Behaviour, Furnishing and Vertical Space Use of Captive Callimico (Callimico goeldii): Implications for Welfare
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
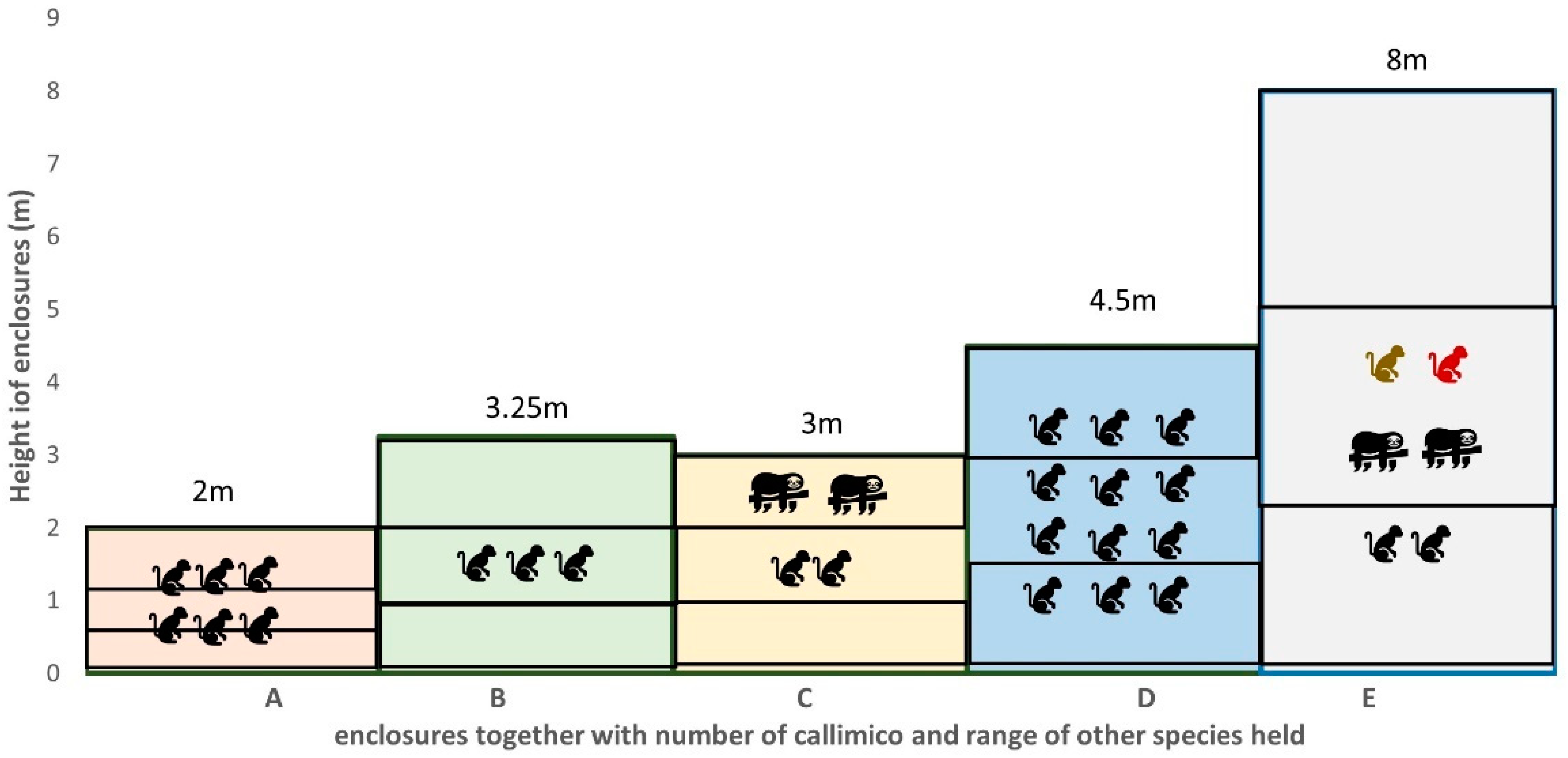
2.1. Study Subjects and Enclosures
2.2. Behavioural Data Collection
2.3. Furnishings
2.4. Data Analysis
2.4.1. Activity Budgets
2.4.2. Spread of Participation
2.4.3. Association between Behaviours, Zone Use and Furnishings
3. Results
3.1. Behaviour Budgets
3.2. Spread of Participation Index
3.3. Association of Behaviour with Vertical Zones
3.4. Association of Behaviour with Furnishings
4. Discussion
4.1. Behaviours
4.2. Vertical Zone Use
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palacios, E.; Wallace, R.B.; Mollinedo, J.M.; Heymann, E.W.; Shanee, S.; Calouro, A.M.; Mollinedo, J.M.; de Valle, E.; Mittermeir, R.A. Callimico goeldii (Amended Version of 2020 Assessment). The IUCN Red List of Threatened Species 2021:E.T3564A191700340. 2021. Available online: https://dx.doi.org/10.2305/IUCN.UK.2021-1.RLTS.T3564A191700340.en (accessed on 26 January 2023).
- Porter, L.M.; Sterr, S.M.; Garber, P.A. Habitat Use and Ranging Behavior of Callimico goeldii. Int. J. Primatol. 2007, 28, 1035–1058. [Google Scholar] [CrossRef]
- Watsa, M.E. 200,000 of Peru’s Primates Trafficked for Pet Trade or Bushmeat Yearly. Available online: https://news.mongabay.com/2015/12/200000-of-perus-primates-trafficked-for-pet-trade-or-bushmeat-yearly/ (accessed on 26 January 2023).
- Bairrão Ruivo, E.; Carroll, J.B.; Desmoulins, A.; Rylands, A.B. Biology and Field Data. In EAZA Best Practice Guidelines for Callitrichidae-3.2 Edition; Bairrão Ruivo, E., Stevenson, M., Eds.; EAZA European Association of Zoos and Aquaria: Amsterdam, The Netherlands, 2022; pp. 22–30. [Google Scholar]
- Bicca-Marques, J.C. Hand Specialization, Sympatry, and Mixed-Species Associations in Callitrichines. J. Hum. Evol. 1999, 36, 349–378. [Google Scholar] [CrossRef]
- Garber, P.A.; Leigh, S.R. Patterns of Positional Behavior in Mixed-Species Troops of Callimico goeldii, Saguinus labiatus, and Saguinus fuscicollis in Northwestern Brazil. Am. J. Primatol. 2001, 54, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.M. Forest Use and Activity Patterns of Callimico goeldii in Comparison to Two Sympatric Tamarins, Saguinus fuscicollis and Saguinus labiatus. Am. J. Phys. Anthropol. 2004, 124, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.; Burn, C.C.; Dallaire, J.A.; Kroshko, J.; McDonald Kinkaid, H.; Jeschke, J.M. Plastic Animals in Cages: Behavioural Flexibility and Responses to Captivity. Anim. Behav. 2013, 85, 1113–1126. [Google Scholar] [CrossRef]
- Veasey, J.S. In Pursuit of Peak Animal Welfare; the Need to Prioritize the Meaningful over the Measurable. Zoo Biol. 2017, 36, 413–425. [Google Scholar] [CrossRef]
- Howell, C.P.; Cheyne, S.M. Complexities of Using Wild versus Captive Activity Budget Comparisons for Assessing Captive Primate Welfare. J. Appl. Anim. Welf. Sci. 2019, 22, 78–96. [Google Scholar] [CrossRef]
- Porter, L.M. The Behavioural Ecology of Callimicos and Tamarins in Northwest Bolivia; Pearson: Hoboken, NJ, USA, 2007. [Google Scholar]
- Pook, A.G.; Pook, G. A Field Study of the Socio-Ecology of the Goeldi’s Monkey (Callimico goeldii) in Northern Bolivia. Folia Primatol. 1981, 35, 288–312. [Google Scholar] [CrossRef]
- Arakaki, P.R.; de Carvalho, F.M.; de Castro, P.H.G.; Muniz, J.A.P.C. Collection, Evaluation, and Coagulum Dissolution of Semen from Goeldi’s Monkey, Callimico goeldii. Folia Primatol. Int. J. Primatol. 2017, 88, 334–343. [Google Scholar] [CrossRef]
- Outer Retinal Degeneration in Two Closely Related Goeldi’s Monkeys (Callimico goeldii)—Kosec—2020—Veterinary Ophthalmology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/vop.12721 (accessed on 26 January 2023).
- Wang, X.; Lim, B.K.; Ting, N.; Hu, J.; Liang, Y.; Roos, C.; Yu, L. Reconstructing the Phylogeny of New World Monkeys (Platyrrhini): Evidence from Multiple Non-Coding Loci. Curr. Zool. 2019, 65, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Melfi, V.A. There Are Big Gaps in Our Knowledge, and Thus Approach, to Zoo Animal Welfare: A Case for Evidence-Based Zoo Animal Management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef]
- Brereton, J.; Rose, P. An Evaluation of the Role of “biological Evidence” in Zoo and Aquarium Enrichment Practices. Anim. Welf. 2022, 31, 13–26. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, L.M.; Beausoleil, N.J.; Embury, A.; Mellor, D.J. An Animal Welfare Risk Assessment Process for Zoos. Animals 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Hosey, G.R.; Melfi, V.A.; Pankhurst, S. Zoo Animals, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- Bacon, H. Behaviour-Based Husbandry—A Holistic Approach to the Management of Abnormal Repetitive Behaviors. Animals 2018, 8, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, L.M.; Rose, P.E. Concepts, Applications, Uses and Evaluation of Environmental Enrichment: Perceptions of Zoo Professionals. J. Zoo Aquar. Res. 2020, 8, 18–28. [Google Scholar] [CrossRef]
- Boere, V. Environmental Enrichment for Neotropical Primates in Captivity. Ciência Rural. 2001, 31, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Vicino, G.A.; Sheftel, J.J.; Radosevich, L.M. Enrichment Is Simple, That’s the Problem: Using Outcome-Based Husbandry to Shift from Enrichment to Experience. Animals 2022, 12, 1293. [Google Scholar] [CrossRef]
- Sha, J.C.M.; Ismail, R.; Marlena, D.; Lee, J.L. Environmental Complexity and Feeding Enrichment Can Mitigate Effects of Space Constraints in Captive Callitrichids. Lab. Anim. 2016, 50, 137–144. [Google Scholar] [CrossRef]
- Dalton, R.; Buchanan-Smith, H.M. A Mixed-Species Exhibit for Goeldi’s Monkeys and Pygmy Marmosets Callimico goeldii and Callithrix pygmaea at Edinburgh Zoo. Int. Zoo Yearb. 2005, 39, 176–184. [Google Scholar] [CrossRef]
- Jones, M.; Pillay, N. Foraging in Captive Hamadryas Baboons: Implications for Enrichment. Appl. Anim. Behav. Sci. 2004, 88, 101–110. [Google Scholar] [CrossRef]
- Meehan, C.L.; Millam, J.R.; Mench, J.A. Foraging Opportunity and Increased Physical Complexity Both Prevent and Reduce Psychogenic Feather Picking by Young Amazon Parrots. Appl. Anim. Behav. Sci. 2003, 80, 71–85. [Google Scholar] [CrossRef]
- Veasey, J.S. Assessing the Psychological Priorities for Optimising Captive Asian Elephant (Elephas maximus) Welfare. Animals 2020, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, C.; Howell, S.M.; Fritz, J.; Nankivell, B. Space Use and Proximity of Captive Chimpanzee (Pan troglodytes) Mother/Offspring Pairs. Zoo Biol. 1994, 13, 61–68. [Google Scholar] [CrossRef]
- Ross, S.R.; Schapiro, S.J.; Hau, J.; Lukas, K.E. Space Use as an Indicator of Enclosure Appropriateness: A Novel Measure of Captive Animal Welfare. Appl. Anim. Behav. Sci. 2009, 121, 42–50. [Google Scholar] [CrossRef]
- Hebert, P.L.; Bard, K. Orangutan Use of Vertical Space in an Innovative Habitat. Zoo Biol. 2000, 19, 239–251. [Google Scholar] [CrossRef]
- Burrell, A.M.; Altman, J.D. The Effect of the Captive Environment on Activity of Captive Cotton-Top Tamarins (Saguinus Oedipus). J. Appl. Anim. Welf. Sci. 2006, 9, 269–276. [Google Scholar] [CrossRef]
- Buchanan-Smith, H.M.; Shand, C.; Morris, K. Cage Use and Feeding Height Preferences of Captive Common Marmosets (Callithrix j. Jacchus) in Two-Tier Cages. J. Appl. Anim. Welf. Sci. 2002, 5, 139–149. [Google Scholar] [CrossRef]
- Price, E.; Coleman, R.; Ahsmann, J.; Glendewar, G.; Hunt, J.; Smith, T.; Wormell, D. Individual, Social, and Environmental Factors Affecting Salivary and Fecal Cortisol Levels in Captive Pied Tamarins (Saguinus bicolor). Am. J. Primatol. 2019, 81, e23033. [Google Scholar] [CrossRef]
- Clubb, R.; Mason, G. Captivity Effects on Wide-Ranging Carnivores. Nature 2003, 425, 473–474. [Google Scholar] [CrossRef]
- Sodaro, V. Social Organization and Housing of Captive Goeldi’s Monkeys. In Callimico Species Survival Plan Husbandry Manual [Online]; Sodaro, V., Ed.; Chicago Zoological Park: Chicago, IL, USA, 2004; pp. 24–37. [Google Scholar]
- Brereton, J.E. Directions in Animal Enclosure Use Studies. J. Zoo Aquar. Res. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Plowman, A.B. A Note on a Modification of the Spread of Participation Index Allowing for Unequal Zones. Appl. Anim. Behav. Sci. 2003, 83, 331–336. [Google Scholar] [CrossRef]
- Browning, H.; Maple, T.L. Developing a Metric of Usable Space for Zoo Exhibits. Front. Psychol. 2019, 10, 791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bateson, M.; Martin, P. Measuring Behaviour. An Introductory Guide, 4th ed.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Rees, P. An Introduction to Zoo Biology and Management; Wiley Blackwell: Chichester, UK, 2011. [Google Scholar]
- Carroll, J.B. The Captive Behaviour and Reproduction of Goeldi’s Monkey Callimico goeldii. Ph.D. Thesis, Open University, Milton Keynes, UK, 1992. [Google Scholar] [CrossRef]
- Ross, M.R.; Niemann, T.; Wark, J.D.; Heintz, M.R.; Horrigan, A.; Cronin, K.A.; Shender, M.A.; Gillespie, K. ZooMonitor (Version 1) [Mobile Application Software]. 2016. Available online: https://zoomonitor.com (accessed on 26 January 2023).
- Melfi, V.; Feistner, A. A Comparison of the Activity Budgets of Wild and Captive Sulawesi Crested Black Macaques (Macaca nigra). Anim. Welf. 2002, 11, 213–222. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. (v4.2.1); R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 26 January 2023).
- Lazaro-Perea, C.; De Fatima Arruda, M.; Snowden, C.T. Grooming as a Reward? Social Function of Grooming between Females in Cooperatively Breeding Marmosets. Anim. Behav. 2004, 67, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Kerridge, F.J. Environmental Enrichment to Address Behavioral Differences between Wild and Captive Black-and-White Ruffed Lemurs (Varecia variegata). Am. J. Primatol. 2005, 66, 71–84. [Google Scholar] [CrossRef]
- Jakob-Hoff, R.; Kingan, M.; Fenemore, C.; Schmid, G.; Cockrem, J.F.; Crackle, A.; Van Bemmel, E.; Connor, R.; Descovich, K. Potential Impact of Construction Noise on Selected Zoo Animals. Animals 2019, 9, 504. [Google Scholar] [CrossRef] [Green Version]
- Jens, W.; Lindsay, N.; Wormell, D. Management in Zoos: Housing and Exhibition of the Callitrichidae. In EAZA Best Practice Guidelines for Callitrichidae-3.2 Edition; Bairrão Ruivo, E., Stevenson, M., Eds.; EAZA European Association of Zoos and Aquaria: Amsterdam, The Netherlands, 2022; pp. 105–112. [Google Scholar]
- Whitehouse, J.; Micheletta, J.; Waller, B.M. Stress Behaviours Buffer Macaques from Aggression. Sci. Rep. 2017, 7, 11083. [Google Scholar] [CrossRef] [Green Version]
- Grothmann, P.; Petit, T.; Cracknell, J. Management in Zoos: Veterinary: Considerations for Health and Welfare. In EAZA Best Practice Guidelines for Callitrichidae-3.2 Edition; Bairrão Ruivo, E., Stevenson, M., Eds.; EAZA European Association of Zoos and Aquaria: Amsterdam, The Netherlands, 2022; pp. 208–217. [Google Scholar]
- Pastorino, G.Q.; Stubbington, T.; Brereton, S.R.; Brereton, J.E.; Gabb, A.; Stoycheva, R.; Ferguson, A.; Preziosi, R. How Do Zoo Evening Events at ZSL London Zoo Affect Sumatran Tiger Behaviour and Enclosure. Acta Sci. Vet. Sci. 2022, 4, 213–222. [Google Scholar] [CrossRef]
- Lopez Goya, A.; Jens, W.; Wormell, D. Management in Zoos: Environmental Enrichment. In EAZA Best Practice Guidelines for Callitrichidae-3.2 Edition; Bairrão Ruivo, E., Stevenson, M., Eds.; EAZA European Association of Zoos and Aquaria: Amsterdam, The Netherlands, 2022; pp. 177–185. [Google Scholar]
- Jablonski, N.G. Social and Affective Touch in Primates and Its Role in the Evolution of Social Cohesion. Neuroscience 2021, 464, 117–125. [Google Scholar] [CrossRef]
- Grandi, L.C. From Sweeping to the Caress: Similarities and Discrepancies between Human and Non-Human Primates’ Pleasant Touch. Front. Psychol. 2016, 7, 1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan-Smith, H.M.; Carroll, J.B. Management in Zoos. Social Structure and Behaviour. In EAZA Best Practice Guidelines for Callitrichidae-3.2 Edition; Bairrão Ruivo, E., Stevenson, M., Eds.; EAZA European Association of Zoos and Aquaria: Amsterdam, The Netherlands, 2022; pp. 22–30. [Google Scholar]
- Martel, F.L.; Nevison, C.M.; Simpson, M.J.A.; Keverne, E.B. Effects of Opioid Receptor Blockade on the Social Behavior of Rhesus Monkeys Living in Large Family Groups. Dev. Psychobiol. 1995, 28, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of Positive Emotions in Animals to Improve Their Welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Mellor, D.J. Updating Animal Welfare Thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojan-Dolar, M.; Heymann, E.W. Vigilance in a Cooperatively Breeding Primate. Int. J. Primatol. 2010, 31, 95–116. [Google Scholar] [CrossRef] [Green Version]
- Schwitzer, C.; Kaumanns, W. Body Weights of Ruffed Lemurs (Varecia variegata) in European Zoos with Reference to the Problem of Obesity. Zoo Biol. 2001, 20, 261–269. [Google Scholar] [CrossRef]
- Plowman, A. Diet Review and Change for Monkeys at Paignton Zoo Environmental Park. J. Zoo Aquar. Res. 2013, 1, 73–77. [Google Scholar]
- Garber, P.A.; Porter, L.M. Trunk-To-Trunk Leaping in Wild Callimico goeldii in Northern Bolivia. Neotrop. Primates 2009, 16, 9–14. [Google Scholar] [CrossRef]
- Epstein, J.; Langan, J.N.; Warneke, M.R.; Allender, M.C.; Kinsel, M.J. Global Retrospective Analysis Of Pathological Findings in Zoo-Managed Goeldi’s Monkeys (Callimico goeldii), 1965–2018. J. Zoo Wildl. Med. 2022, 53, 339–348. [Google Scholar] [CrossRef]
- Tidière, M.; Gaillard, J.-M.; Berger, V.; Müller, D.W.H.; Bingaman Lackey, L.; Gimenez, O.; Clauss, M.; Lemaître, J.-F. Comparative Analyses of Longevity and Senescence Reveal Variable Survival Benefits of Living in Zoos across Mammals. Sci. Rep. 2016, 6, 36361. [Google Scholar] [CrossRef] [Green Version]
- Bacon, H. Quality of Life Assessments. EAZA Animal Welfare Webinar. 2020. Available online: https://www.youtube.com/watch?v=GpFDtGyXJD0&ab_channel=EAZAvideo (accessed on 26 January 2023).
- Wormell, D.; Hunt, J.; Ruivo, E.B.; Price, E. Enriched Environments for Callitrichids. In Solitaire: Issue 23; Price, E., Ed.; Durrell Wildlife Conservation Trust: Jersey, NY, USA, 2012. [Google Scholar]
- Morgan, K.N.; Tromborg, C.T. Sources of Stress in Captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Rudebeck, P.H.; Izquierdo, A. Foraging with the Frontal Cortex: A Cross-Species Evaluation of Reward-Guided Behavior. Neuropsychopharmacology 2022, 47, 134–146. [Google Scholar] [CrossRef]
- Rapaport, L.G.; Brown, G.R. Social Influences on Foraging Behavior in Young Nonhuman Primates: Learning What, Where, and How to Eat. Evol. Anthropol. Issues News Rev. 2008, 17, 189–201. [Google Scholar] [CrossRef]
- Kierulff, M.C.M.; Ruiz-Miranda, C.R.; de Oliveira, P.P.; Beck, B.B.; Martins, A.; Dietz, J.M.; Rambaldi, D.M.; Baker, A.J. The Golden Lion Tamarin Leontopithecus Rosalia: A Conservation Success Story. Int. Zoo Yearb. 2012, 46, 36–45. [Google Scholar] [CrossRef]
- Cabana, F. What Is Nutritional Welfare and How Can I Make Sure My Animals Have It. EAZA Animal Welfare Forum. 2020. Available online: https://www.youtube.com/watch?v=D80IlyYV5nE&ab_channel=EAZAvideo (accessed on 27 January 2023).
- Shora, J.; Myhill, M.; Brereton, J.E. Should Zoo Foods Be Coati Chopped. J. Zoo Aquar. Res. 2018, 6, 22–25. [Google Scholar] [CrossRef]
- Brereton, J.E. Should Zoo Foods be Chopped or Should We ‘Lemur’ them Whole? Medp. Nutr. Food Sci. 2022, 1, 202211002. [Google Scholar]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and Measuring the Quality of Fresh-Cut Fruit and Vegetables: A Review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Hancocks, D. The History and Principles of Zoo Exhibition. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management; Kleiman, D.G., Thompson, K.V., Baer, C.K., Eds.; The University of Chicago Press, Ltd.: London, UK, 2012; pp. 121–136. [Google Scholar]
- Ferrari, S.F.; Ferrari, M.A.L. Predator Avoidance Behaviour in the Buffy-Headed Marmoset, Callithrix Flaviceps. Primates 1990, 31, 323–338. [Google Scholar] [CrossRef]
- Peres, C.A. Diet and Feeding Ecology of Saddle-Back (Saguinus fuscicollis) and Moustached (S. mystax) Tamarins in an Amazonian Terra Firme Forest. J. Zool. 1993, 230, 567–592. [Google Scholar] [CrossRef]
- Caine, N.G. Visual Scanning by Tamarins. Folia Primatol. 1984, 43, 59–67. [Google Scholar] [CrossRef]
- Barros, M.; Alencar, C.; Tomaz, C. Differences in Aerial and Terrestrial Visual Scanning in Captive Black Tufted-Ear Marmosets (Callithrix penicillata) Exposed to a Novel Environment. Folia Primatol. 2004, 75, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A. Visual Scanning by Common Marmosets (Callithrix jacchus): Functional Aspects and the Specialrrole of Adult Males. Primates 1998, 39, 85–90. [Google Scholar] [CrossRef]
- Nekaris, K.A.I. Foraging Behaviour of the Slender Loris (Loris lydekkerianus lydekkerianus): Implications for Theories of Primate Origins. J. Hum. Evol. 2005, 49, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.D.; Tung, J.; Camden, J.B.; Leal, M.; Drea, C.M. Seeing Red: Behavioral Evidence of Trichromatic Color Vision in Strepsirrhine Primates. Behav. Ecol. 2009, 20, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Trapanese, C.; Meunier, H.; Masi, S. What, Where and When: Spatial Foraging Decisions in Primates. Biol. Rev. 2019, 94, 483–502. [Google Scholar] [CrossRef]
- Whitham, J.C.; Wielebnowski, N. New Directions for Zoo Animal Welfare Science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar] [CrossRef]
- Pomerantz, O.; Meiri, S.; Terkel, J. Socio-Ecological Factors Correlate with Levels of Stereotypic Behavior in Zoo-Housed Primates. Behav. Process. 2013, 98, 85–91. [Google Scholar] [CrossRef]
- Addessi, E.; Chiarotti, F.; Visalberghi, E.; Anzenberger, G. Response to Novel Food and the Role of Social Influences in Common Marmosets (Callithrix jacchus) and Goeldi’s Monkeys (Callimico goeldii). Am. J. Primatol. 2007, 69, 1210–1222. [Google Scholar] [CrossRef]
- Bartlett, A. A Comparative Multi Zoo Survey Investigating the Housing and Husbandry of Callimico goeldii. Bachelor’s Thesis, Sparsholt University Centre, Winchester, UK, 2021. [Google Scholar]





| Activity during Feeding Enrichment, Ex-Situ [25] | Mixed Species Exhibit Study, Ex-Situ [26] | In Situ Field Study [11] | General Callitrichid Ex-Situ ‘Best Practice’ Guidelines [4] | |
|---|---|---|---|---|
| Locomotion | 23.3% | 22% | 17% | |
| Foraging | 20.6% * | 19% | 6% | Up to 37% |
| Grooming | 27% | 7% | ||
| Scanning | 60% |
| Collection | Observation Dates | Study Subjects | Enclosure |
|---|---|---|---|
| A | 17 May 2022–22 May 2022 | ♀ 16y.6m ♂ 6y.11m ♀ 6y.6m ♂ 5y.11m ♂ 5y.0m ♂ 4y.6m | Open fronted external enclosure with moat and glass fronted internal enclosure. Internal enclosure 11.5 m3/external enclosure 100 m3 |
| B | 4 September 22–9 September 22 | ♂ 27y.2m ♂ 21y.4m ♂ 18y.1m | Enclosure is part of a glasshouse rainforest exhibit; 51.19 m3 |
| C | 12 September 22–17 September 22 | ♀ 1y.10m ♂ 8y.2m | Enclosure shared with pair of southern two-toed sloth (Choloepus didactylus); 136 m3 |
| D | 9 May 2022–14 May 2022 | ♀ 9y.11m ♂ 3y.0m ♀ 3y.0m ♂ 2y.6m ♀ 5y.1m ♂ 2y.6m ♀ 1y.4m ♂ 1y.10m ? 0.10m ♂ 1y.6m ? 0.4m ♂ 0.11m | large planted outside area and glass fronted internal area; 67.5 m3 |
| E | 18 April 2022–23 April 2022 | ♀ 18y.9m (nonbreeding) ♂ 9y.11m (introduced 3 weeks before observations) | Rainforest themed ‘walk-through’ enclosure with multiple South American mixed species (including red titi monkeys (Callicebus cupreus), golden headed lion tamarin (Leontopithecus chrysomelas), southern two toed sloths (Choloepus didactylus) and tamandua (Tamandua tetradactylac)); 2780 m3 |
| Behaviours | Definitions |
|---|---|
| Clinging | Subject is in a fixed position within the enclosure, holding on with claws to a vertical surface. There may be head movement but not ‘scanning’. |
| Perching | Subject is in a fixed position on a largely horizontal surface which may include but is not limited to shelving, nest boxes, ropes and branches. There may be head movement, but not ‘scanning’. |
| Lying | Subject is spread across a surface—can be facing downwards or on side—or may have hind legs tucked under—no scanning movement of the head |
| Scanning | Involves subject adopting a still posture while moving the head in a distinct, vigilant, motion as if monitoring the area. This movement is often directed downwards but may be observed with the head tilted upwards. |
| Grooming | Self-grooming which may include but is not limited to the subject scratching themselves with fore or rear claws or rubbing themselves against bark other surface. |
| Allo-grooming | Grooming occurring between subjects including but not limited to one subject manipulating or picking through or the fur of the other. |
| Conspecific interaction | Any contact between subjects that does not constitute grooming. It may involve but is not limited to play, aggression, food sharing or sexual activity. |
| Allospecific | Any contact between callimico and other animals within the enclosure (for mixed species exhibits). |
| Locomotion | Movement around the enclosure by the subject using a leaping, bounding or clinging motion and can include, but is not restricted to, passage across shelving, wire, panels, branches and ropes. |
| Foraging | The active seeking of food by the subject which may include the manipulation of a substrate but does not include the consumption of food or the simple act of lifting food from a bowl/platform—but can include actively sorting through food bowl contents. |
| Feeding | The active consumption of food. |
| Out of Sight | Subject cannot be observed by the researcher. |
| Other | Any activity not expressly noted in the ethogram which include drinking, vocalisation or interaction with any species in an adjoining enclosure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartlett, A.; Grinsted, L.; Freeman, M.S. Behaviour, Furnishing and Vertical Space Use of Captive Callimico (Callimico goeldii): Implications for Welfare. Animals 2023, 13, 2147. https://doi.org/10.3390/ani13132147
Bartlett A, Grinsted L, Freeman MS. Behaviour, Furnishing and Vertical Space Use of Captive Callimico (Callimico goeldii): Implications for Welfare. Animals. 2023; 13(13):2147. https://doi.org/10.3390/ani13132147
Chicago/Turabian StyleBartlett, Amanda, Lena Grinsted, and Marianne Sarah Freeman. 2023. "Behaviour, Furnishing and Vertical Space Use of Captive Callimico (Callimico goeldii): Implications for Welfare" Animals 13, no. 13: 2147. https://doi.org/10.3390/ani13132147
APA StyleBartlett, A., Grinsted, L., & Freeman, M. S. (2023). Behaviour, Furnishing and Vertical Space Use of Captive Callimico (Callimico goeldii): Implications for Welfare. Animals, 13(13), 2147. https://doi.org/10.3390/ani13132147








