Enclosure Background Preferences Differ between Sexes and Color Morphs in the Gouldian Finch
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Analysis
2.3. Ethics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karpestam, E.; Merilaita, S.; Forsman, A. Size variability effects on visual detection are influenced by colour pattern and perceived size. Anim. Behav. 2018, 143, 131–138. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Castagneyrol, B.; Cornelissen, T.; Forsman, A.; Hernández-Agüero, J.A.; Klemola, T.; Paolucci, L.; Polo, V.; Salinas, N.; Theron, K.J.; et al. Opposite latitudinal patterns for bird and arthropod predation revealed in experiments with differently colored artificial prey. Ecol. Evol. 2019, 9, 14273–14285. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.A.; Wang, Y.; Fang, R.; Lu, Y.; Dakin, R. How conspicuous are peacock eyespots and other colorful feathers in the eyes of mammalian predators? PLoS ONE 2019, 14, e0210924. [Google Scholar] [CrossRef]
- Xiao, F.; Cuthill, E.C. Background complexity and the detectability of camouflaged targets by birds and humans. Proc. R. Soc. B 2016, 283, 20161527. [Google Scholar] [CrossRef]
- Price, N.; Green, S.; Troscianko, J.; Tregenza, T.; Stevens, M. Background matching and disruptive coloration as habitat specific strategies for camouflage. Sci. Rep. 2019, 9, 7840. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Sota, T. Evolutionary fine-tuning of background-matching camouflage among geographical populations in the sandy beach tiger beetle. Proc. R. Soc. B 2020, 287, 20202315. [Google Scholar] [CrossRef]
- Murali, G.; Mallick, S.; Kodandaramaiah, U. Background complexity and optimal background matching camouflage. Behav. Ecol. Evol. 2021, 75, 69. [Google Scholar] [CrossRef]
- Mark, C.J.; O’Hanlon, J.C.; Holwell, G.I. Camouflage in lichen moths: Field predation experiments and avian vision modelling demonstrate the importance of wing pattern elements and background for survival. J. Anim. Ecol. 2022, 91, 2358–2369. [Google Scholar] [CrossRef]
- Magelan, K.; Swartz, E.R. Crypsis in a heterogeneous environment: Relationships between changeable polymorphic colour patterns and behaviour in a galaxiid fish. Freshw. Biol. 2013, 58, 793–799. [Google Scholar] [CrossRef]
- Nafus, M.G.; Germano, J.M.; Perry, J.A.; Todd, B.D.; Walsh, A.; Swaisgood, R.R. Hiding in plain sight: A study on camouflage and habitat selection in a slow-moving desert herbivore. Behav. Ecol. 2015, 26, 1389–1394. [Google Scholar] [CrossRef]
- Michalis, C.; Scott-Samuel, N.E.; Gibson, D.P.; Cuthill, I.C. Optimal background matching camouflage. Proc. R. Soc. B 2017, 284, 20170709. [Google Scholar] [CrossRef]
- Toh, K.B.; Todd, P. Camouflage that is spot on! Optimization of spot size in prey-background matching. Evol. Ecol. 2017, 31, 447–461. [Google Scholar] [CrossRef]
- Siegenthaler, A.; Mastin, A.; Dufaut, C.; Mondal, D.; Benvenuto, C. Background matching in the brown shrimp Crangon crangon: Adaptive camouflage and behavioural plasticity. Sci. Rep. 2018, 8, 3292. [Google Scholar] [CrossRef]
- Salisbury, J.W.; Peters, R.A. Non-random perch selection by cryptic lizards, Amphibolurus muricatus. Behav. Ecol. Sociobiol. 2019, 73, 115. [Google Scholar] [CrossRef]
- Endler, J.A. A predator’s view of animal color patterns. In Evolutionary Biology; Hecht, M.K., Steere, W.C., Wallace, B., Eds.; Plenum Press: New York, NY, USA; London, UK, 1979; Volume 2, pp. 319–364. [Google Scholar]
- Xiao, F.; Yang, C.; Shi, H.; Wang, J.; Sun, L.; Lin, L. Background matching and camouflage efficiency predict population density in four-eyed turtle (Sacalia quadriocellata). Behav. Proc. 2016, 131, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.B.; Michalis, C.; Scott-Samuel, N.E.; Cuthill, C. Colour pattern variation forms local background matching camouflage in a leaf-mimicking toad. J. Evol. Biol. 2021, 34, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Merilaita, S.; Scott-Samuel, N.E.; Cuthill, I.C. How camouflage works. Philos. Trans. R. Soc. B 2017, 372, 20160341. [Google Scholar] [CrossRef] [PubMed]
- Koskenpato, K.; Lehikoinen, A.; Lindstedt, C.; Karell, P. Gray plumage color is more cryptic than brown in snowy landscapes in a resident color polymorphic bird. Ecol. Evol. 2020, 10, 1751–1761. [Google Scholar] [CrossRef]
- Nokelainen, O.; Brito, J.C.; Scott-Samuel, N.E.; Valkonen, J.K.; Boratyński, Z. Camouflage accuracy in Sahara–Sahel desert rodents. J. Anim. Ecol. 2020, 89, 1658–1669. [Google Scholar] [CrossRef]
- Solonen, T. Significance of plumage colour for winter survival in the Tawny Owl (Strix aluco): Revisiting the camouflage hypothesis. IBIS 2021, 163, 1437–1442. [Google Scholar] [CrossRef]
- Steinhoff, P.O.M.; Warfen, B.; Voigt, S.; Uhl, G.; Dammhahn, M. Individual differences in risk-taking affect foraging across different landscapes of fear. Oikos 2020, 129, 1891–1902. [Google Scholar] [CrossRef]
- Rowe, Z.W.; Austin, D.J.D.; Chippington, N.; Flynn, W.; Starkey, F.; Wightman, E.J.; Scott-Samuel, N.E.; Cuthill, I.C. Background complexity can mitigate poor camouflage. Proc. R. Soc. B 2021, 288, 20212029. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, P.Z.; Diniz, P.; Helena, M.; Spyrides, C.; Pessoa, D.M.A. The effect of pelage, background, and distance on predator detection and the evolution of primate color vision. Am. J. Primatol. 2021, 83, e23230. [Google Scholar] [CrossRef]
- Karpestam, E.; Merilaita, S.; Forsman, A. Reduced predation risk for melanistic pygmy grasshoppers in post-fire environments. Evol. Evol. 2012, 2, 2204–2212. [Google Scholar] [CrossRef]
- Cheng, W.; Xing, S.; Chen, Y.; Lin, R.; Bonebrake, T.C.; Nakamura, A. Dark butterflies camouflaged from predation in dark tropical forest understories. Ecol. Entomol. 2018, 43, 304–309. [Google Scholar] [CrossRef]
- Sowersby, W.; Lehtonen, T.K.; Wong, B.B.M. Background matching ability and the maintenance of a colour polymorphism in the red devil cichlid. J. Evol. Biol. 2015, 28, 395–402. [Google Scholar] [CrossRef]
- Cournoyer, B.L.; Cohen, J.H. Cryptic coloration as a predator avoidance strategy in seagrass arrow shrimp colormorphs. J. Exp. Mar. Biol. Ecol. 2011, 402, 27–34. [Google Scholar] [CrossRef]
- Isaac, L.A.; Gregory, P.T. Can snakes hide in plain view? Chromatic and achromatic crypsis of two colour forms of the Western Terrestrial Garter Snake (Thamnophis elegans). Biol. J. Linn. Soc. 2013, 108, 756–772. [Google Scholar] [CrossRef]
- Tonkins, B.M.; Tyers, A.M.; Cooke, G.M. Cuttlefish in captivity: An investigation into housing and husbandry for improving welfare. Appl. Anim. Behav. Sci. 2015, 168, 77–83. [Google Scholar] [CrossRef]
- Holmes, A.M.; Emmans, C.J.; Jones, N.; Coleman, R.; Smith, T.E.; Hosie, C.A. Impact of tank background on the welfare of the African clawed frog, Xenopus laevis (Daudin). Appl. Anim. Behav. Sci. 2016, 185, 131–136. [Google Scholar] [CrossRef]
- Wilby, D.; Riches, S.; Daly, I.M.; Bird, A.; Wheelwright, M.; Foster, J.J. Hermit crabs (Pagurus bernhardus) use visual contrast in self-assessment of camouflage. J. Exp. Biol. 2018, 221, jeb173831. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, K.; Mettke-Hofmann, C. Colour polymorphic Gouldian finches avoid complex backgrounds but prefer simple camouflage colours over white backgrounds. Appl. Anim. Behav. Sci. 2018, 206, 102–108. [Google Scholar] [CrossRef]
- Dimitrova, M.; Merilaita, S. Prey concealment: Visual background complexity and prey contrast distribution. Behav. Ecol. 2010, 21, 176–181. [Google Scholar] [CrossRef]
- Dimitrova, M.; Merilaita, S. Prey pattern regularity and background complexity affect detectability of background-matching prey. Behav. Ecol. 2012, 23, 384–390. [Google Scholar] [CrossRef]
- Kjernsmo, K.; Merilaita, S. Background choice as an anti-predator strategy: The roles of background matching and visual complexity in the habitat choice of the least killifish. Proc. R. Soc. B Lond. 2012, 279, 4192–4198. [Google Scholar] [CrossRef]
- Delhey, K.; Peters, A. Conservation implications of anthropogenic impacts on visual communication and camouflage. Conserv. Biol. 2017, 31, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Matchette, S.R.; Cuthill, I.C.; Scott-Samuel, N.E. Concealment in a dynamic world: Dappled light and caustics mask movement. Anim. Behav. 2018, 143, 51–57. [Google Scholar] [CrossRef]
- Hughes, A.; Liggins, E.; Stevens, M. Imperfect camouflage: How to hide in a variable world? Proc. R. Soc. B 2019, 286, 20190646. [Google Scholar] [CrossRef]
- Dimitrova, M.; Merilaita, S. Hide and seek: Properties of prey and background patterns affect prey detection by blue tits. Behav. Ecol. 2014, 25, 402–408. [Google Scholar] [CrossRef]
- Maend, T.; Tammaru, T.; Mappes, J. Size dependent predation risk in cryptic and conspicuous insects. Evol. Ecol. 2007, 21, 485–498. [Google Scholar] [CrossRef]
- Brush, A.H.; Seifried, H. Pigmentation and Feather Structure in Genetic Variants of the Gouldian Finch, Poephila gouldiae. Auk 1968, 85, 416–430. [Google Scholar] [CrossRef]
- Dostine, P.L.; Johnson, G.C.; Franklin, D.C.; Zhang, Y.; Hempel, C. Seasonal use of savanna landscapes by the Gouldian finch, Erythrura gouldiae, in the Yinberrie Hills area, Northern Territory. Wildl. Res. 2001, 28, 445–458. [Google Scholar] [CrossRef]
- EPBC. Environment Protection and Biodiversity Conservation Act. Species Profile and Threat Database. Gouldian Finch. 2023. Available online: http://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=413 (accessed on 21 January 2023).
- Nicolai, J.; Steinbacher, J. Prachtfinken: Australien, Ozeanien, Suedostasien; Ulmer Publisher: Stuttgart, Germany, 2001. [Google Scholar]
- Franklin, D.C.; Burbidge, A.H.; Destine, P.L. The harvest of wild birds for aviculture: An historical perspective on finch trapping in the Kimberley with special emphasis on the Gouldian Finch. Aust. Zool. 1999, 31, 92–109. [Google Scholar] [CrossRef]
- Pryke, S.R. Fiery red heads: Female dominance among head color morphs in the Gouldian finch. Behav. Ecol. 2007, 18, 621–627. [Google Scholar] [CrossRef]
- Pryke, S.R.; Griffith, S.C. Red Dominates Black: Agonistic Signalling among Head Morphs in the Colour Polymorphic Gouldian Finch. Biol. Sci. 2006, 273, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.J.; King, A.J.; Mettke-Hofmann, C. Colourful characters: Head colour reflects personality in a social bird, the Gouldian finch, Erythrura gouldiae. Anim. Behav. 2012, 84, 159–165. [Google Scholar] [CrossRef]
- Mettke-Hofmann, C. The effect of head colour and age on personality traits in a social setting. Ethology 2012, 118, 906–916. [Google Scholar] [CrossRef]
- Mettke-Hofmann, C.; Eccles, G.R.; Greggor, A.L.; Bethell, E.J. Cognition in a changing world: Red-headed Gouldian finches enter spatially unfamiliar habitats more readily than do black-headed birds. Front. Ecol. Evol. 2020, 8, 498347. [Google Scholar] [CrossRef]
- Eccles, G.R.; Bethell, E.J.; Greggor, A.L.; Mettke-Hofmann, C. Individual variation in dietary wariness Is predicted by head color in a specialist feeder, the Gouldian Finch. Front. Ecol. Evol. 2021, 9, 772812. [Google Scholar] [CrossRef]
- Brazill-Boast, J.; Pryke, S.R.; Griffith, S.C. Nest-site utilisation and niche overlap in two sympatric, cavity-nesting finches. Emu 2010, 110, 170–177. [Google Scholar] [CrossRef]
- Brazill-Boast, J.; Dessmann, J.K.; Davies, G.T.O.; Pryke, S.R.; Griffith, S.C. Selection of breeding habitat by the endangered Gouldian finch (Erythrura gouldiae) at two spatial scales. Emu 2011, 111, 304–311. [Google Scholar] [CrossRef]
- Guidelines for the Use of Animals. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2018, 135, I–X. [Google Scholar] [CrossRef]
- Karpestam, E.; Merilaita, S.; Forsman, A. Detection experiments with humans implicate visual predation as a driver of colour polymorphism dynamics in pygmy grasshoppers. BMC Ecol. 2013, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Karpestam, E.; Merilaita, S.; Forsman, A. Natural levels of colour polymorphism reduce performance of visual predators searching for camouflaged prey. Biol. J. Linn. Soc. 2014, 112, 546–555. [Google Scholar] [CrossRef]
- Doktorovova, L.; Exnerova, A.; Hotova Svadova, A.; Stys, P.; Adamova-Jezova, D.; Zverev, V.; Kozlov, M.V.; Zvereva, E.L. Differential bird responses to colour morphs of an aposematic leaf beetle may affect variation in morph frequencies in polymorphic prey populations. Evol. Biol. 2019, 46, 35–46. [Google Scholar] [CrossRef]
- Morgans, C.L.; Ord, T.J. Natural selection in novel environments: Predation selects for background matching in the body colour of a land fish. Anim. Behav. 2013, 86, 1241–1249. [Google Scholar] [CrossRef]
- O’Reilly, A.O.; Hofmann, G.; Mettke-Hofmann, C. Gouldian finches are followers with black-headed females taking the lead. PLoS ONE 2019, 14, e0214531. [Google Scholar] [CrossRef] [PubMed]
- Merilaita, S.; Jormalainen, V. Evolution of sex differences in microhabitat choice and colour polymorphism in Idotea baltica. Anim. Behav. 1997, 54, 769–778. [Google Scholar] [CrossRef]
- Hatton, L. Predation as a Mechanism Maintaining Polymorphism: Evidence for Disruptive Selection in the Gouldian Finch. Master’s Thesis, Macquarie University, Sydney, Australia, 2013. [Google Scholar]
- Mettke-Hofmann, C. Morph composition matters in the Gouldian finch (Chloebia gouldiae): Involvement of red-headed birds increases vigilance. Birds 2021, 2, 404–414. [Google Scholar] [CrossRef]
- Mettke-Hofmann, C. Is vigilance a personality trait? Plasticity is key alongside some contextual consistency. PLoS ONE 2022, 17, e0279066. [Google Scholar] [CrossRef]

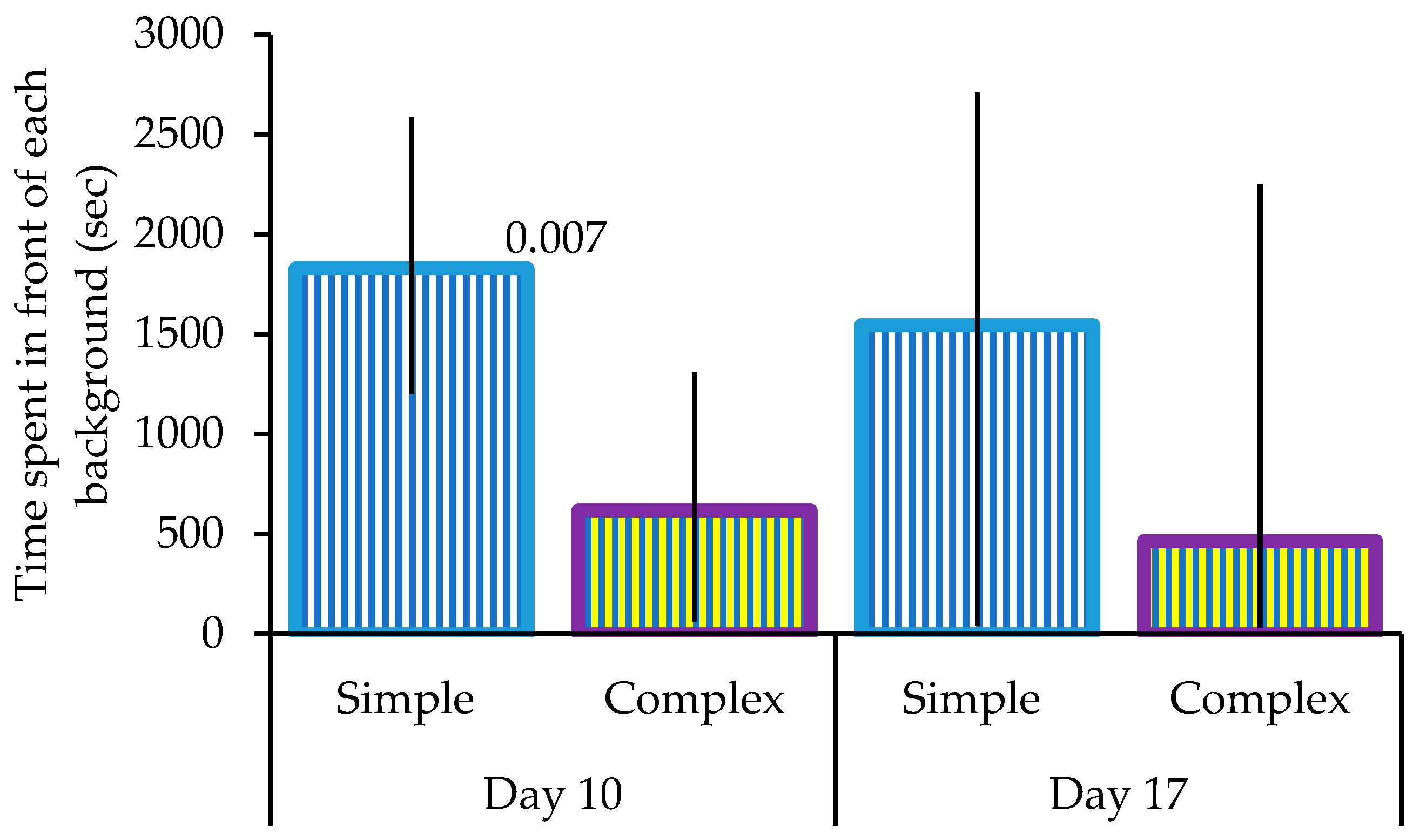
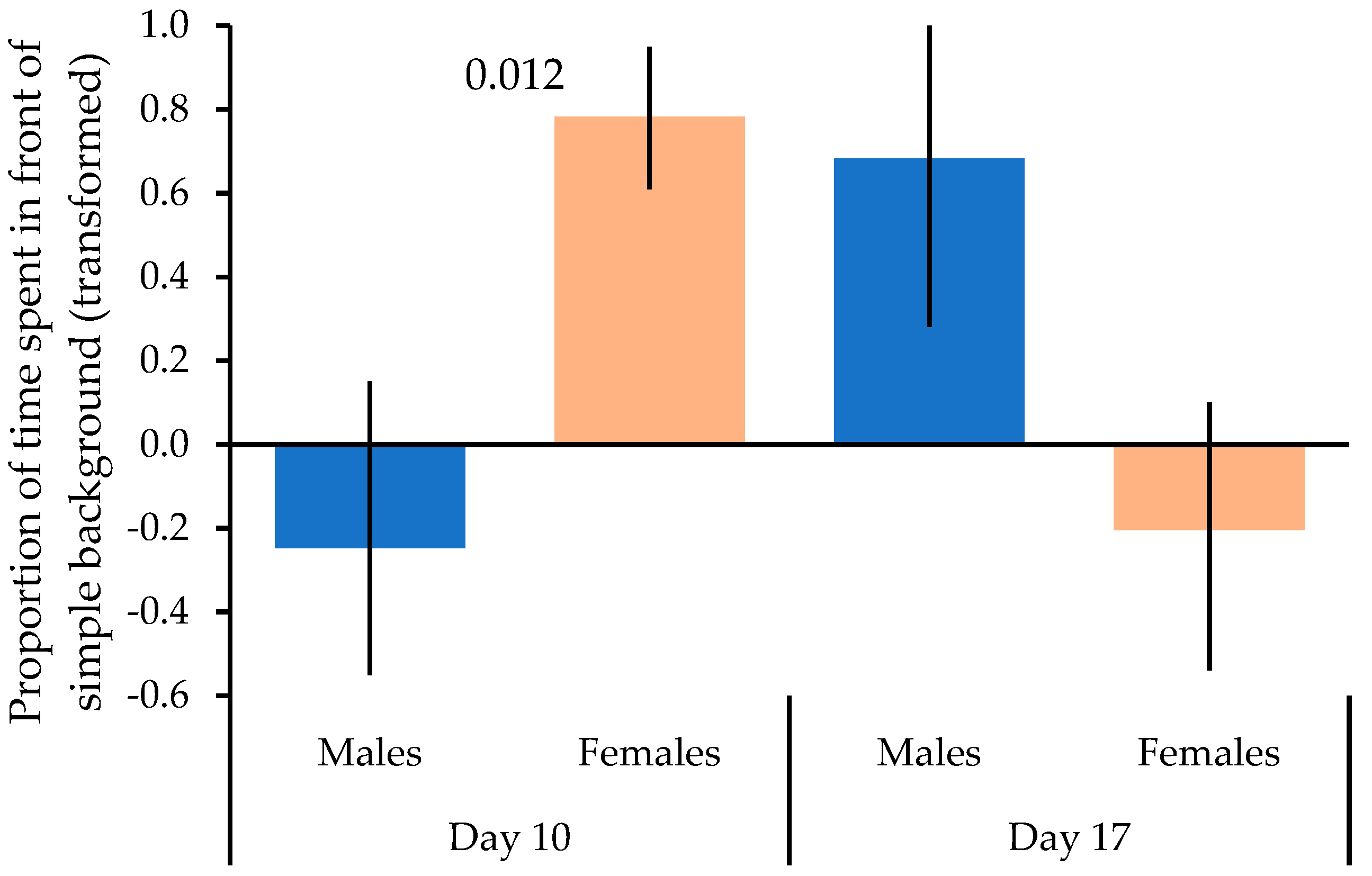

| Multivariate Tests | ||||
|---|---|---|---|---|
| Effects | F Value | Hypothesis df | Error df | Significance |
| Phase | 0.005 | 1 | 13 | 0.946 |
| Phase × sex | 7.292 | 1 | 13 | 0.018 |
| Phase × head colour | 0.871 | 1 | 13 | 0.368 |
| Phase × age class | 1.476 | 3 | 13 | 0.267 |
| Tests of between-subject effects | ||||
| Intercept | 3.945 | 1 | 0.069 | |
| Sex | 0.564 | 1 | 0.466 | |
| Head colour | 5.187 | 1 | 0.040 | |
| Age class | 1.610 | 3 | 0.235 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moise, R.I.; Eccles, G.R.; Mettke-Hofmann, C. Enclosure Background Preferences Differ between Sexes and Color Morphs in the Gouldian Finch. Animals 2023, 13, 1353. https://doi.org/10.3390/ani13081353
Moise RI, Eccles GR, Mettke-Hofmann C. Enclosure Background Preferences Differ between Sexes and Color Morphs in the Gouldian Finch. Animals. 2023; 13(8):1353. https://doi.org/10.3390/ani13081353
Chicago/Turabian StyleMoise, Robert I., Georgina R. Eccles, and Claudia Mettke-Hofmann. 2023. "Enclosure Background Preferences Differ between Sexes and Color Morphs in the Gouldian Finch" Animals 13, no. 8: 1353. https://doi.org/10.3390/ani13081353
APA StyleMoise, R. I., Eccles, G. R., & Mettke-Hofmann, C. (2023). Enclosure Background Preferences Differ between Sexes and Color Morphs in the Gouldian Finch. Animals, 13(8), 1353. https://doi.org/10.3390/ani13081353







