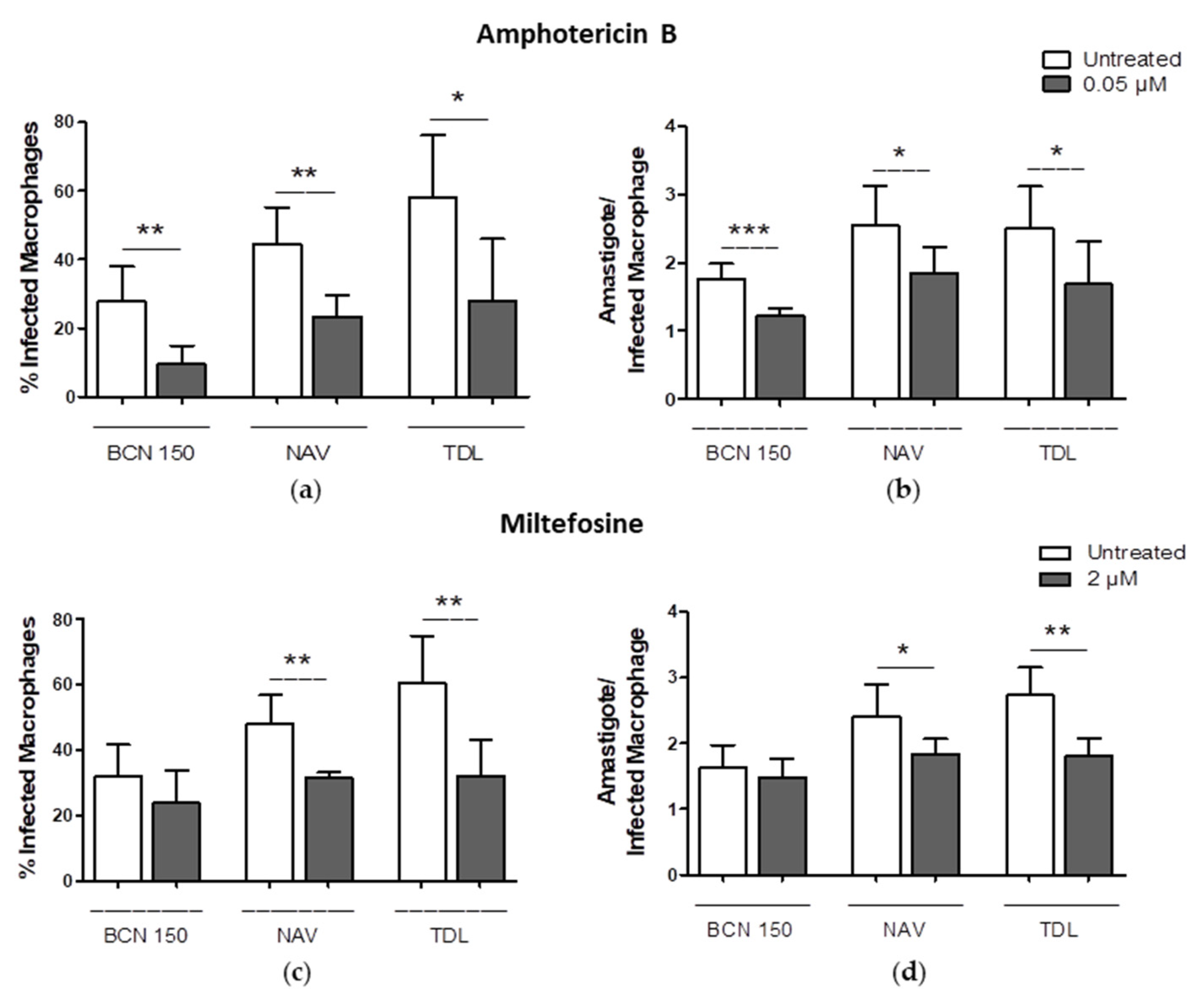
3.7. Amphotericin B and Miltefosine Were Active against Intracellular Amastigotes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO|Neglected Tropical Diseases. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 10 December 2021).
- Cohen, A.; Azas, N. Challenges and Tools for In Vitro Leishmania Exploratory Screening in the Drug Development Process: An Updated Review. Pathogens 2021, 10, 1608. [Google Scholar] [CrossRef]
- King, L. Combating the Triple Threat: The Need for a One Health Approach. Microbiol. Spectr. 2013, 1, OH-0012-2012. [Google Scholar] [CrossRef]
- Ruiz-Postigo, J.A.; Jain, S.; Mikhailov, A.; Maia-Elkhoury, A.N.; Valadas, S.; Warusavithana, S.; Osman, M.; Lin, Z.; Beshah, A.; Yajima, A.; et al. Global Leishmaniasis Surveillance: 2019–2020, a Baseline for the 2030 Roadmap. Wkly. Epidemiol. Rec. 2021, 35, 401–419. [Google Scholar]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Rubio, C.; Larrea, E.; Guerrero, J.P.; Herrero, E.S.; Gamboa, I.; Berrio, C.; Plano, D.; Amin, S.; Sharma, A.K.; Nguewa, P.A. Leishmanicidal Activity of Isoselenocyanate Derivatives. Antimicrob. Agents Chemother. 2019, 63, e00904-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemayehu, B.; Alemayehu, M. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Health Sci. J. 2017, 11, 519. [Google Scholar] [CrossRef]
- Miró, G.; López-Vélez, R. Clinical Management of Canine Leishmaniosis versus Human Leishmaniasis Due to Leishmania infantum: Putting “One Health” Principles into Practice. Vet. Parasitol. 2018, 254, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Gherasim, A.; Jimenez, B.C.; Granados, M.; San Martín, J.V.; Aparicio, P. Epidemiological Changes in Leishmaniasis in Spain According to Hospitalization-Based Records, 1997–2011: Raising Awareness towards Leishmaniasis in Non-HIV Patients. PLoS Negl. Trop. Dis. 2015, 9, e0003594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ready, P.D. Leishmaniasis Emergence in Europe. Eurosurveillance 2010, 15, 29–39. [Google Scholar] [CrossRef]
- Monge-Maillo, B.; Norman, F.F.; Cruz, I.; Alvar, J.; López-Vélez, R. Visceral Leishmaniasis and HIV Coinfection in the Mediterranean Region. PLoS Negl. Trop. Dis. 2014, 8, e3021. [Google Scholar] [CrossRef]
- Berriatua, E.; Maia, C.; Conceição, C.; Özbel, Y.; Töz, S.; Baneth, G.; Pérez-Cutillas, P.; Ortuño, M.; Muñoz, C.; Jumakanova, Z.; et al. Leishmaniases in the European Union and Neighboring Countries. Emerg. Infect. Dis. 2021, 27, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, M.; Rugna, G.; Calzolari, M.; Bellini, R.; Albieri, A.; Angelini, P.; Cagarelli, R.; Landini, M.P.; Charrel, R.N.; Varani, S. Phlebotomine Sand Fly–Borne Pathogens in the Mediterranean Basin: Human Leishmaniasis and Phlebovirus Infections. PLoS Negl. Trop. Dis. 2017, 11, e0005660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Rutte, E.A.; van Straten, R.; Overgaauw, P.A.M. Awareness and Control of Canine Leishmaniosis: A Survey among Spanish and French Veterinarians. Vet. Parasitol. 2018, 253, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hide, M.; Bañuls, A.L. Species-Specific PCR Assay for L. infantum/L. donovani Discrimination. Acta Trop. 2006, 100, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, R.; Montoya, A.; Cruz, I.; Fernández, C.; Martín, O.; Checa, R.; Chicharro, C.; Migueláñez, S.; Marino, V.; Miró, G. Latest Trends in Leishmania infantum Infection in Dogs in Spain, Part I: Mapped Seroprevalence and Sand Fly Distributions. Parasites Vectors 2020, 13, 204. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Cotrina, J.; Iniesta, V.; Belinchón-Lorenzo, S.; Muñoz-Madrid, R.; Serrano, F.; Parejo, J.C.; Gómez-Gordo, L.; Soto, M.; Alonso, C.; Gómez-Nieto, L.C. Experimental Model for Reproduction of Canine Visceral Leishmaniosis by Leishmania infantum. Vet. Parasitol. 2013, 192, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Mas, A.; Martínez-Rodrigo, A.; Orden, J.A.; Molina, R.; Jiménez, M.; Jiménez, M.; Carrión, J.; Domínguez-Bernal, G. Properties of Virulence Emergence of Leishmania infantum Isolates from Phlebotomus perniciosus Collected during the Human Leishmaniosis Outbreak in Madrid, Spain. Hepatic Histopathology and Immunological Parameters as Virulence Markers in the Mouse Model. Transbound. Emerg. Dis. 2021, 68, 704–714. [Google Scholar] [CrossRef]
- Calvo, A.; Moreno, E.; Larrea, E.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Berberine-Loaded Liposomes for the Treatment of Leishmania infantum-Infected BALB/c Mice. Pharmaceutics 2020, 12, 858. [Google Scholar] [CrossRef]
- Medina-Acosta, E.; Cross, G.A.M. Rapid Isolation of DNA from Trypanosomatid Protozoa Using a Simple “mini-Prep” Procedure. Mol. Biochem. Parasitol. 1993, 59, 327–329. [Google Scholar] [CrossRef]
- Oshaghi, M.A.; Ravasan, N.M.; Hide, M.; Javadian, E.A.; Rassi, Y.; Sedaghat, M.M.; Mohebali, M.; Hajjaran, H. Development of Species-Specific PCR and PCR-Restriction Fragment Length Polymorphism Assays for L. infantum/L. donovani Discrimination. Exp. Parasitol. 2009, 122, 61–65. [Google Scholar] [CrossRef]
- Alexander, J.; Coombs, G.; Mottram, J. Leishmania Mexicana Cysteine Proteinase-Deficient Mutants Have Attenuated Virulence for Mice and Potentiate a Th1 Response. J Immunol. 1998, 161, 6794–6801. [Google Scholar] [CrossRef] [PubMed]
- Sukmee, T.; Siripattanapipong, S.; Mungthin, M.; Worapong, J.; Rangsin, R.; Samung, Y.; Kongkaew, W.; Bumrungsana, K.; Chanachai, K.; Apiwathanasorn, C.; et al. A Suspected New Species of Leishmania, the Causative Agent of Visceral Leishmaniasis in a Thai Patient. Int. J. Parasitol. 2008, 38, 617–622. [Google Scholar] [CrossRef]
- Alanazi, A.D.; Alouffi, A.S.; Alyousif, M.S.; Rahi, A.A.; Ali, M.A.; Abdullah, H.H.A.M.; Brayner, F.A.; Mendoza-Roldan, J.A.; Bezerra-Santos, M.A.; Otranto, D. Molecular Characterization of Leishmania Species from Stray Dogs and Human Patients in Saudi Arabia. Parasitol. Res. 2021, 120, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Rotureau, B.; Ravel, C.; Couppié, P.; Pratlong, F.; Nacher, M.; Dedet, J.P.; Carme, B. Use of PCR-Restriction Fragment Length Polymorphism Analysis to Identify the Main New World Leishmania Species and Analyze Their Taxonomic Properties and Polymorphism by Application of the Assay to Clinical Samples. J. Clin. Microbiol. 2006, 44, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfurt, J.; Nasereddin, A.; Niederwieser, I.; Jaffe, C.L.; Beck, H.P.; Felger, I. Identification and Differentiation of Leishmania Species in Clinical Samples by PCR Amplification of the Miniexon Sequence and Subsequent Restriction Fragment Length Polymorphism Analysis. J. Clin. Microbiol. 2003, 41, 3147–3153. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, A.M.; Fraga, J.; Monzote, L.; Montano, I.; De Doncker, S.; Dujardin, J.C.; Van Der Auwera, G. Heat-Shock Protein 70 PCR-RFLP: A Universal Simple Tool for Leishmania Species Discrimination in the New and Old World. Parasitology 2010, 137, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Fraga, J.; Montalvo, A.M.; Van der Auwera, G.; Maes, I.; Dujardin, J.C.; Requena, J.M. Evolution and Species Discrimination According to the Leishmania Heat-Shock Protein 20 Gene. Infect. Genet. Evol. 2013, 18, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Patino, L.H.; Muskus, C.; Ramírez, J.D. Transcriptional Responses of Leishmania (Leishmania) Amazonensis in the Presence of Trivalent Sodium Stibogluconate. Parasites Vectors 2019, 12, 348. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Sen, A.; Das, P.; Saha, P. Leishmania Donovani Cyclin 1 (LdCyc1) Forms a Complex with Cell Cycle Kinase Subunit CRK3 (LdCRK3) and Is Possibly Involved in S-Phase-Related Activities. FEMS Microbiol. Lett. 2006, 256, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Espada, C.R.; Levatti, E.V.C.; Boité, M.C.; Lamounier, D.; Alvar, J.; Cupolillo, E.; Costa, C.H.N.; Rode, J.; Uliana, S.R.B. In Vitro Susceptibility to Miltefosine of Leishmania infantum (syn. L. chagasi) Isolates from Different Geographical Areas in Brazil. Microorganisms 2021, 9, 1228. [Google Scholar] [CrossRef]
- Keighobadi, M.; Emami, S.; Lagzian, M.; Fakhar, M.; Rafiei, A.; Valadan, R. Molecular Modeling and Structural Stability of Wild-Type and Mutant CYP51 from Leishmania major: In Vitro and In Silico Analysis of a Laboratory Strain. Molecules 2018, 23, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| GAPDH | ACCACCATCCACTCCTACA | CGTGCTCGGGATGATGTTTA |
| CYCA | CCCCAACACCGCTGACTAAT | TCCGACTGGCGGCTCATGTA |
| CYC6 | AGTACCCTGCACGCCTACTA | TTGTTGTTGGCGCAGGAAAG |
| APG9 | TCACTCTCGTTTGGTGGCTC | AAAGGTCGTCGTGATGTGCT |
| Compound | BCN 150 | NAV | TDL |
|---|---|---|---|
| EC50 (µM) | EC50 (µM) | EC50 (µM) | |
| Amphotericin B | 0.043 ± 0.002 | 0.02 ± 0.01 ** | 0.018 ± 0.002 ** |
| Miltefosine | 41.1 ± 3.5 | 16.7 ± 3.3 ** | 9.2 ± 1.7 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burguete-Mikeo, A.; Fernández-Rubio, C.; Peña-Guerrero, J.; El-Dirany, R.; Gainza, L.; Carasa Buj, B.; Nguewa, P.A. Characterization of Leishmania Parasites Isolated from Naturally Infected Mammals. Animals 2023, 13, 2153. https://doi.org/10.3390/ani13132153
Burguete-Mikeo A, Fernández-Rubio C, Peña-Guerrero J, El-Dirany R, Gainza L, Carasa Buj B, Nguewa PA. Characterization of Leishmania Parasites Isolated from Naturally Infected Mammals. Animals. 2023; 13(13):2153. https://doi.org/10.3390/ani13132153
Chicago/Turabian StyleBurguete-Mikeo, Aroia, Celia Fernández-Rubio, José Peña-Guerrero, Rima El-Dirany, Leonardo Gainza, Belen Carasa Buj, and Paul A. Nguewa. 2023. "Characterization of Leishmania Parasites Isolated from Naturally Infected Mammals" Animals 13, no. 13: 2153. https://doi.org/10.3390/ani13132153
APA StyleBurguete-Mikeo, A., Fernández-Rubio, C., Peña-Guerrero, J., El-Dirany, R., Gainza, L., Carasa Buj, B., & Nguewa, P. A. (2023). Characterization of Leishmania Parasites Isolated from Naturally Infected Mammals. Animals, 13(13), 2153. https://doi.org/10.3390/ani13132153






