Predicting Suitable Areas for African Swine Fever Outbreaks in Wild Boars in South Korea and Their Implications for Managing High-Risk Pig Farms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Status of ASF Outbreaks and Dataset Construction
2.2. Environmental Variables
2.3. Data Analysis
2.3.1. Predicting Suitable Areas for ASF Outbreaks
2.3.2. Predicting the Shortest Path for an ASF Outbreaks
2.3.3. Pig Farm Density
3. Results
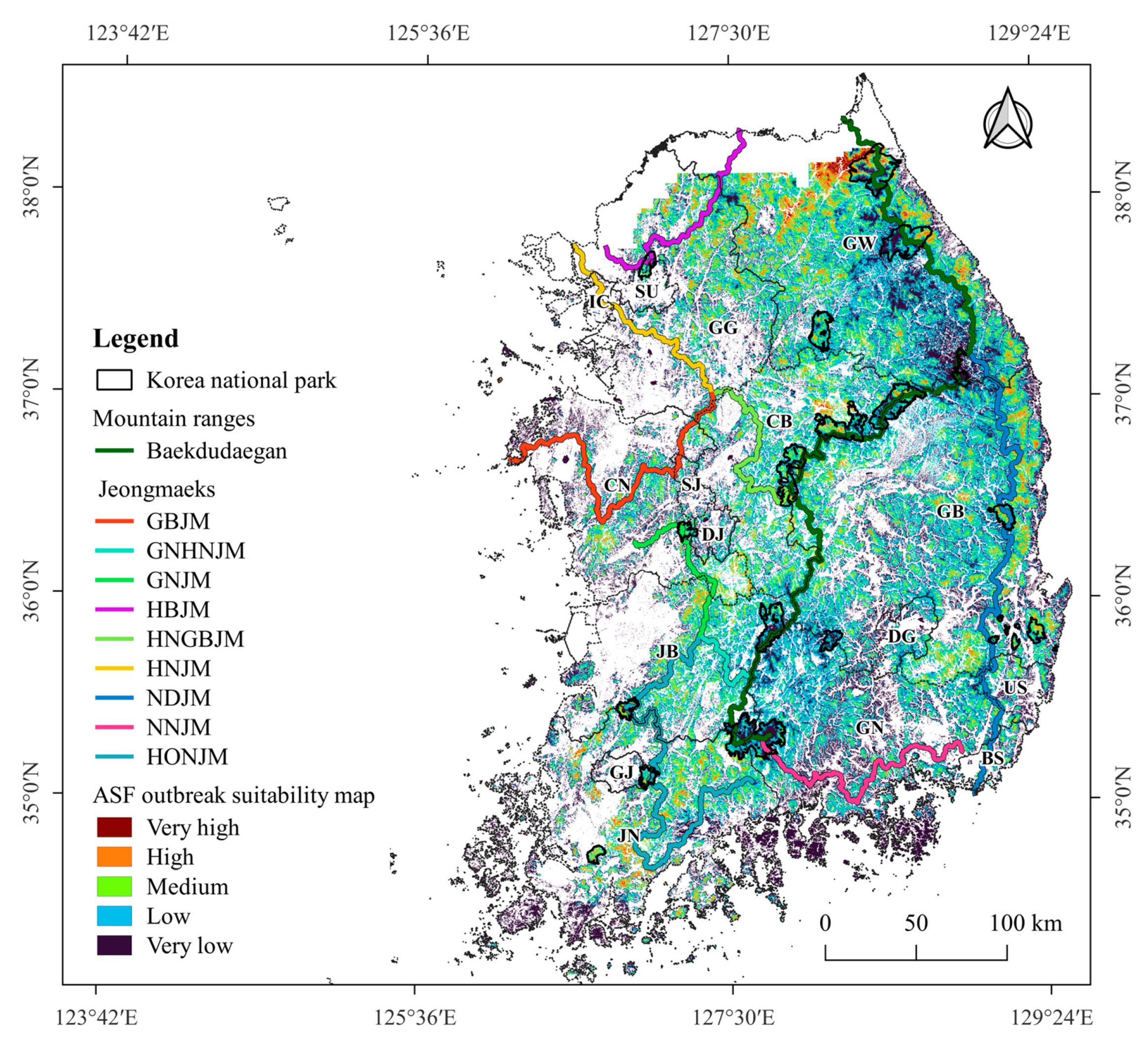
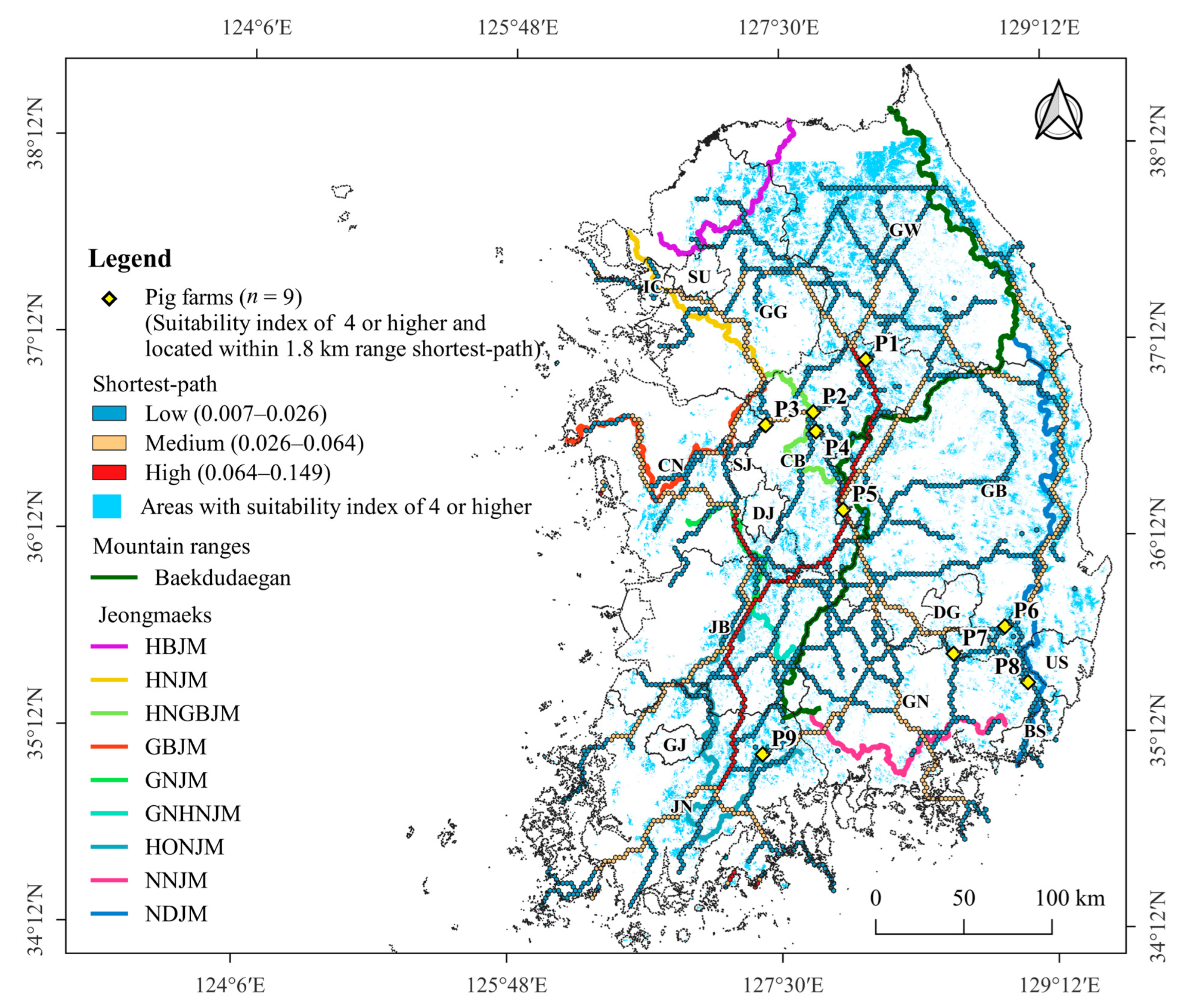
3.1. Suitable Areas for ASF Outbreaks
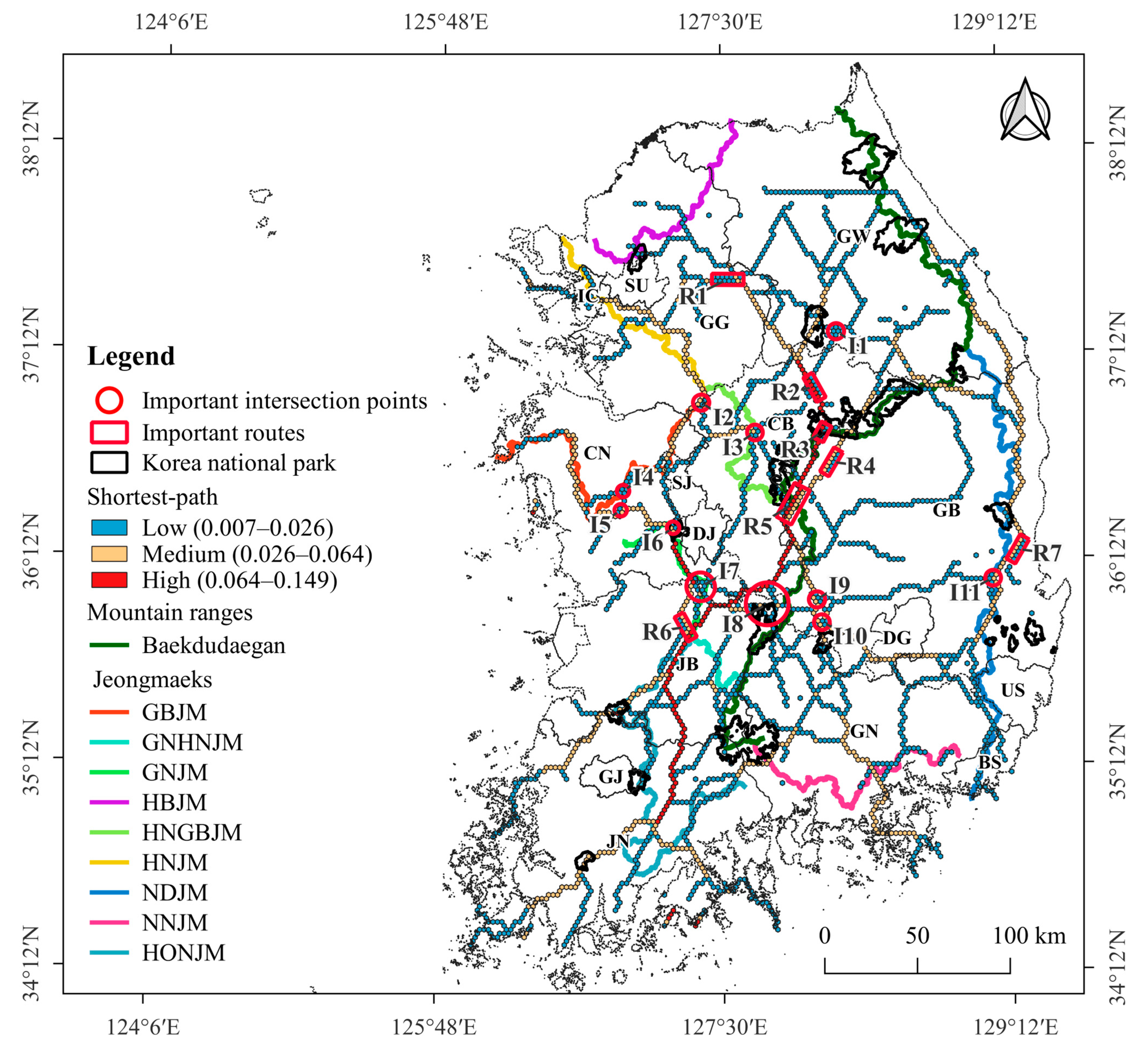
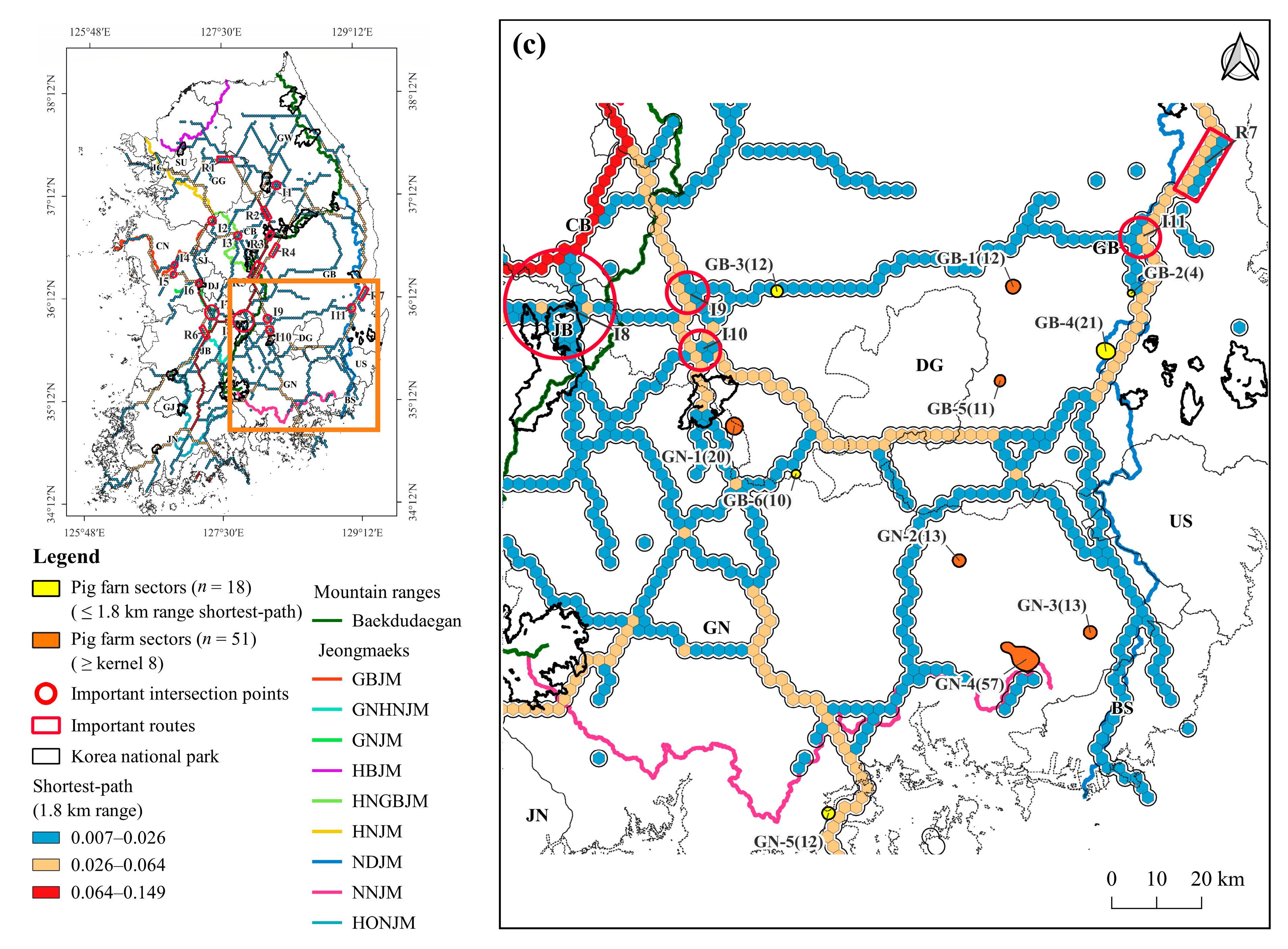
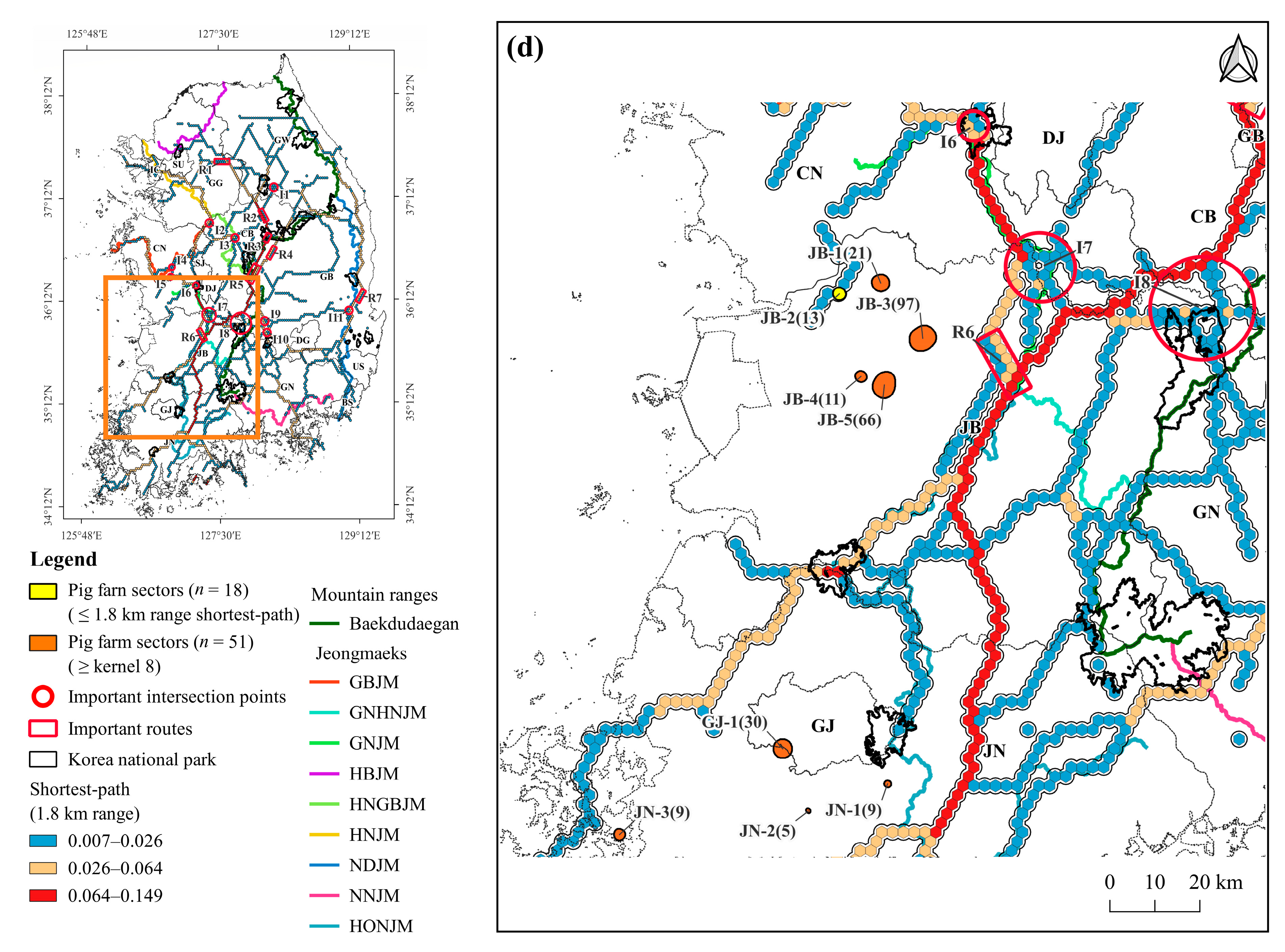
3.2. Shortest-Path of ASF Outbreak
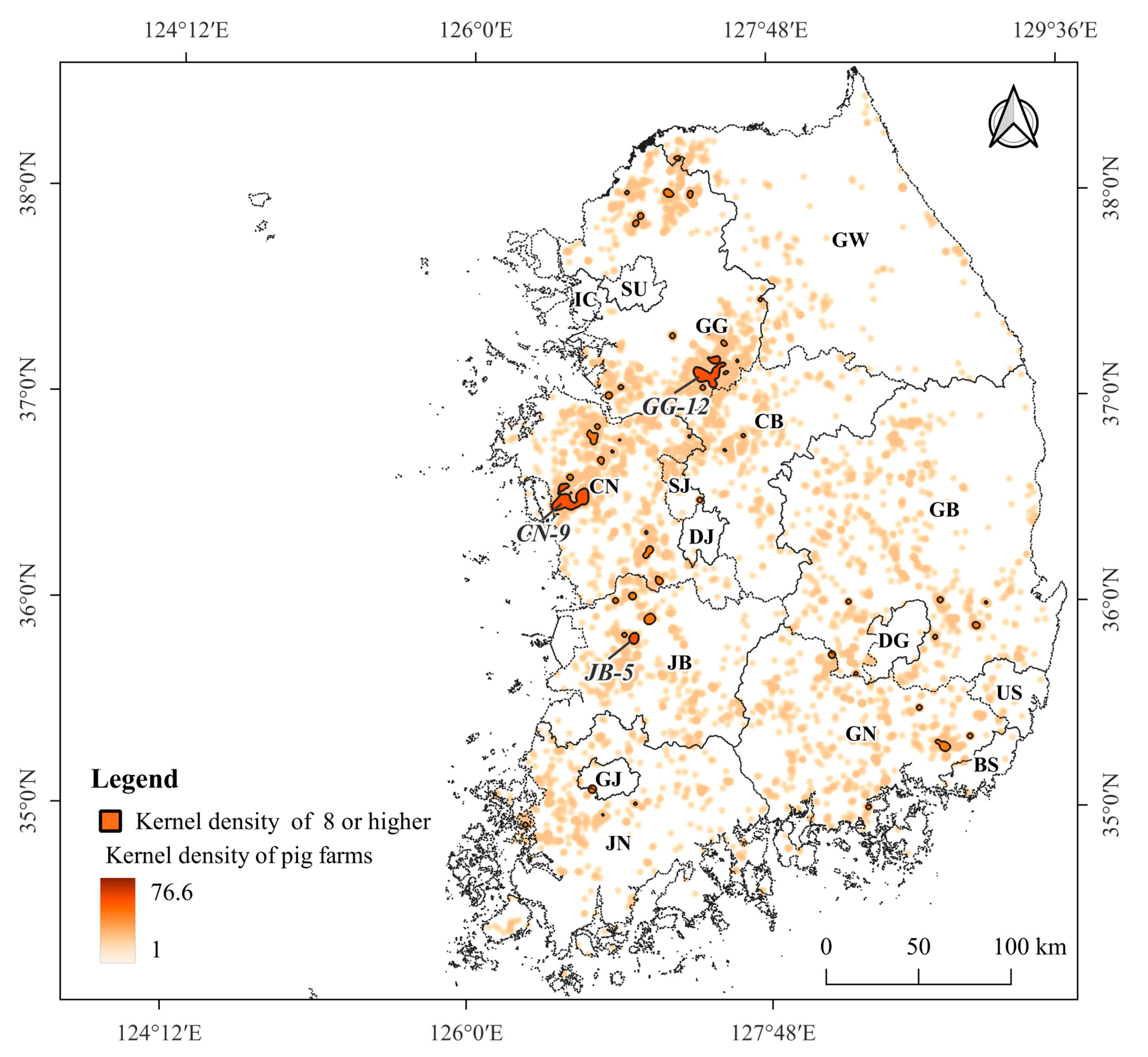
3.3. Density of Pig Farms
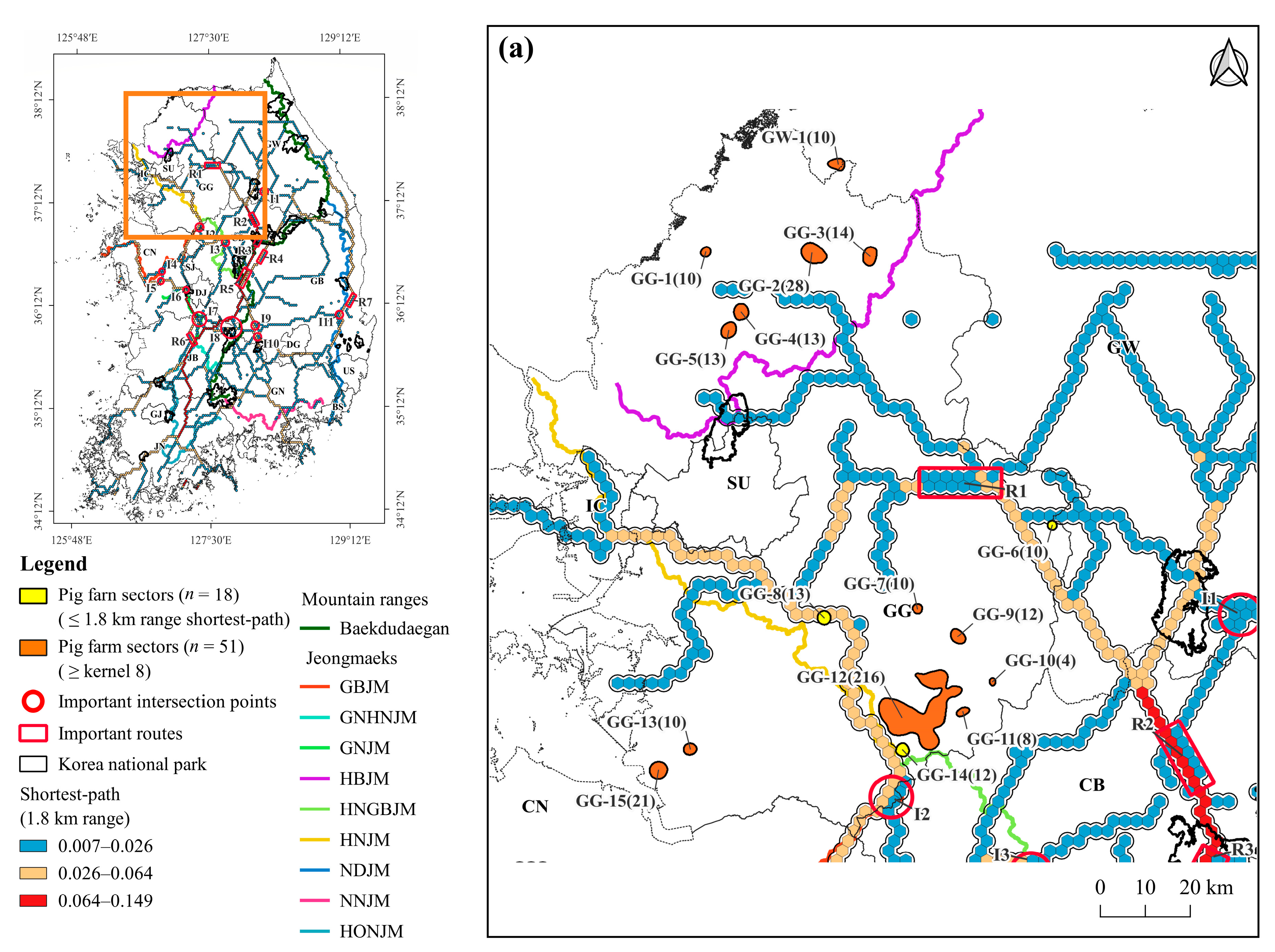
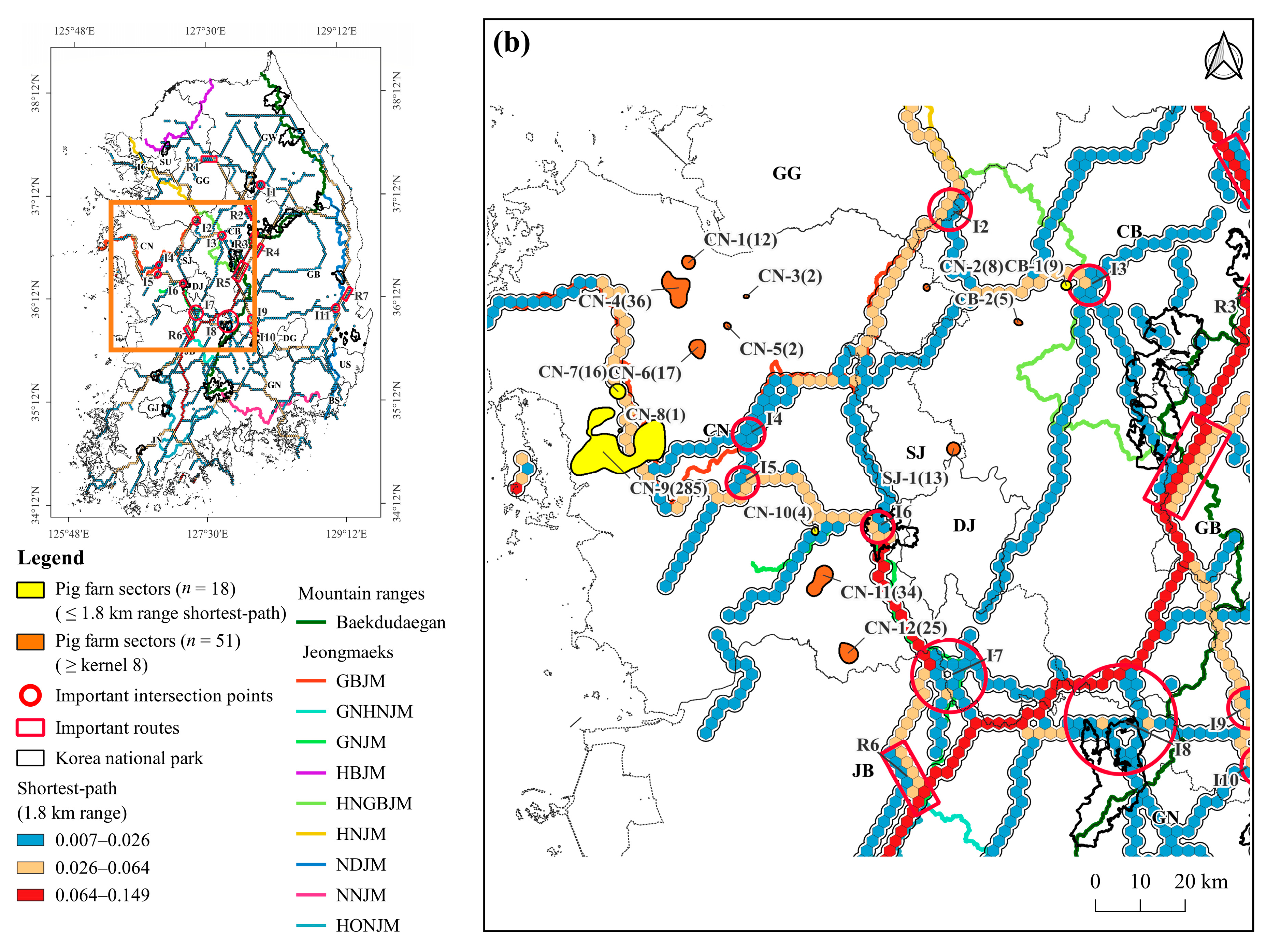
3.4. Pig Farms at High-Risk of ASF Outbreak
4. Discussion
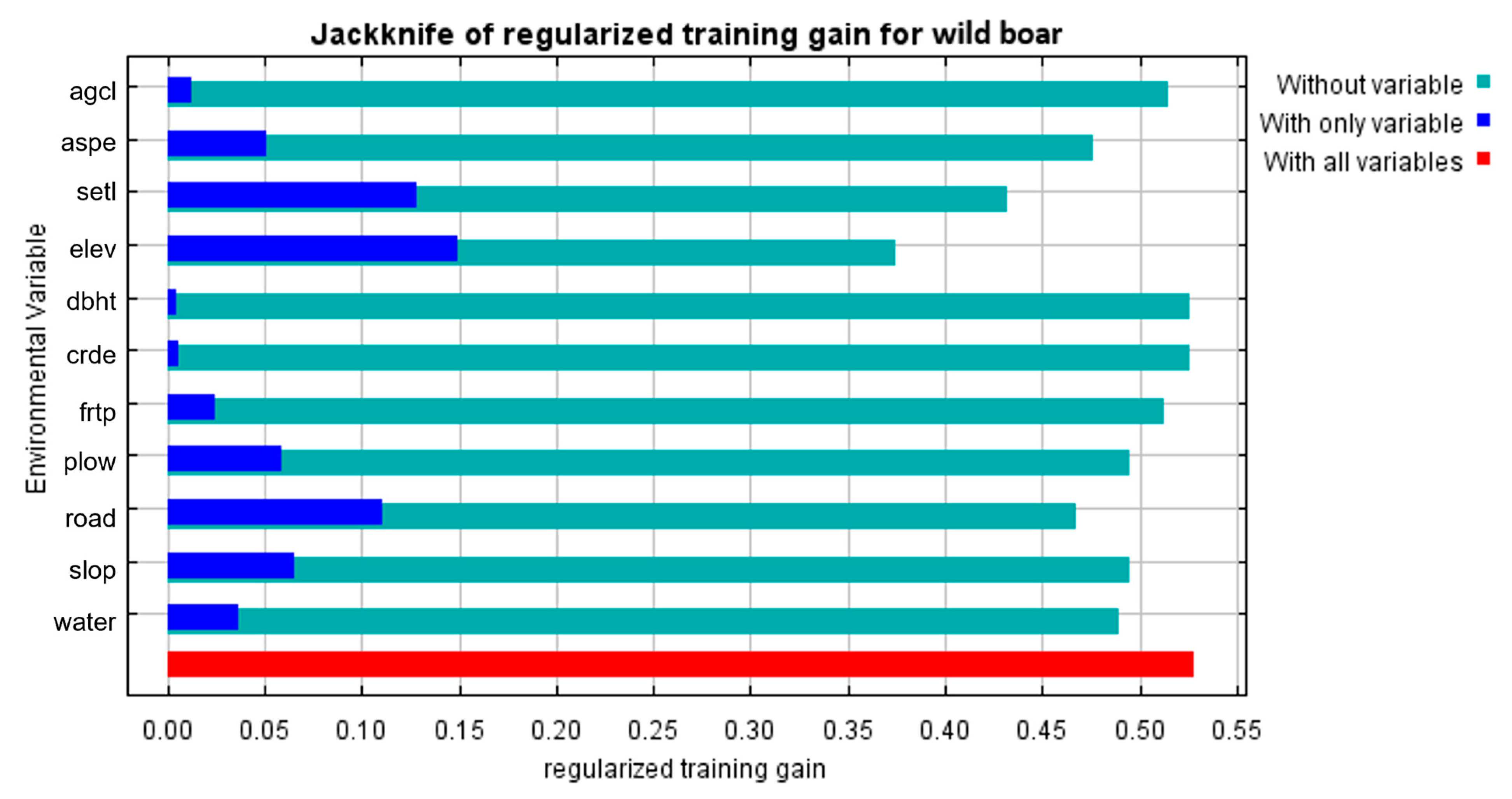
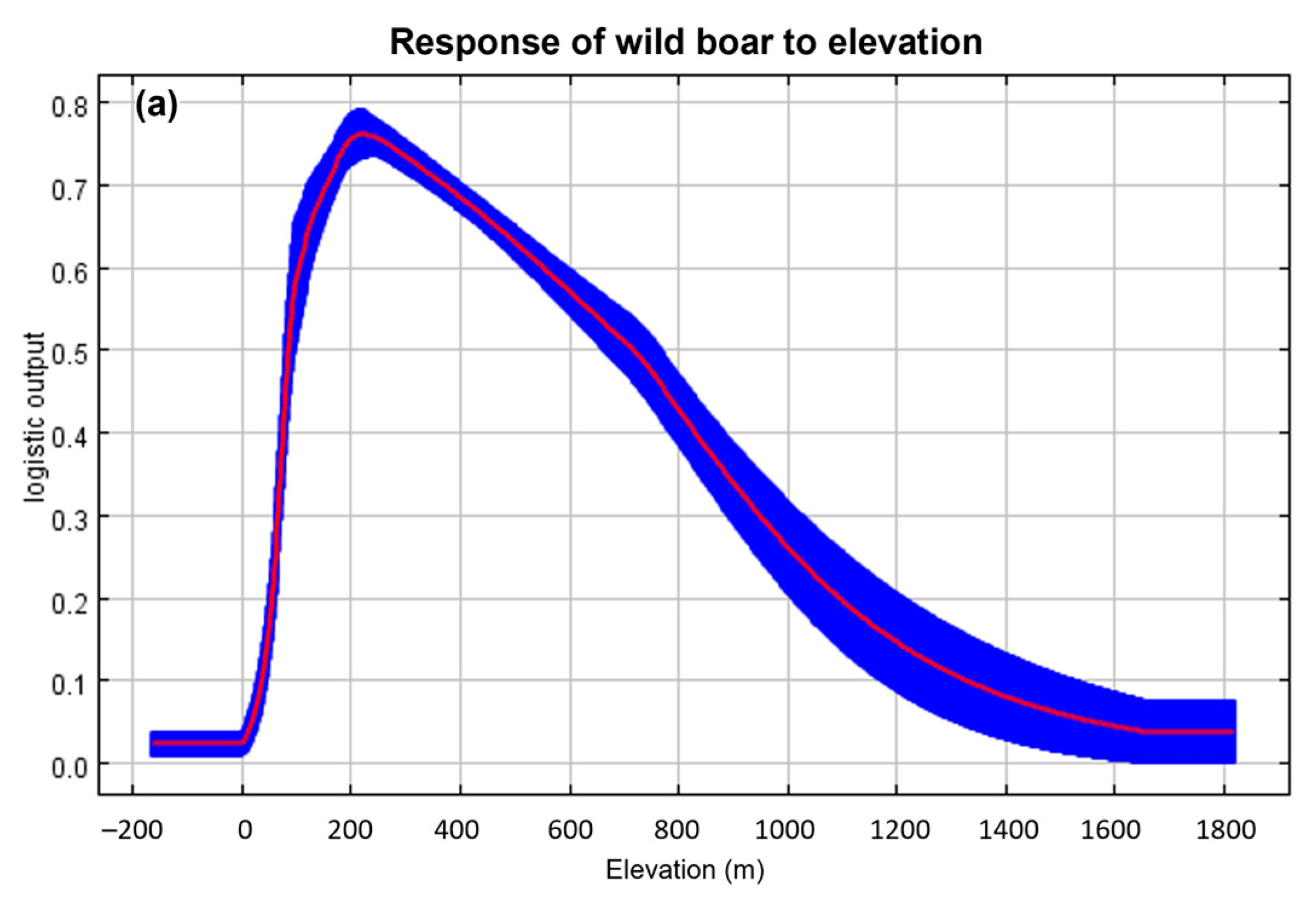
4.1. Suitable Areas for ASF Outbreaks in Wild Boars
4.2. Shortest-Path for ASF Outbreaks in Wild Boars
4.3. Pig Farming Sectors at High Risk of ASF Spillover and Its Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, J.; Vergne, T.; Pak, S.; Kim, E. Modelling the Spatial Distribution of ASF-Positive wild boar carcasses in South Korea using 2019–2020 national surveillance data. Animals 2021, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Blome, S.; Malogolovkin, A.; Parilov, S.; Kolbasov, D.; Teifke, J.P.; Beer, M. Characterization of African swine fever virus Caucasus isolate in European wild boars. Emerg. Infect. Dis. 2011, 17, 2342. [Google Scholar] [CrossRef] [PubMed]
- Guinat, C.; Reis, A.L.; Netherton, C.L.; Goatley, L.; Pfeiffer, D.U.; Dixon, L. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Vet. Res. 2014, 45, 93. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, J.; Guinat, C.; Beer, M.; Pronin, V.; Tauscher, K.; Petrov, A.; Keil, G.; Blome, S. Course and transmission characteristics of oral low-dose infection of domestic pigs and European wild boar with a Caucasian African swine fever virus isolate. Arch. Virol. 2015, 160, 1657–1667. [Google Scholar] [CrossRef]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Ståhl, K. Epidemiological considerations on African swine fever in Europe 2014–2018. Porc. Health Manag. 2019, 5, 6. [Google Scholar] [CrossRef]
- Gallardo, C.; Soler, A.; Nieto, R.; Sánchez, M.A.; Martins, C.; Pelayo, V.; Carrascosa, A.; Revilla, Y.; Simón, A.; Briones, V. Experimental transmission of African swine fever (ASF) low virulent isolate NH/P68 by surviving pigs. Transbound. Emerg. Dis. 2015, 62, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Gogin, A.; Gerasimov, V.; Malogolovkin, A.; Kolbasov, D. African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus Res. 2013, 173, 198–203. [Google Scholar] [CrossRef]
- Pejsak, Z.; Niemczuk, K.; Frant, M.; Pomorska-Mól, M.; Ziętek-Barszcz, A.; Bocian, Ł.; Łyjak, M.; Borowska, D.; Woźniakowski, G. Four years of African swine fever in Poland. New insights into epidemiology and prognosis of future disease spread. Pol. J. Vet. Sci. 2018, 21, 835–841. [Google Scholar]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African swine fever status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current state of global African swine fever vaccine development under the prevalence and transmission of ASF in China. Vaccines 2020, 8, 531. [Google Scholar] [CrossRef]
- Wyckoff, A.C.; Henke, S.E.; Campbell, T.A.; Hewitt, D.G.; VerCauteren, K.C. Feral swine contact with domestic swine: A serologic survey and assessment of potential for disease transmission. J. Wildl. Dis. 2009, 45, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oganesyan, A.S.; Petrova, O.N.; Korennoy, F.I.; Bardina, N.S.; Gogin, A.E.; Dudnikov, S.A. African swine fever in the Russian Federation: Spatio-temporal analysis and epidemiological overview. Virus Res. 2013, 173, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, H.; Schulz, K.; Conraths, F.J.; Sauter-Louis, C. A review of environmental risk factors for African Swine Fever in European wild boar. Animals 2021, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Oļševskis, E.; Guberti, V.; Seržants, M.; Westergaard, J.; Gallardo, C.; Rodze, I.; Depner, K. African swine fever virus introduction into the EU in 2014: Experience of Latvia. Res. Vet. Sci. 2016, 105, 28–30. [Google Scholar] [CrossRef]
- Guinat, C.; Gogin, A.; Blome, S.; Keil, G.; Pollin, R.; Pfeiffer, D.U.; Dixon, L. Transmission routes of African swine fever virus to domestic pigs: Current knowledge and future research directions. Vet. Rec. 2016, 178, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Boklund, A.; Dhollander, S.; Chesnoiu Vasile, T.; Abrahantes, J.C.; Bøtner, A.; Gogin, A.; Gonzalez Villeta, L.C.; Gortázar, C.; More, S.J.; Papanikolaou, A. Risk factors for African swine fever incursion in Romanian domestic farms during 2019. Sci. Rep. 2020, 10, 10215. [Google Scholar] [CrossRef]
- Nurmoja, I.; Mõtus, K.; Kristian, M.; Niine, T.; Schulz, K.; Depner, K.; Viltrop, A. Epidemiological analysis of the 2015–2017 African swine fever outbreaks in Estonia. Prev. Vet. Med. 2020, 181, 104556. [Google Scholar] [CrossRef]
- Zani, L.; Dietze, K.; Dimova, Z.; Forth, J.H.; Denev, D.; Depner, K.; Alexandrov, T. African swine fever in a Bulgarian backyard farm—A case report. Vet. Sci. 2019, 6, 94. [Google Scholar] [CrossRef] [Green Version]
- Olesen, A.S.; Belsham, G.J.; Bruun Rasmussen, T.; Lohse, L.; Bødker, R.; Halasa, T.; Boklund, A.; Bøtner, A. Potential routes for indirect transmission of African swine fever virus into domestic pig herds. Transbound. Emerg. Dis. 2020, 67, 1472–1484. [Google Scholar] [CrossRef]
- Gervasi, V.; Marcon, A.; Guberti, V. Estimating the risk of environmental contamination by forest users in African Swine Fever endemic areas. Acta Vet. Scand. 2022, 64, 16. [Google Scholar] [CrossRef]
- Probst, C.; Gethmann, J.; Amler, S.; Globig, A.; Knoll, B.; Conraths, F.J. The potential role of scavengers in spreading African swine fever among wild boar. Sci. Rep. 2019, 9, 11450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco-Garcia, R.; Barroso, P.; Perez-Olivares, J.; Montoro, V.; Vicente, J. Consumption of big game remains by scavengers: A potential risk as regards disease transmission in central Spain. Front. Vet. Sci. 2018, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.J.; Han, S.H.; Park, J.Y.; Kim, N.H.; Namgung, H.; Oh, Y.; Park, Y.C. Wildlife as Potential Vectors of African Swine Fever Virus. J. For. Environ. Sci. 2022, 38, 55–63. [Google Scholar]
- EFSA Panel on Animal Health and Welfare (AHAW) Scientific opinion on African swine fever. EFSA J. 2014, 12, 3628.
- CFSPH (The Centre for Food Security and Public Health). African Swine Fever. Available online: https://www.cfsph.iastate.edu/diseaseinfo/disease/?disease=african-swine-fever&lang=en (accessed on 10 December 2021).
- Kukielka, E.; Barasona, J.A.; Cowie, C.E.; Drewe, J.A.; Gortazar, C.; Cotarelo, I.; Vicente, J. Spatial and temporal interactions between livestock and wildlife in South Central Spain assessed by camera traps. Prev. Vet. Med. 2013, 112, 213–221. [Google Scholar] [CrossRef]
- Probst, C.; Globig, A.; Knoll, B.; Conraths, F.J.; Depner, K. Behaviour of free ranging wild boar towards their dead fellows: Potential implications for the transmission of African swine fever. R. Soc. Open Sci. 2017, 4, 170054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend Peterson, A.; Papeş, M.; Eaton, M. Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Blackburn, J.K. Integrating geographic information systems and ecological niche modeling into disease ecology: A case study of Bacillus anthracis in the United States and Mexico. In Emerging and Endemic Pathogens: Advances in Surveillance, Detection and Identification; Springer: Dordrecht, Germany, 2010; pp. 59–88. [Google Scholar]
- Banerjee, A.K.; Mukherjee, A.; Guo, W.; Liu, Y.; Huang, Y. Spatio-temporal patterns of climatic niche dynamics of an invasive plant Mikania micrantha Kunth and its potential distribution under projected climate change. Front. Ecol. Evol. 2019, 7, 291. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, X.; An, Q.; Sun, Z.; Wang, H. Ecological niche modeling based on ensemble algorithms to predicting current and future potential distribution of African swine fever virus in China. Sci. Rep. 2022, 12, 15614. [Google Scholar] [CrossRef]
- Chavy, A.; Ferreira Dales Nava, A.; Luz, S.L.B.; Ramírez, J.D.; Herrera, G.; Vasconcelos dos Santos, T.; Ginouves, M.; Demar, M.; Prévot, G.; Guégan, J. Ecological niche modelling for predicting the risk of cutaneous leishmaniasis in the Neotropical moist forest biome. PLoS Negl. Trop. Dis. 2019, 13, e0007629. [Google Scholar] [CrossRef] [Green Version]
- Ron, S.R. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World 1. Biotropica J. Biol. Conserv. 2005, 37, 209–221. [Google Scholar]
- Blackburn, J.K.; McNyset, K.M.; Curtis, A.; Hugh-Jones, M.E. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. Am. J. Trop. Med. Hyg. 2007, 77, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Mullins, J.C.; Garofolo, G.; Van Ert, M.; Fasanella, A.; Lukhnova, L.; Hugh-Jones, M.E.; Blackburn, J.K. Ecological niche modeling of Bacillus anthracis on three continents: Evidence for genetic-ecological divergence? PLoS ONE 2013, 8, e72451. [Google Scholar] [CrossRef] [PubMed]
- Haverkost, T.R.; Gardner, S.L.; Townsend Peterson, A. Predicting the distribution of a parasite using the ecological niche model, GARP. Rev. Mex. Biodivers. 2010, 81, 895–902. [Google Scholar]
- Peterson, A.T.; Sánchez-Cordero, V.; Beard, C.B.; Ramsey, J.M. Ecologic niche modeling and potential reservoirs for Chagas disease, Mexico. Emerg. Infect. Dis. 2002, 8, 662. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.A.; Xiao, X.; Peterson, A.T. Continent-wide association of H5N1 outbreaks in wild and domestic birds in Europe. Geospat. Health 2011, 5, 247. [Google Scholar] [CrossRef] [Green Version]
- Sallam, M.F.; Michaels, S.R.; Riegel, C.; Pereira, R.M.; Zipperer, W.; Lockaby, B.G.; Koehler, P.G. Spatio-temporal distribution of vector-host contact (VHC) ratios and ecological niche modeling of the West Nile virus mosquito vector, Culex quinquefasciatus, in the City of New Orleans, LA, USA. Int. J. Environ. Res. Public Health 2017, 14, 892. [Google Scholar] [CrossRef] [Green Version]
- Pratumchart, K.; Suwannatrai, K.; Sereewong, C.; Thinkhamrop, K.; Chaiyos, J.; Boonmars, T.; Suwannatrai, A.T. Ecological Niche Model based on Maximum Entropy for mapping distribution of Bithynia siamensis goniomphalos, first intermediate host snail of Opisthorchis viverrini in Thailand. Acta Trop. 2019, 193, 183–191. [Google Scholar] [CrossRef]
- Peterson, A.T.; Martínez-Campos, C.; Nakazawa, Y.; Martínez-Meyer, E. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 647–655. [Google Scholar] [CrossRef]
- Rogers, D.J. Models for vectors and vector-borne diseases. Adv. Parasitol. 2006, 62, 1–35. [Google Scholar]
- Adjemian, J.C.; Girvetz, E.H.; Beckett, L.; Foley, J.E. Analysis of Genetic Algorithm for Rule-Set Production (GARP) modeling approach for predicting distributions of fleas implicated as vectors of plague, Yersinia pestis, in California. J. Med. Entomol. 2006, 43, 93–103. [Google Scholar] [PubMed]
- Moffett, A.; Shackelford, N.; Sarkar, S. Malaria in Africa: Vector species’ niche models and relative risk maps. PLoS ONE 2007, 2, e824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Thoisy, B.; Silva, N.I.O.; Sacchetto, L.; de Souza Trindade, G.; Drumond, B.P. Spatial epidemiology of yellow fever: Identification of determinants of the 2016–2018 epidemics and at-risk areas in Brazil. PLoS Negl. Trop. Dis. 2020, 14, e0008691. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Korennoy, F.I.; Gulenkin, V.M.; Malone, J.B.; Mores, C.N.; Dudnikov, S.A.; Stevenson, M.A. Spatio-temporal modeling of the African swine fever epidemic in the Russian Federation, 2007–2012. Spat. Spatio-Temporal Epidemiol. 2014, 11, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.; Brugger, K.; Rubel, F. Usutu virus induced mass mortalities of songbirds in Central Europe: Are habitat models suitable to predict dead birds in unsampled regions? Prev. Vet. Med. 2018, 159, 162–170. [Google Scholar] [CrossRef]
- Morelle, K.; Jezek, M.; Licoppe, A.; Podgorski, T. Deathbed choice by ASF-infected wild boar can help find carcasses. Transbound. Emerg. Dis. 2019, 66, 1821–1826. [Google Scholar] [CrossRef]
- Estrada, E.; Bodin, Ö. Using network centrality measures to manage landscape connectivity. Ecol. Appl. 2008, 18, 1810–1825. [Google Scholar] [CrossRef] [Green Version]
- Carroll, C.; McRAE, B.H.; Brookes, A. Use of linkage mapping and centrality analysis across habitat gradients to conserve connectivity of gray wolf populations in western North America. Conserv. Biol. 2012, 26, 78–87. [Google Scholar] [CrossRef]
- Silva, M.P.; Massi, K.G.; Negri, R.G.; Pedrosa, F. Jaguars and wild pigs indicate protected area connectivity in the south-east Atlantic Forest (Brazil). Environ. Conserv. 2023, 50, 22–30. [Google Scholar] [CrossRef]
- Walsh, M.G.; Mor, S.M. Interspecific network centrality, host range and early-life development are associated with wildlife hosts of Rift Valley fever virus. Transbound. Emerg. Dis. 2018, 65, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Borgatti, S.P.; Everett, M.G. A graph-theoretic perspective on centrality. Soc. Netw. 2006, 28, 466–484. [Google Scholar] [CrossRef]
- Chetkiewicz, C.B.; St. Clair, C.C.; Boyce, M.S. Corridors for conservation: Integrating pattern and process. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 317–342. [Google Scholar] [CrossRef] [Green Version]
- Cushman, S.A.; McRae, B.; Adriaensen, F.; Beier, P.; Shirley, M.; Zeller, K. Biological corridors and connectivity. In Key Topics in Conservation Biology 2; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 384–404. [Google Scholar]
- Almpanidou, V.; Mazaris, A.D.; Mertzanis, Y.; Avraam, I.; Antoniou, I.; Pantis, J.D.; Sgardelis, S.P. Providing insights on habitat connectivity for male brown bears: A combination of habitat suitability and landscape graph-based models. Ecol. Model. 2014, 286, 37–44. [Google Scholar] [CrossRef]
- Silk, M.J.; Croft, D.P.; Delahay, R.J.; Hodgson, D.J.; Boots, M.; Weber, N.; McDonald, R.A. Using social network measures in wildlife disease ecology, epidemiology, and management. Bioscience 2017, 67, 245–257. [Google Scholar] [CrossRef]
- Gao, S.; Xu, G.; Zeng, Z.; Lv, J.; Huang, L.; Wang, H.; Wang, X. Transboundary spread of peste des petits ruminants virus in western China: A prediction model. PLoS ONE 2021, 16, e0257898. [Google Scholar] [CrossRef]
- GIS Developer. Korea Map. Available online: http://www.gisdeveloper.co.kr/?p=2332 (accessed on 5 November 2021).
- Fekede, R.J.; Van Gils, H.; Huang, L.; Wang, X. High probability areas for ASF infection in China along the Russian and Korean borders. Transbound. Emerg. Dis. 2019, 66, 852–864. [Google Scholar] [CrossRef]
- Fekede, R.J.; HaoNing, W.; Hein, V.G.; Xiaolong, W. Could wild boar be the Trans-Siberian transmitter of African swine fever? Transbound. Emerg. Dis. 2021, 68, 1465–1475. [Google Scholar] [CrossRef]
- Tiwari, S.; Dhakal, T.; Tiwari, I.; Jang, G.; Oh, Y. Spatial proliferation of African swine fever virus in South Korea. PLoS ONE 2022, 17, e0277381. [Google Scholar] [CrossRef]
- Taylor, R.A.; Podgórski, T.; Simons, R.R.; Ip, S.; Gale, P.; Kelly, L.A.; Snary, E.L. Predicting spread and effective control measures for African swine fever—Should we blame the boars? Transbound. Emerg. Dis. 2021, 68, 397–416. [Google Scholar] [CrossRef]
- Díaz-Cao, J.M.; Grossmann, N.; Goodman, S.M.; Bosch, J.; Guis, H.; Rasamoelina, M.; Rakotoarivony, R.; Jori, F.; Martínez-López, B. A maximum entropy species distribution model to estimate the distribution of bushpigs on Madagascar and its implications for African Swine Fever. Transbound. Emerg. Dis. 2023, 2023, 7976252. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Forestry, and Fisheries. Disclose the Status of an Outbreak of Animal Spotted Fever (ASF). Available online: https://www.mafra.go.kr/FMD-AI2/2145/subview.do (accessed on 20 November 2022).
- Rondinini, C.; Di Marco, M.; Chiozza, F.; Santulli, G.; Baisero, D.; Visconti, P.; Hoffmann, M.; Schipper, J.; Stuart, S.N.; Tognelli, M.F. Global habitat suitability models of terrestrial mammals. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2633–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef] [Green Version]
- Préau, C.; Trochet, A.; Bertrand, R.; Isselin-Nondereu, F. Modeling potential distributions of three European amphibian species comparing ENFA and Maxent. Herpetol. Conserv. Biol. 2018, 13, 91–104. [Google Scholar]
- Sirois-Delisle, C.; Kerr, J.T. Climate change-driven range losses among bumblebee species are poised to accelerate. Sci. Rep. 2018, 8, 14464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Kushwaha, S.; Saran, S.; Xu, J.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, S.; Li, H.; Zhang, B.; Lv, J. Research on geographical environment unit division based on the method of natural breaks (Jenks). Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2013, 3, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.K. Kernel density estimation and K-means clustering to profile road accident hotspots. Accid. Anal. Prev. 2009, 41, 359–364. [Google Scholar] [CrossRef]
- MAFRA (Ministry of Agriculture, Food, and Rural Affairs). Standard Operating Procedure (SOP) for African Swine Fever. Available online: https://www.mafra.go.kr/home/5248/subview.do (accessed on 10 December 2021).
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.Y.; van Langevelde, F.; Honer, K.J.; Naguib, M.; de Boer, W.F. Regional level risk factors associated with the occurrence of African swine fever in West and East Africa. Parasites Vectors 2017, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Ma, T.; Hao, M.; Ding, F.; Sun, K.; Wang, Q.; Kang, T.; Wang, D.; Zhao, S.; Li, M. Quantifying risk factors and potential geographic extent of African swine fever across the world. PLoS ONE 2022, 17, e0267128. [Google Scholar] [CrossRef]
- Primatika, R.A.; Sudarnika, E.; Sumiarto, B.; Basri, C. Estimation of the probability risks of African swine fever outbreaks using the maximum entropy method in North Sumatra Province, Indonesia. Vet. World 2022, 15, 1814–1820. [Google Scholar] [CrossRef]
- Rho, P. Using habitat suitability model for the wild boar (Sus scrofa Linnaeus) to select wildlife passage sites in extensively disturbed temperate forests. J. Ecol. Environ. 2015, 38, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Kazmi, S.J.H.; Usery, E.L. Application of remote sensing and GIS for the monitoring of diseases: A unique research agenda for geographers. Remote Sens. Rev. 2001, 20, 45–70. [Google Scholar] [CrossRef]
- Palaniyandi, M. The role of remote sensing and GIS for spatial prediction of vector-borne diseases transmission: A systematic review. J. Vector Borne Dis. 2012, 49, 197. [Google Scholar] [PubMed]
- Orusa, T.; Viani, A.; Moyo, B.; Cammareri, D.; Borgogno-Mondino, E. Risk Assessment of Rising Temperatures Using Landsat 4–9 LST Time Series and Meta® Population Dataset: An Application in Aosta Valley, NW Italy. Remote Sens. 2023, 15, 2348. [Google Scholar] [CrossRef]
- Viani, A.; Orusa, T.; Borgogno-Mondino, E.; Orusa, R. Snow Metrics as Proxy to Assess Sarcoptic Mange in Wild Boar: Preliminary Results in Aosta Valley (Italy). Life 2023, 13, 987. [Google Scholar] [CrossRef]
- Erasmi, S.; Kappas, M.; Twele, A.; Ardiansyah, M. From global to regional scale: Remote sensing-based concepts and methods for mapping land-cover and land-cover change in tropical regions. In Stability of Tropical Rainforest Margins; Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2007; pp. 435–460. [Google Scholar]
- Liang, R.; Lu, Y.; Qu, X.; Su, Q.; Li, C.; Xia, S.; Liu, Y.; Zhang, Q.; Cao, X.; Chen, Q. Prediction for global African swine fever outbreaks based on a combination of random forest algorithms and meteorological data. Transbound. Emerg. Dis. 2020, 67, 935–946. [Google Scholar] [CrossRef]
- Ferdousi, T.; Moon, S.A.; Self, A.; Scoglio, C. Generation of swine movement network and analysis of efficient mitigation strategies for African swine fever virus. PLoS ONE 2019, 14, e0225785. [Google Scholar] [CrossRef] [PubMed]
- NIE. EcoBank (Internationl Ecological Information Bank). Available online: https://www.nie-ecobank.kr/othbcData/othbcData/OthbcDataInqireCoUpdt.do (accessed on 20 February 2023).
- Bosch, J.; Iglesias, I.; Muñoz, M.J.; De la Torre, A. A cartographic tool for managing African swine fever in Eurasia: Mapping wild boar distribution based on the quality of available habitats. Transbound. Emerg. Dis. 2017, 64, 1720–1733. [Google Scholar] [CrossRef]
- ENETWILD-consortium; Gavier-Widen, D.; Ferroglio, E.; Smith, G.; Gonçalves, C.; Vada, R.; Zanet, S.; Gethöffer, F.; Keuling, O.; Staubach, C. Recommendations and technical specifications for sustainable surveillance of zoonotic pathogens where wildlife is implicated. EFSA Support. Publ. 2023, 20, 7812E. [Google Scholar]
- Kim, E.; Lim, J.; Pak, S. Mechanistic modelling for African swine fever transmission in the Republic of Korea. J. Vet. Sci. 2023, 24, e21. [Google Scholar] [CrossRef]
- KFS (Korea Forest Service). Statistical Yearbook of Forestry. Available online: https://kfss.forest.go.kr/stat/ptl/fyb/frstyYrBookList.do?curMenu=9854 (accessed on 20 February 2023).
- NGII (National Geographic Information Institute). The National Atlas of Korea. Available online: http://nationalatlas.ngii.go.kr/pages/page_1159.php (accessed on 20 February 2023).
- Kim, S.; Kwon, K.; Kim, T.; Ko, H.; Jang, G. An analysis on aspects of farm lands damaged by the wild boar (Sus scrofa) in Gyeongnam Province, Korea. J. Korean Soc. Environ. Restor. Technol. 2014, 17, 17–27. [Google Scholar]
- Lim, S.J.; Kwon, J.H.; Namgung, H.; Park, J.Y.; Kim, E.K.; Park, Y.C. Long-Term Monitoring of the Barrier Effect of the Wild Boar Fence. J. For. Environ. Sci. 2022, 38, 128–132. [Google Scholar]
- Chenais, E.; Ståhl, K.; Guberti, V.; Depner, K. Identification of wild boar–habitat epidemiologic cycle in African swine fever epizootic. Emerg. Infect. Dis. 2018, 24, 810. [Google Scholar] [CrossRef] [Green Version]
- Zani, L.; Masiulis, M.; Bušauskas, P.; Dietze, K.; Pridotkas, G.; Globig, A.; Blome, S.; Mettenleiter, T.; Depner, K.; Karvelienė, B. African swine fever virus survival in buried wild boar carcasses. Transbound. Emerg. Dis. 2020, 67, 2086–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gervasi, V.; Guberti, V. African swine fever endemic persistence in wild boar populations: Key mechanisms explored through modelling. Transbound. Emerg. Dis. 2021, 68, 2812–2825. [Google Scholar] [CrossRef]
- Cadenas-Fernández, E.; Ito, S.; Aguilar-Vega, C.; Sánchez-Vizcaíno, J.M.; Bosch, J. The Role of the Wild Boar Spreading African Swine Fever Virus in Asia: Another Underestimated Problem. Front. Vet. Sci. 2022, 453, 844209. [Google Scholar] [CrossRef]
- Podgórski, T.; Śmietanka, K. Do wild boar movements drive the spread of African Swine Fever? Transbound. Emerg. Dis. 2018, 65, 1588–1596. [Google Scholar] [CrossRef]


| Category | Variable | Abbreviation | Source |
|---|---|---|---|
| Forest environment | Forest type | Frtp | Forest Type Map (1:5000) from Korea Forest Service (accessed on 24 November 2021) https://www.forest.go.kr/ |
| Age classes of trees | Agcl | ||
| Diameter at breast height of trees | Dbht | ||
| Crown density | Crde | ||
| Topographic | Elevation | Elev | Digital Elevation Model from National Geographic Information Institute of the Republic of Korea (accessed on 5 November 2021) http://data.nsdi.go.kr/ |
| Slope | Slop | ||
| Aspect | Aspe | ||
| Water | Water | Stream Order Map from Water Resources Management Information System of the Republic of Korea (accessed on 23 November 2021) http://www.wamis.go.kr/ | |
| Anthropogenic | Settlement | Setl | Subdivision Land Cover Map from Ministry of Environment of the Republic of Korea (accessed on 25 November 2021) https://egis.me.go.kr/ |
| Plow | Plow | ||
| Road | Road | Road map of Korea from Ministry of Land of the Republic of Korea (accessed on 21 November 2021) https://www.its.go.kr/ |
| Level of Suitability Value | Area (km2) at Suitability Levels | Percentage of Area in Each Suitability Level out of the Total Area (16,469.1 km2) | No. of ASF OutBreaks at Suitability Level | Percentage of ASF Outbreaks at Suitability Level out of the Total ASF Outbreaks (252 Cases) | No. ASF OutBreaks/km2 |
|---|---|---|---|---|---|
| Very high (0.8–1) | 15.8 | 0.1 | 2 | 0.8 | 0.127 |
| High (0.6–0.8) | 760.7 | 4.6 | 59 | 23.4 | 0.078 |
| Medium (0.4–0.6) | 4047.7 | 24.6 | 112 | 44.4 | 0.028 |
| Low (0.2–0.4) | 7482.4 | 45.4 | 73 | 29.0 | 0.010 |
| Very low (0–0.2) | 4162.5 | 25.3 | 6 | 2.4 | 0.001 |
| Total | 16,469.1 | 100 | 252 | 100 | 0.015 |
| Distance from Shortest-Path | Level of Shortest-Path BC | Area Size (km2) | No. of ASF Outbreaks | No. of ASF Outbreaks/km2 |
|---|---|---|---|---|
| 1.8 km | Low (0.007~0.026) | 3329 | 87 | 0.026 |
| Medium (0.026~0.064) | 1457 | 43 | 0.030 | |
| High (0.064~0.149) | 505 | 37 | 0.073 | |
| 1.8~3.6 km | Low (0.007~0.026) | 3373 | 26 | 0.008 |
| Medium (0.026~0.064) | 1312 | 34 | 0.026 | |
| High (0.064~0.149) | 394 | 6 | 0.015 |
| Local Region | Pig Farm Sector | Locality | No. of Pig Farms | No. of Heads | Distance from the Shortest Path (km) | Overlap Area * (km2) |
|---|---|---|---|---|---|---|
| Gyeonggi-do (GG) | GG-1 | Yeoncheon-gun | 10 | 17,555 | 5.8 | |
| GG-2 | Pocheon-si | 28 | 82,880 | 5.9 | 0.11 | |
| GG-3 | Pocheon-si | 14 | 14,735 | 8.6 | 0.56 | |
| GG-4 | Dongducheon-si ** | 13 | 24,140 | 1.2 | 0.01 | |
| GG-5 | Yangju-si | 13 | 25,720 | 5.0 | ||
| GG-6 * | Yangpyeong-gun | 10 | 21,710 | 0.0 | 0.49 | |
| GG-7 | Icheon-si | 10 | 17,340 | 6.0 | 0.00 | |
| GG-8 * | Cheoin-gu ** | 13 | 9932 | 0.0 | 0.38 | |
| GG-9 | Icheon-si ** | 12 | 33,795 | 15.5 | ||
| GG-10 | Yeoju-si | 4 | 6024 | 11.5 | ||
| GG-11 | Icheon-si | 8 | 14,900 | 10.9 | 0.00 | |
| GG-12 | Cheoin-gu ** | 216 | 364,060 | 0.6 | 1.93 | |
| GG-13 | Pyeongtaek-si ** | 10 | 6373 | 11.9 | ||
| GG-14 * | Anseong-si | 12 | 17,700 | 0.0 | 0.02 | |
| GG-15 | Pyeongtaek-si ** | 21 | 48,546 | 14.8 | ||
| Gangwon-do (GW) | GW-1 | Cheorwon-gun ** | 10 | 36,414 | 25.5 | |
| Chungcheongbuk-do (CB) | CB-1 | Goesan-gun | 9 | 14,490 | 0.0 | |
| CB-2 | Cheongwon-gun ** | 5 | 10,195 | 3.9 | ||
| Chungcheongnam-do (CN) | CN-1 | Dangjin-si | 12 | 16,785 | 12.5 | |
| CN-2 | Dongnam-gu ** | 8 | 25,830 | 3.8 | 0.08 | |
| CN-3 | Asan-si | 2 | 3200 | 16.6 | ||
| CN-4 | Dangjin-si ** | 36 | 72,427 | 6.7 | ||
| CN-5 | Asan-si ** | 2 | 6000 | 12.7 | ||
| CN-6 | Yesan-gun | 17 | 36,004 | 11.6 | ||
| CN-7 * | Hongseong-gun ** | 16 | 39,220 | 0.0 | 0.02 | |
| CN-8 | Hongseong-gun | 1 | 2000 | 0.0 | ||
| CN-9 * | Boryeong-si ** | 285 | 604,359 | 0.0 | 0.80 | |
| CN-10 | Gongju-si | 4 | 6550 | 0.0 | ||
| CN-11 | Gongju-si ** | 34 | 33,483 | 2.9 | ||
| CN-12 | Nonsan-si | 25 | 37,540 | 9.9 | 1.12 | |
| Sejong-si (SJ) | SJ-1 | Yuseong-gu ** | 13 | 26,230 | 13.6 | 0.35 |
| Jeollabuk-do (JB) | JB-1 | Iksan-si | 21 | 19,750 | 3.1 | |
| JB-2 | Gunsan-si ** | 13 | 22,900 | 0.0 | ||
| JB-3 | Iksan-si ** | 97 | 80,999 | 8.9 | ||
| JB-4 | Gimje-si | 11 | 25,630 | 13.4 | ||
| JB-5 | Gimje-si ** | 66 | 133,895 | 16.1 | ||
| Jeollanam-do (JN) | JN-1 | Hwasun-gun | 9 | 13,773 | 8.3 | 0.32 |
| JN-2 | Naju-si | 5 | 16,150 | 12.2 | ||
| JN-3 | Muan-gun | 9 | 16,150 | 3.0 | ||
| Gwangju-si (GJ) | GJ-1 | Gwangsan-gu ** | 30 | 38,482 | 11.9 | |
| Gyeongsangbuk-do (GB) | GB-1 | Yeongcheon-si | 12 | 28,900 | 4.7 | 0.03 |
| GB-2 * | Yeongcheon-si | 4 | 7400 | 0.0 | 0.21 | |
| GB-3 * | Seongju-gun | 12 | 12,860 | 0.0 | 1.34 | |
| GB-4 * | Gyeongju-si ** | 21 | 29,985 | 0.0 | 0.01 | |
| GB-5 | Gyeongsan-si | 11 | 20,442 | 8.5 | ||
| GB-6 * | Hapcheon-gun ** | 10 | 26,200 | 0.0 | 0.04 | |
| Gyeongsangnam-do (GN) | GN-1 | Hapcheon-gun ** | 20 | 33,510 | 0.1 | 1.80 |
| GN-2 | Miryang-si | 13 | 10,280 | 6.0 | 0.35 | |
| GN-3 | Yangsan-si | 13 | 17,896 | 4.0 | 0.44 | |
| GN-4 | Gimhae-si | 57 | 97,584 | 0.1 | 1.26 | |
| GN-5 * | Goseong-gun | 12 | 18,578 | 0.0 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H.; Namgung, H.; Lim, S.J.; Kim, E.K.; Oh, Y.; Park, Y.C. Predicting Suitable Areas for African Swine Fever Outbreaks in Wild Boars in South Korea and Their Implications for Managing High-Risk Pig Farms. Animals 2023, 13, 2148. https://doi.org/10.3390/ani13132148
Choi JH, Namgung H, Lim SJ, Kim EK, Oh Y, Park YC. Predicting Suitable Areas for African Swine Fever Outbreaks in Wild Boars in South Korea and Their Implications for Managing High-Risk Pig Farms. Animals. 2023; 13(13):2148. https://doi.org/10.3390/ani13132148
Chicago/Turabian StyleChoi, Ju Hui, Hun Namgung, Sang Jin Lim, Eui Kyeong Kim, Yeonsu Oh, and Yung Chul Park. 2023. "Predicting Suitable Areas for African Swine Fever Outbreaks in Wild Boars in South Korea and Their Implications for Managing High-Risk Pig Farms" Animals 13, no. 13: 2148. https://doi.org/10.3390/ani13132148
APA StyleChoi, J. H., Namgung, H., Lim, S. J., Kim, E. K., Oh, Y., & Park, Y. C. (2023). Predicting Suitable Areas for African Swine Fever Outbreaks in Wild Boars in South Korea and Their Implications for Managing High-Risk Pig Farms. Animals, 13(13), 2148. https://doi.org/10.3390/ani13132148







